Clinical Evidence for Microbiome-Based Strategies in Cancer Immunotherapy: A State-of-the-Art Review
Abstract
1. Introduction
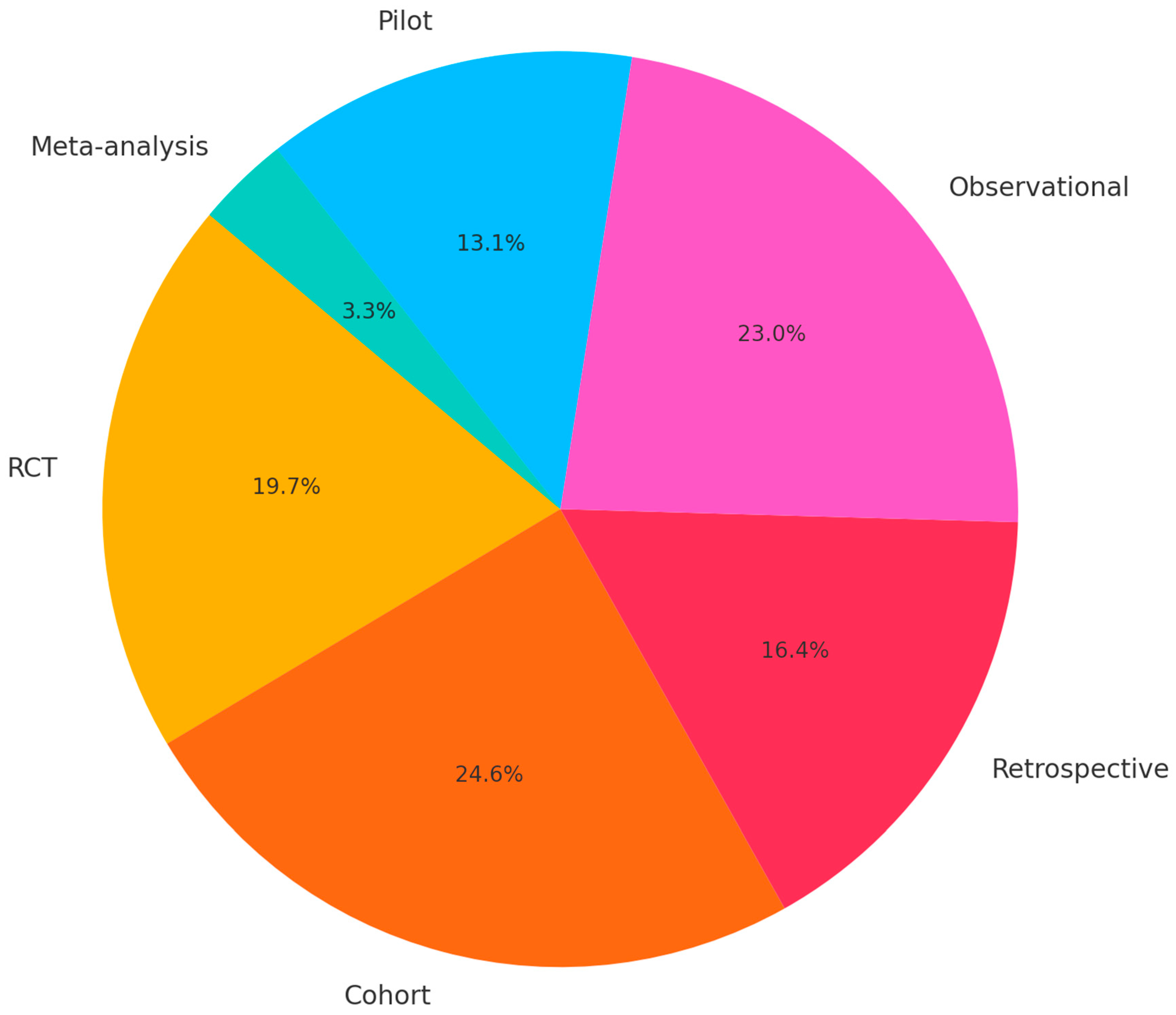
2. Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Synthesis
2.3. Risk of Bias Assessment
2.4. Ethics and Limitations
3. Results
3.1. Antibiotic Exposure
3.2. Fecal Microbiota Transplantation (FMT)
3.3. Dietary Fiber
3.4. Probiotics
