The Diagnostic Role of Tumor and Inflammatory Biomarkers in Ascitic Fluid: A Systematic Review
Abstract
1. Introduction
- Do tumor markers measured in ascitic fluid offer clinically useful diagnostic and prognostic accuracy for malignant ascites?
- Are inflammatory markers in ascitic fluid effective in differentiating between inflammatory and malignant ascites?
- Does combining tumor and inflammatory markers enhance the ability to distinguish between malignant and non-malignant ascites?
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection
2.5. Data Collection and Extraction
- Study characteristics (author, year, country, sample size, age, duration and setting);
- Type of marker(s) evaluated (tumor and inflammatory);
- Fluid analyzed (ascitic fluid);
- Diagnostic performance (sensitivity, specificity and AUC, if available);
- Prognostic significance (if available);
- Study design (e.g., prospective, retrospective).
2.6. Summary Measures
2.7. Synthesis of Results
2.8. Reporting Standards and Ethical Statement
3. Results
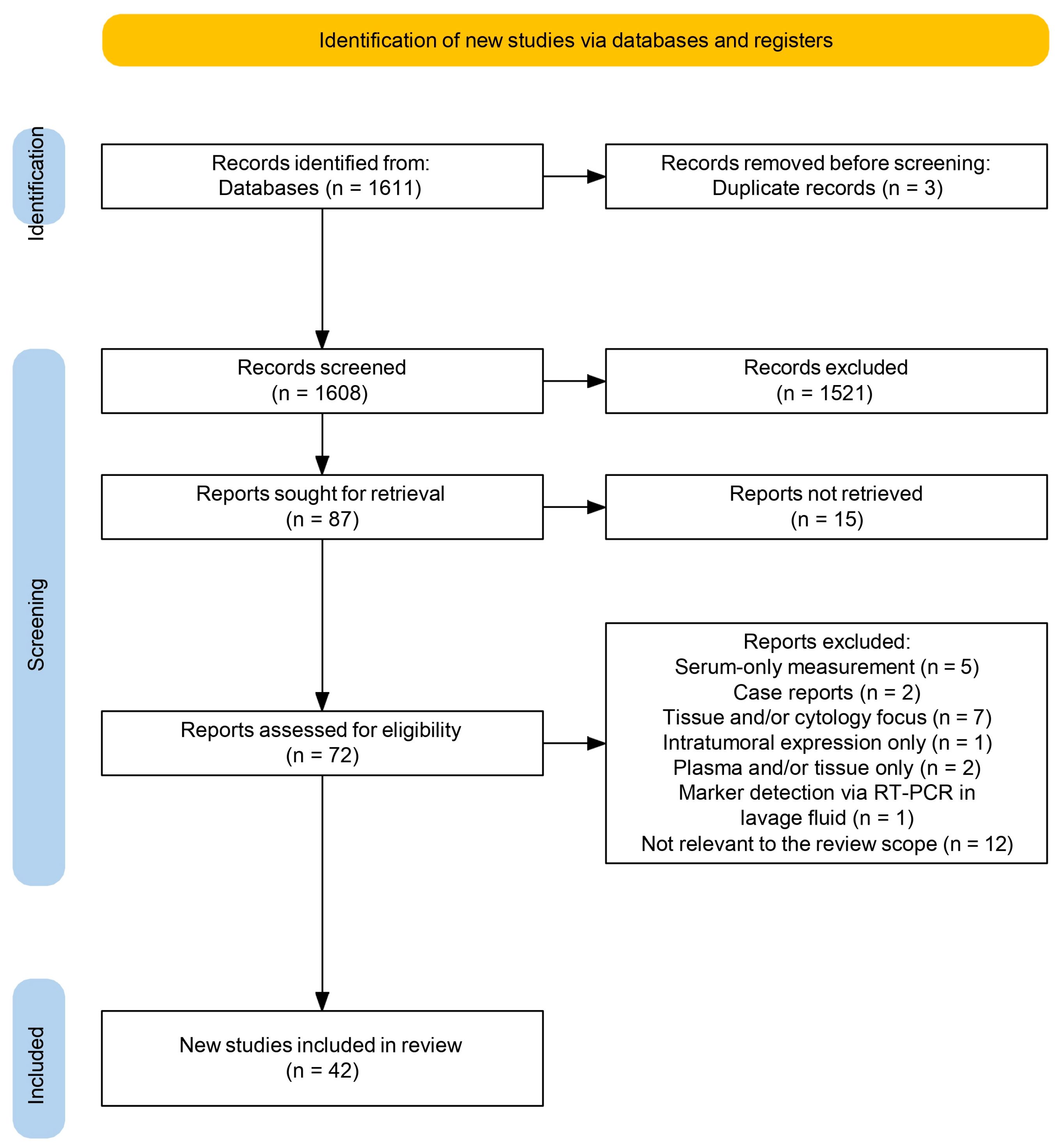
3.1. Study Selection and Overview