3.5. Vidutolimod Plus Nivolumab
3.6. Microbiome Diversity and Predictive Models
3.7. Diet–Microbiome Interactions
3.8. Traditional Chinese Medicine (TCM)
3.9. Multi-Omics Integration
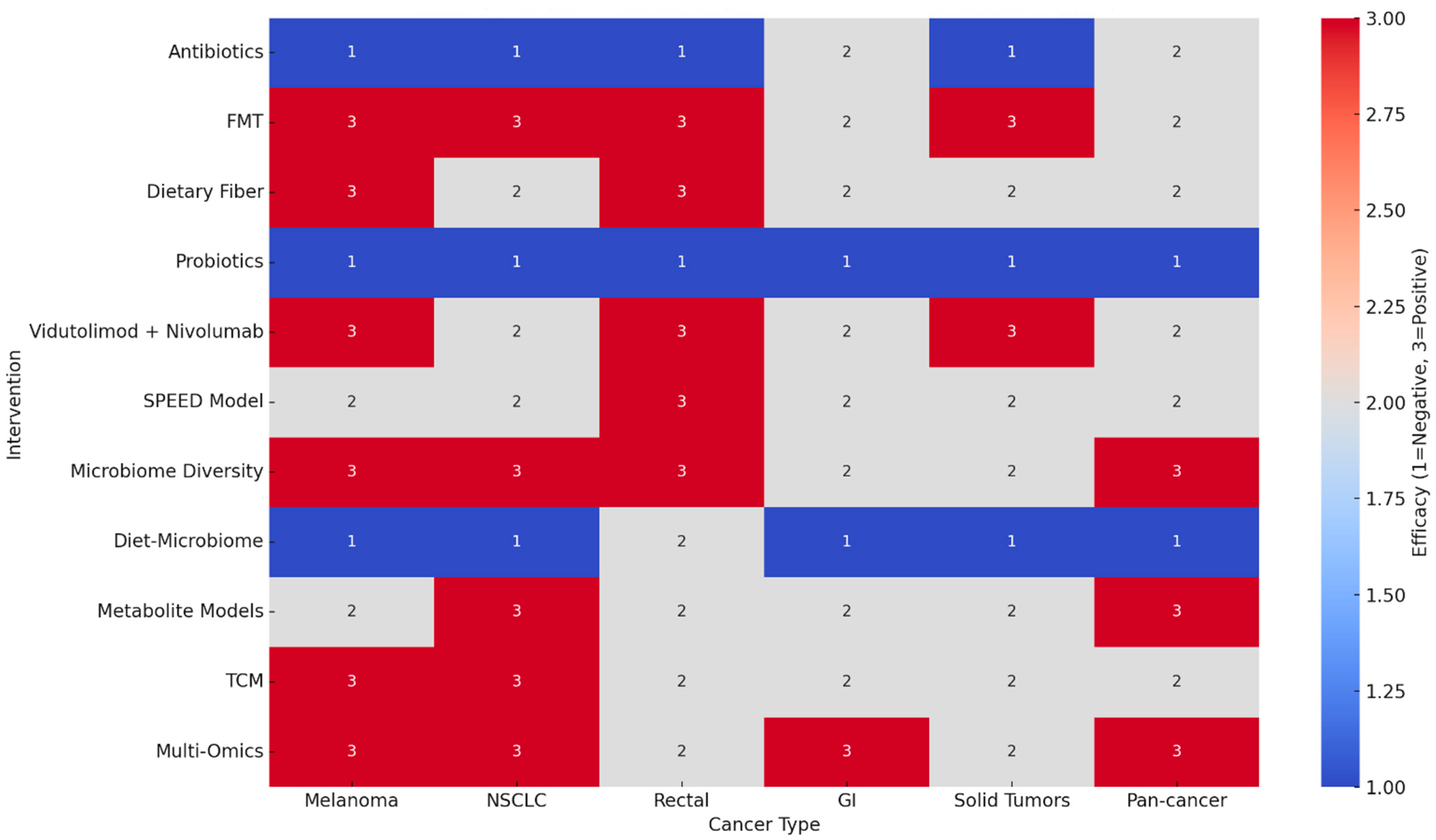
4. Cross-Intervention Synthesis
5. GRADE Evidence
- High-certainty evidence was not reached in any domain due to methodological heterogeneity, open-label designs, and reliance on observational cohorts.
- Moderate certainty: The strongest evidence supports the negative impact of antibiotics and probiotics and the positive impact of FMT, dietary fiber, vidutolimod combinations, and baseline microbiome diversity.
- Low certainty: Diet–microbiome interactions, TCM, and multi-omics approaches remain promising but exploratory, requiring validation in larger RCTs.
- Consistency: Across tumor types, the microbiome signal was remarkably concordant, particularly regarding antibiotic harm and fiber/FMT benefit.
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Zuo, T.; Ng, S.C. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma. J. Immunother. Cancer 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Fidelle, M.; Iebba, V.; Alla, L.; Pasolli, E.; Segata, N.; Desnoyer, A.; Pietrantonio, F.; Ferrere, G.; et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 2020, 78, 195–206. [Google Scholar] [CrossRef]
- Wilson, B.E.; Routy, B.; Nagrial, A.; Chin, V.T. The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: A systematic review and meta-analysis of observational studies. Cancer Immunol. Immunother. 2020, 69, 343–354. [Google Scholar] [CrossRef]
- Giampazolias, E.; da Costa, M.P.; Lam, K.C.; Lim, K.H.J.; Cardoso, A.; Piot, C.; Chakravarty, P.; Blasche, S.; Patel, S.; Biram, A.; et al. Vitamin D regulates microbiome-dependent cancer immunity. Science 2024, 384, 428–437. [Google Scholar] [CrossRef]
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022, 28, 704–712. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, J.; Han, J.; Li, A.; Liu, G.; Sun, Y.; Zheng, J.; Zhang, J.; Chen, G.; Xu, R.; et al. Gut microbiome model predicts response to neoadjuvant immunotherapy plus chemoradiotherapy in rectal cancer. Med 2024, 5, 1293–1306.e4. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Albiges, L.; Haanen, J.B.A.G.; Larkin, J.M.; Uemura, M.; Pal, S.K.; Gravis, G.; Campbell, M.T.; Penkov, K.; Lee, J.-L.; et al. The gut microbiome (GM) and probiotics influence response to immune checkpoint blockade (ICB) in melanoma. J. Clin. Oncol. 2019, 37 (Suppl. 15), 101. [Google Scholar] [CrossRef]
- Ribas, A.; Medina, T.; Kummar, S.; Amin, A.; Kalbasi, A.; Drabick, J.J.; Barve, M.; Daniels, G.A.; Wong, D.J.; Schmidt, E.V.; et al. SD-101 in combination with pembrolizumab in advanced melanoma: Results of a phase 1b, multicenter study. Cancer Discov. 2018, 8, 1250–1257. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8+ T Cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guan, D.; Wang, Z.; Li, X.; Dong, S.; Huang, J.; Zhou, W. Microbiota in cancer: Molecular mechanisms and therapeutic interventions. MedComm 2023, 4, e417. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Duan, G.; Wei, D.; Zhao, C.; Ma, Y. The Bile Acid Membrane Receptor TGR5 in Cancer: Friend or Foe? Molecules 2022, 27, 5292. [Google Scholar] [CrossRef]
- Elkrief, A.; Derosa, L.; Kroemer, G.; Zitvogel, L.; Routy, B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: A new independent prognostic factor? Ann. Oncol. 2019, 30, 1572–1579. [Google Scholar] [CrossRef]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019, 5, 1774–1778. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- McCulloch, J.A.; Davar, D.; Rodrigues, R.R.; Badger, J.H.; Fang, J.R.; Cole, A.M.; Balaji, A.K.; Vetizou, M.; Prescott, S.M.; Fernandes, M.R. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, N.; Xu, L.; Jia, X.; Liu, F.; Huang, J.; Li, X.; Wang, Y.; Lai, C.; Shen, Y.; et al. Integrative multi-omics analysis reveals the LncRNA 60967.1-PLCD4-ATRA axis as a key regulator of colorectal cancer progression and immune response. Mol. Cancer 2025, 24, 164. [Google Scholar] [CrossRef]
- Rosario, S.R.; Long, M.D.; Chilakapati, S.; Gomez, E.C.; Battaglia, S.; Singh, P.K.; Wang, J.; Wang, K.; Attwood, K.; Hess, S.M.; et al. Integrative multi-omics analysis uncovers tumor-immune-gut axis influencing immunotherapy outcomes in ovarian cancer. Nat. Commun. 2024, 15, 10609. [Google Scholar] [CrossRef]
- Ma, J.; Sun, S.; Cheng, X.; Meng, C.; Zhao, H.; Fu, W.; Gao, Y.; Ma, L.; Yang, Z.; Yao, H.; et al. Unraveling the role of gut microbiome in predicting adverse events in neoadjuvant therapy for rectal cancer. Hum. Vaccin. Immunother. 2024, 20, 2430087. [Google Scholar] [CrossRef]
- Ullern, A.; Holm, K.; Røssevold, A.H.; Andresen, N.K.; Bang, C.; Lingjærde, O.C.; Naume, B.; Hov, J.R.; Kyte, J.A. Gut microbiota diversity is prognostic and associated with benefit from chemo-immunotherapy in metastatic triple-negative breast cancer. Mol. Oncol. 2025, 19, 1229–1243. [Google Scholar] [CrossRef]
- Sitthideatphaiboon, P.; Somlaw, N.; Zungsontiporn, N.; Ouwongprayoon, P.; Sukswai, N.; Korphaisarn, K.; Poungvarin, N.; Aporntewan, C.; Hirankarn, N.; Vinayanuwattikun, C.; et al. Dietary pattern and the corresponding gut microbiome in response to immunotherapy in Thai patients with advanced non-small cell lung cancer (NSCLC). Sci. Rep. 2024, 14, 27791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bzura, A.; Baitei, E.Y.; Zhou, Z.; Spicer, J.B.; Poile, C.; Rogel, J.; Branson, A.; King, A.; Barber, S.; et al. A gut microbiota rheostat forecasts responsiveness to PD-L1 and VEGF blockade in mesothelioma. Nat. Commun. 2024, 15, 7187. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, S.; Kajiwara, Y.; Taniguchi, K.; Hida, A.I.; Miyoshi, Y.; Kin, T.; Yamamoto, M.; Takabatake, D.; Kubo, S.; Hikino, H.; et al. Baseline gut microbiota as a predictive marker for the efficacy of neoadjuvant chemotherapy in patients with early breast cancer. Breast Cancer Res. Treat. 2024, 208, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Bukavina, L.; Ginwala, R.; Eltoukhi, M.; Sindhani, M.; Prunty, M.; Geynisman, D.M.; Ghatalia, P.; Valentine, H.; Calaway, A.; Correa, A.F.; et al. Role of gut microbiome in neoadjuvant chemotherapy response in urothelial carcinoma: A multi-institutional prospective cohort evaluation. Cancer Res. Commun. 2024, 4, 1505–1516. [Google Scholar] [CrossRef]
- Glitza, I.C.; Seo, Y.D.; Spencer, C.N.; Wortman, J.R.; Burton, E.M.; Alayli, F.A.; Loo, C.P.; Gautam, S.; Damania, A.; Densmore, J.; et al. Randomized placebo-controlled, biomarker-stratified phase Ib microbiome modulation in melanoma: Impact of antibiotic preconditioning. Cancer Discov. 2024, 14, 1161–1175. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H., Jr.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Punčochář, M.; et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: A phase I trial. Nat. Med. 2023, 29, 2121–2132. [Google Scholar] [CrossRef]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.-J.; Hanna, E.; et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G.; Kim, S.; Cho, B.; Kim, S.-Y.; Do, E.-J.; Bae, D.-J.; Kim, S.; Kweon, M.-N.; Song, J.S.; et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe 2024, 32, 1380–1393.e9. [Google Scholar] [CrossRef]
- Cascone, T.; Leung, C.H.; Weissferdt, A.; Pataer, A.; Carter, B.W.; Godoy, M.C.B.; Feldman, H.; William, W.N., Jr.; Xi, Y.; Basu, S.; et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: The phase 2 NEOSTAR trial. Nat. Med. 2023, 29, 593–604. [Google Scholar] [CrossRef]
- Olson, D.J.; Eroglu, Z.; Brockstein, B.; Poklepovic, A.S.; Bajaj, M.; Babu, S.; Hallmeyer, S.; Velasco, M.; Lutzky, J.; Higgs, E.; et al. Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J. Clin. Oncol. 2021, 39, 2647–2655. [Google Scholar] [CrossRef] [PubMed]
- Glitza Oliva, I.C.; Ferguson, S.D.; Bassett, R., Jr.; Foster, A.P.; John, I.; Hennegan, T.D.; Rohlfs, M.; Richard, J.; Iqbal, M.; Dett, T.; et al. Concurrent intrathecal and intravenous nivolumab in leptomeningeal disease: Phase 1 trial interim results. Nat. Med. 2023, 29, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomized phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Gunjur, A.; Shao, Y.; Rozday, T.; Klein, O.; Mu, A.; Haak, B.W.; Markman, B.; Kee, D.; Carlino, M.S.; Underhill, C.; et al. A gut microbial signature for combination immune checkpoint blockade across cancer types. Nat. Med. 2024, 30, 797–809. [Google Scholar] [CrossRef]
- Naqash, A.R.; Kihn-Alarcón, A.J.; Stavraka, C.; Kerrigan, K.; Maleki Vareki, S.; Pinato, D.J.; Puri, S. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann. Transl. Med. 2021, 9, 1034. [Google Scholar] [CrossRef]
- Chang, C.W.; Lee, H.C.; Li, L.H.; Chang, Y.H.; Wu, M.S.; Lin, J.T.; Wang, H.P.; Chen, C.C. Fecal microbiota transplantation alleviates immune checkpoint inhibitor-induced colitis. Clin. Transl. Gastroenterol. 2020, 11, e00134. [Google Scholar] [CrossRef]
- Wang, F.; Yin, Q.; Chen, L.; Davis, M.M.; Zhang, C. Gut microbiome composition modulates the antitumor effect of PD-1 blockade in murine models. Cell. Rep. 2018, 23, 3891–3903. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut microbiome affects the response to anti–PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer. 2019, 7, 193. [Google Scholar] [CrossRef]
- Li, H.; Zandberg, D.P.; Kulkarni, A.; Chiosea, S.I.; Santos, P.M.; Isett, B.R.; Joy, M.; Sica, G.L.; Contrera, K.J.; Tatsuoka, C.M.; et al. Distinct CD8(+) T cell dynamics associate with response to neoadjuvant cancer immunotherapies. Cancer Cell. 2025, 43, 757–775.e8. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Wilson, M.; Moran, U.; Pavlick, A.; Izsak, A.; Wechter, T.; Weber, J.S.; Osman, I.; Ahn, J. Relating the gut microbiome and outcomes in patients with advanced melanoma treated with immune checkpoint inhibitors. J. Natl. Cancer Inst. 2019, 111, 616–622. [Google Scholar] [CrossRef]
- Lee, K.A.; Thomas, A.M.; Bolte, L.A.; Björk, J.R.; de Ruijter, L.K.; Armanini, F.; de Vos, W.M.; Reijnders, D.; Adriaans, D.; Dekker, E.; et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 2022, 28, 1620–1631. [Google Scholar] [CrossRef]
- Davar, D.; Morrison, R.M.; Dzutsev, A.K.; Karunamurthy, A.; Chauvin, J.-M.; Amatore, F.; Deutsch, J.S.; Neves, R.X.D.; Rodrigues, R.R.; McCulloch, J.A.; et al. Neoadjuvant vidutolimod and nivolumab in high-risk resectable melanoma: A prospective phase II trial. Cancer Cell. 2024, 42, 1898–1918.e12. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.; Feng, X.; Xu, W.; Luo, R.; Zhu, Z.; Zeng, Q.; He, Z. Advances in efficacy prediction and monitoring of neoadjuvant immunotherapy for non-small cell lung cancer. Front. Oncol. 2023, 13, 1145128. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non–small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Elkrief, A.; El Raichani, L.; Richard, C.; Messaoudene, M.; Belkaid, W.; Malo, J.; Belanger, K.; Miller, W.; Jamal, R.; Letarte, N.; et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology 2019, 8, e1568812. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.-S.; Derosa, L.; Khan, M.A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C.; et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, H.; Xia, L.; Yang, Y.; Zhu, Y.; Shen, Y.; Zheng, H.; Yao, C.; Wang, Y.; Lu, S. The diversity of gut microbiome is associated with favorable responses to anti–PD-1 immunotherapy in Chinese patients with NSCLC. J. Thorac. Oncol. 2019, 14, 1378–1389. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.-F.; Li, J.; Lu, M.; et al. The gut microbiome is associated with clinical response to anti–PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Coutzac, C.; Jouniaux, J.M.; Paci, A.; Tang, Y.; Ji, J.; Wang, H.; Karim, R.; Rosas, C.; Huang, Y.; Zhai, Y. Antibiotics are associated with attenuated efficacy of immune checkpoint blockers in patients with advanced renal cell and non–small-cell lung cancer. Ann. Oncol. 2019, 30, 651–659. [Google Scholar] [CrossRef]
- Hakozaki, T.; Richard, C.; Elkrief, A.; Hosomi, Y.; Benlaïfaoui, M.; Mimpen, I.; Terrisse, S.; Derosa, L.; Zitvogel, L.; Routy, B.; et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced NSCLC. Cancer Immunol. Res. 2020, 8, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, F.S.; Kasper, D.L. The gut microbiome and cancer response to immune checkpoint inhibitors. J. Clin. Investig. 2025, 135, e178897. [Google Scholar] [CrossRef]
- Botticelli, A.; Zizzari, I.G.; Mazzuca, F.; Ascierto, P.A.; Putignani, L.; Marchetti, L.; Napoletano, C.; Nuti, M.; Marchetti, P. Cross-talk between microbiota and immune fitness to steer and control response to anti–PD-1/PD-L1 treatment. J. Exp. Clin. Cancer Res. 2019, 38, 264. [Google Scholar] [CrossRef]
- Raziq, M.F.; Manzoor, H.; Kayani, M.U.R. Non-small Cell Lung Cancer, Immunotherapy and the Influence of Gut Microbiome. Curr. Microbiol. 2025, 82, 419. [Google Scholar] [CrossRef]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; DuPont, H.L.; Jiang, Z.-D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.-C.; et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor–associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef]
- Kaderbhai, C.; Richard, C.; Fumet, J.D.; Aarnink, A.; Foucher, P.; Coudert, B.; Favier, L.; Lagrange, A.; Limagne, E.; Boidot, R.; et al. Antibiotic Use Does Not Appear to Influence Response to Nivolumab. Anticancer Res. 2017, 37, 3195–3200. [Google Scholar] [CrossRef]
- Grenda, A.; Iwan, E.; Kuźnar-Kamińska, B.; Bomba, A.; Bielińska, K.; Krawczyk, P.; Chmielewska, I.; Frąk, M.; Szczyrek, M.; Rolska-Kopińska, A.; et al. Gut microbial predictors of first-line immunotherapy efficacy in advanced NSCLC patients. Sci. Rep. 2025, 15, 6139. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhu, X.; Wang, Y.; Lu, S. The gut microbiota improves the efficacy of immune-checkpoint inhibitor immunotherapy against tumors: From association to cause and effect. Cancer Lett. 2024, 598, 217123. [Google Scholar] [CrossRef]
- Petrelli, F.; Iaculli, A.; Signorelli, D.; Ghidini, A.; Dottorini, L.; Perego, G.; Cabiddu, M.; Borgonovo, K.; Parati, M.C.; Ghilardi, M.; et al. Survival of patients treated with antibiotics and immunotherapy for cancer: A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 1458. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Nagatomo, R.; Doi, K.; Shimizu, J.; Baba, K.; Saito, T.; Matsumoto, S.; Inoue, K.; Muto, M. Association of short-chain fatty acids in the gut with response to nivolumab in advanced non–small cell lung cancer. Transl. Lung. Cancer Res. 2020, 9, 1900–1909. [Google Scholar] [CrossRef]
- Li, Y.; Tinoco, R.; Elmén, L.; Segota, I.; Xian, Y.; Fujita, Y.; Sahu, A.; Zarecki, R.; Marie, K.; Feng, Y.; et al. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5–/– mice. Nat. Commun. 2019, 10, 1492. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; Federici, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Atarashi, K.; Honda, K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 2016, 16, 295–309. [Google Scholar] [CrossRef]
- Rosshart, S.P.; Herz, J.; Vassallo, B.G.; Hunter, A.; Wall, M.K.; Badger, J.H.; McCulloch, J.A.; Anastasakis, D.G.; Sarshad, A.A.; Leonardi, I.; et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 2019, 365, eaaw4361. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Ben-Betzalel, G.; Elinav, E. Fecal microbiota transplantation for refractory melanoma patients: Mechanisms and predictive biomarkers. Cell Host Microbe 2021, 29, 844–853.e5. [Google Scholar] [CrossRef]
- Ma, W.; Mao, Q.; Xia, W.; Dong, G.; Yu, C.; Jiang, F. Gut microbiota shapes the efficiency of cancer therapy. Front. Microbiol. 2019, 10, 1050. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Pleiotropic Effects of Metformin on the Antitumor Efficiency of Immune Checkpoint Inhibitors. Front. Immunol. 2021, 11, 586760. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Oh, B.; Boyle, F.; Pavlakis, N.; Clarke, S.; Eade, T.; Hruby, G.; Lamoury, G.; Carroll, S.; Morgia, M.; Kneebone, A.; et al. The gut microbiome and cancer immunotherapy. Cancers 2021, 13, 4824. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Helmink, B.A.; Wargo, J.A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019, 20, e77–e91. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Sholl, L.M.; Hirsch, F.R.; Hwang, D.; Botling, J.; Lopez-Rios, F.; Bubendorf, L.; Mino-Kenudson, M.; Roden, A.C.; Beasley, M.B.; Borczuk, A.; et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: A perspective from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1409–1424. [Google Scholar] [CrossRef] [PubMed]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lichterman, J.N.; Coughlin, L.A.; Poulides, N.; Li, W.; Del Valle, P.; Palmer, S.N.; Gan, S.; Kim, J.; Zhan, X.; et al. Immune checkpoint blockade induces gut microbiota translocation that augments extraintestinal antitumor immunity. Sci. Immunol. 2023, 8, eabo2003. [Google Scholar] [CrossRef] [PubMed]
- Cass, S.; White, M.G. The Influence of the Microbiome on Metastatic Colorectal Cancer. Clin. Colon Rectal Surg. 2023, 36, 112–119. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.; Ren, Y.; Weng, S.; Weng, S.; Liu, L.; Guo, C.; Wang, L.; Han, X.; Ren, J.; et al. Antitumor effects of fecal microbiota transplantation: Implications for microbiome modulation in cancer treatment. Front. Immunol. 2022, 13, 949490. [Google Scholar] [CrossRef]
- Michikawa, C.; Gopalakrishnan, V.; Harrandah, A.M.; Karpinets, T.V.; Garg, R.R.; Chu, R.A.; Park, Y.P.; Chukkapallia, S.S.; Yadlapalli, N.; Erikson-Carter, K.C.; et al. Fusobacterium is enriched in oral cancer and promotes induction of programmed death-ligand 1 (PD-L1). Neoplasia 2022, 31, 100813. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Brunner, V.; Tschurtschenthaler, M. Microbiota and Colorectal Cancer: From Gut to Bedside. Front. Pharmacol. 2021, 12, 760280. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Weiner, B.; Ford, C.B.; Sellman, B.R.; Hammond, S.A.; Freeman, D.J.; Dennis, P.; Soria, J.-C.; Wortman, J.R.; Henn, M.R. Intervention strategies for microbial therapeutics in cancer immunotherapy. Immunooncol. Technol. 2020, 6, 9–17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrelli, F.; Ghidini, A.; Dottorini, L.; Ghidini, M.; Zaniboni, A.; Tomasello, G. Clinical Evidence for Microbiome-Based Strategies in Cancer Immunotherapy: A State-of-the-Art Review. Medicina 2025, 61, 1595. https://doi.org/10.3390/medicina61091595
Petrelli F, Ghidini A, Dottorini L, Ghidini M, Zaniboni A, Tomasello G. Clinical Evidence for Microbiome-Based Strategies in Cancer Immunotherapy: A State-of-the-Art Review. Medicina. 2025; 61(9):1595. https://doi.org/10.3390/medicina61091595
Chicago/Turabian StylePetrelli, Fausto, Antonio Ghidini, Lorenzo Dottorini, Michele Ghidini, Alberto Zaniboni, and Gianluca Tomasello. 2025. "Clinical Evidence for Microbiome-Based Strategies in Cancer Immunotherapy: A State-of-the-Art Review" Medicina 61, no. 9: 1595. https://doi.org/10.3390/medicina61091595
APA StylePetrelli, F., Ghidini, A., Dottorini, L., Ghidini, M., Zaniboni, A., & Tomasello, G. (2025). Clinical Evidence for Microbiome-Based Strategies in Cancer Immunotherapy: A State-of-the-Art Review. Medicina, 61(9), 1595. https://doi.org/10.3390/medicina61091595