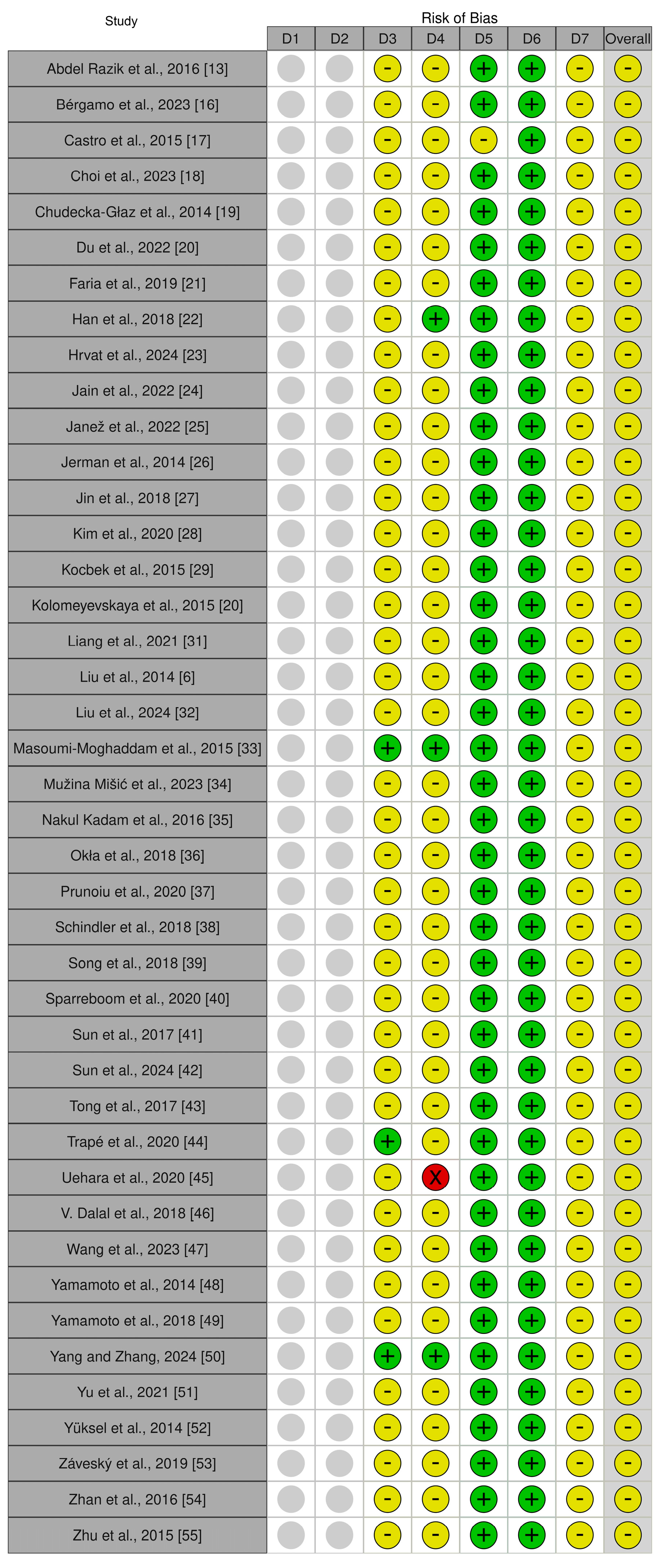
3.2. Risk-of-Bias Assessment
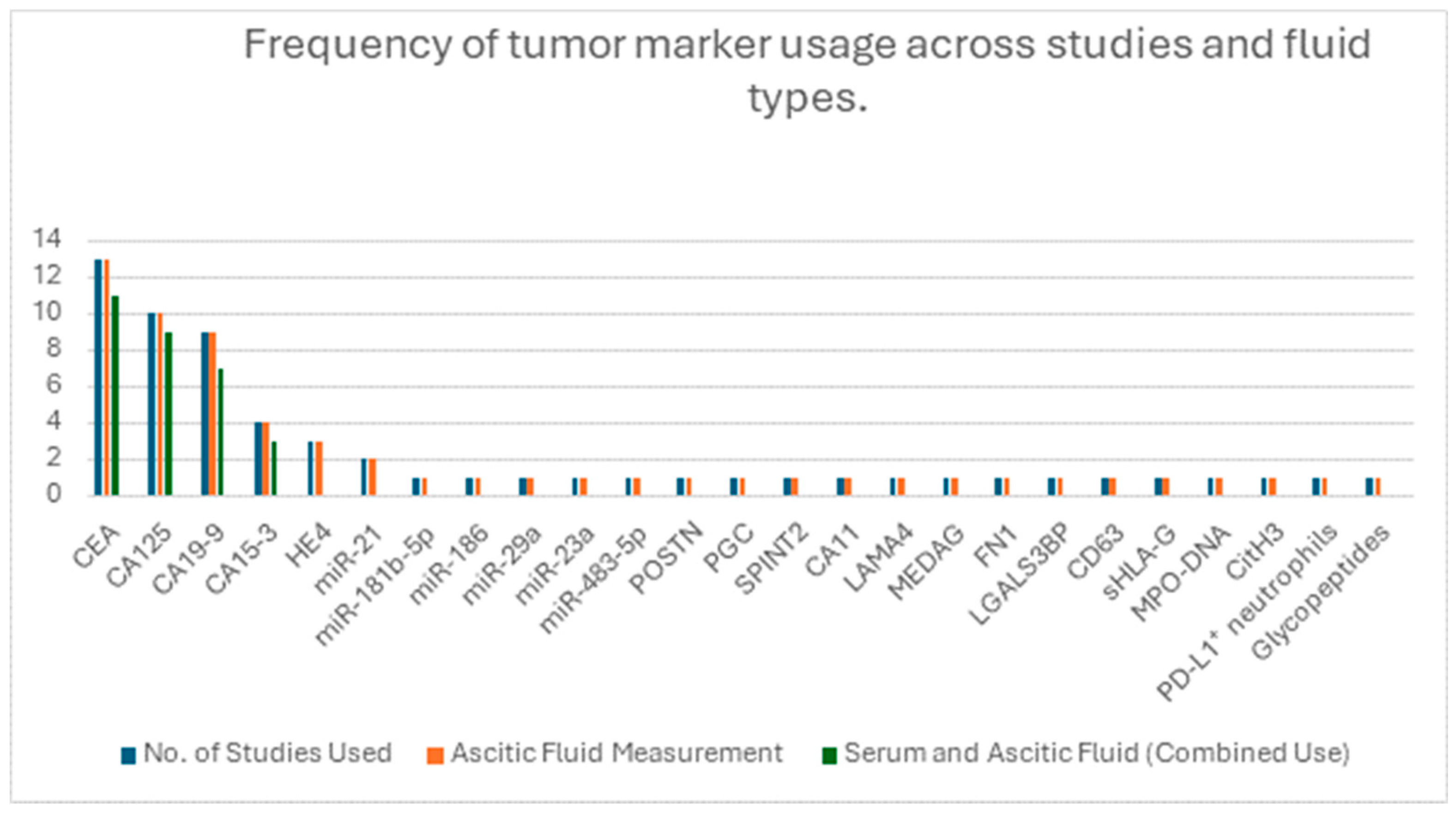
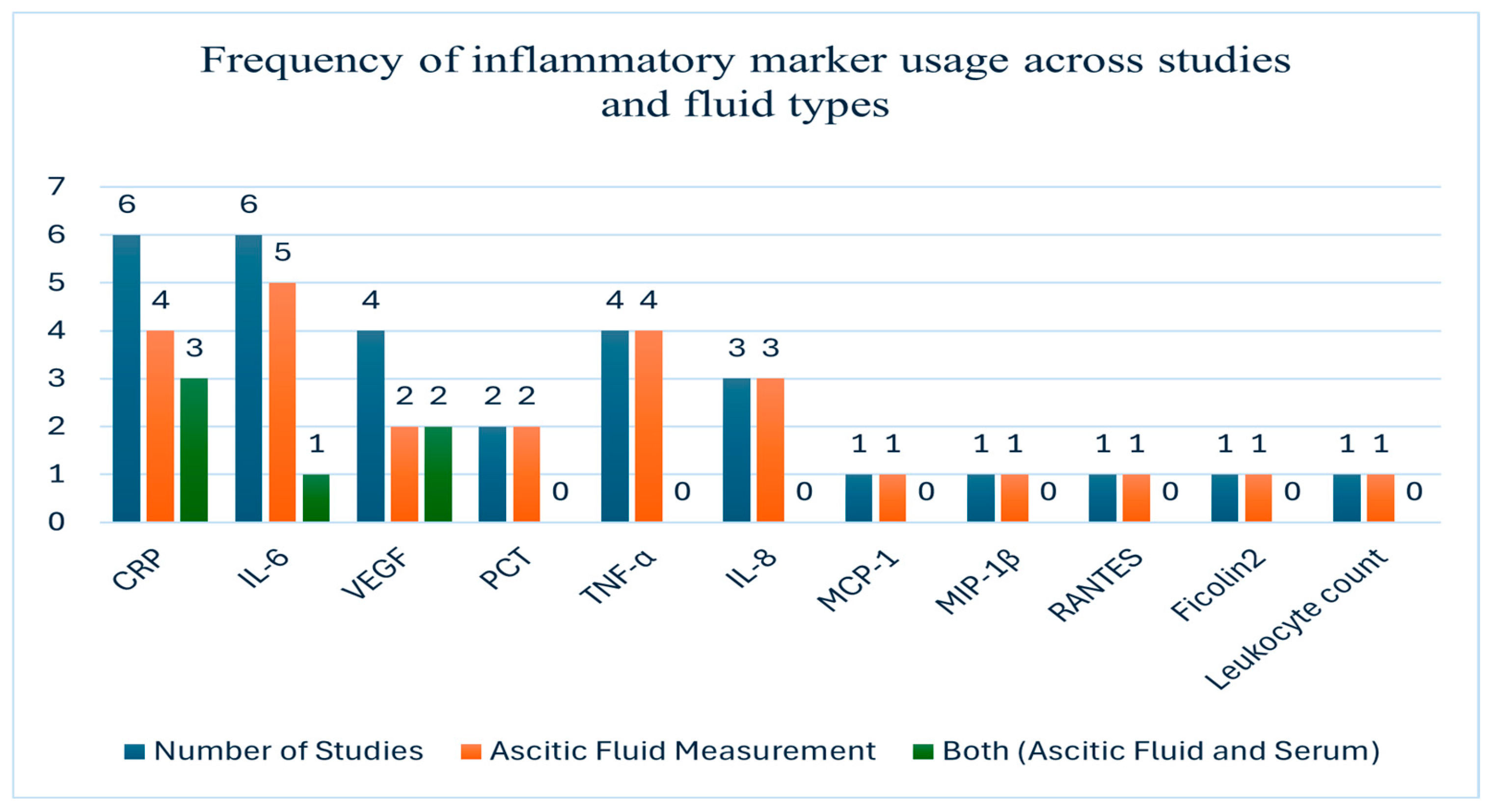
3.3. Biomarker Evaluation in Ascitic Fluid
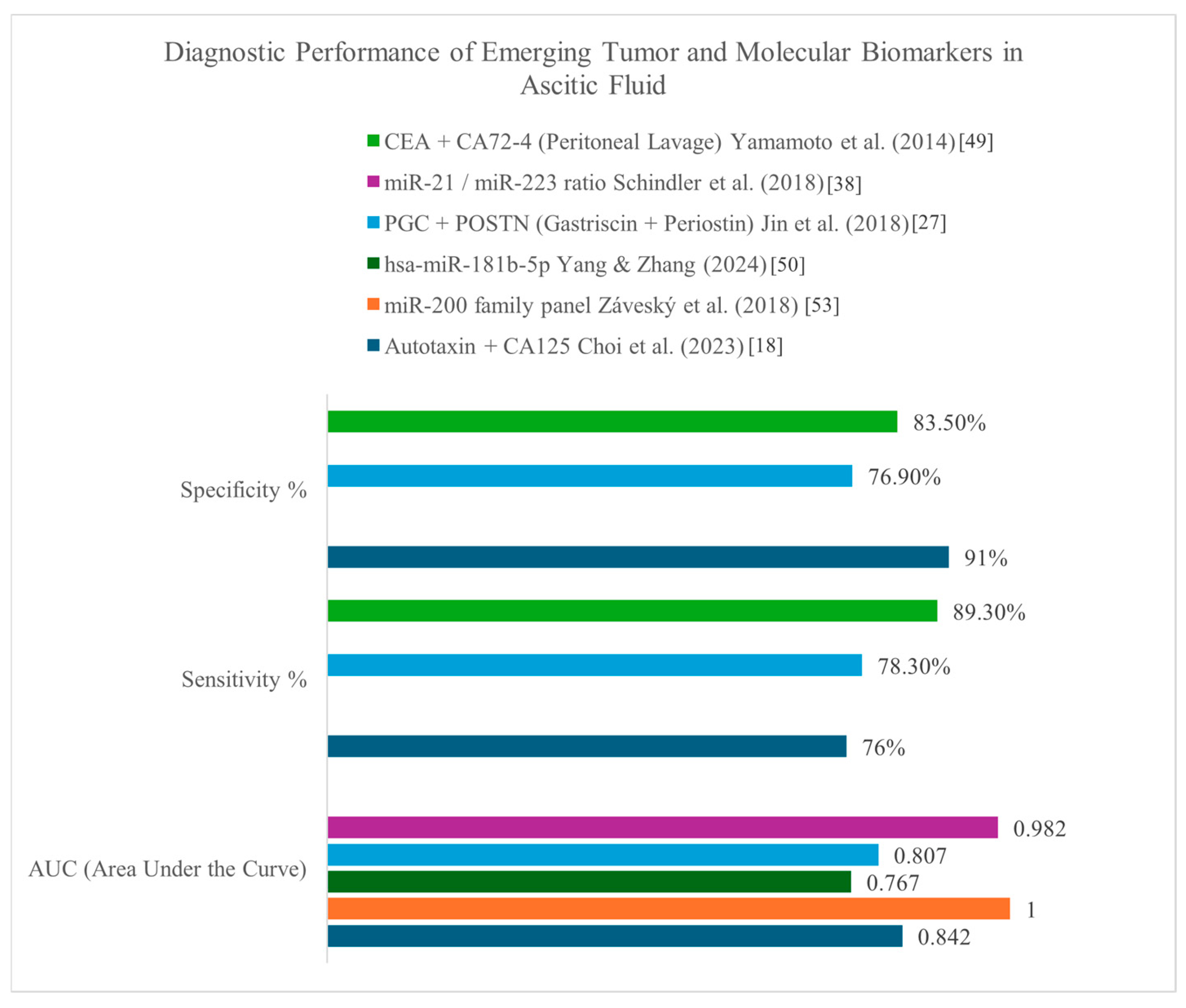
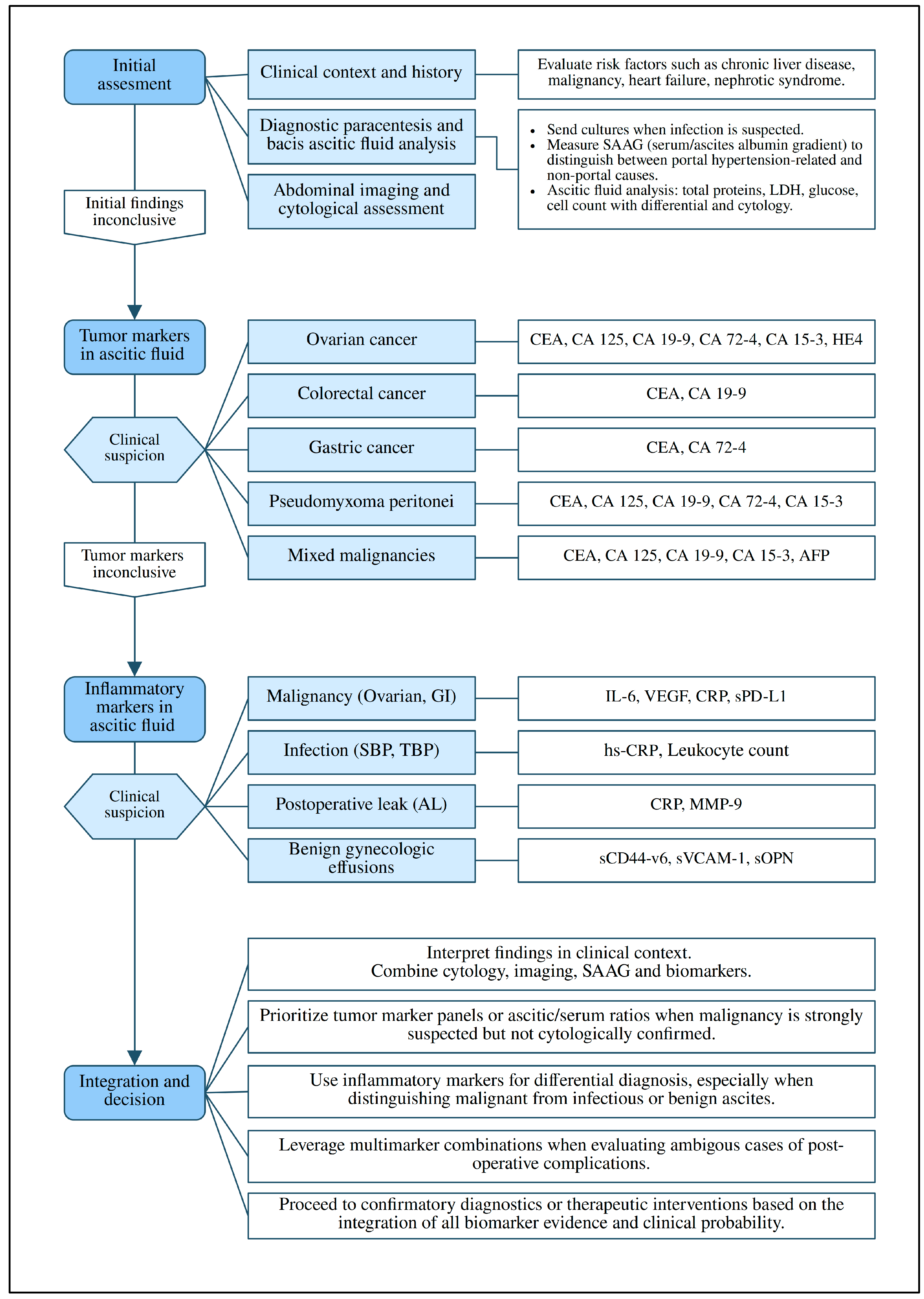
3.4. Diagnostic Algorithm and Interpretation
4. Discussion
4.1. Summary of Main Findings
4.2. Interpretation of Findings in Context
4.3. Clinical Implications
4.4. Strengths and Limitations
4.5. Research Gaps and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROBINS-I | Risk Of Bias In Non-randomized Studies—of Interventions |
| VEGF | Vascular endothelial growth factor |
| VEGF-A | Vascular endothelial growth factor A |
| LDH | Lactate dehydrogenase |
| CRP | C-reactive protein |
| hs-CRP | High-sensitivity C-reactive protein |
| PD-L1 | Programmed Death-Ligand 1 |
| CT | Computed Tomography |
| PET | Positron Emission Tomography |
| FDG | Fluorodeoxyglucose |
| ROC | Receiver Operating Characteristic |
| RNA | Ribonucleic Acid |
| RT-PCR | Reverse transcription polymerase chain reaction |
| HCC | Hepatocellular carcinoma |
| EV | Extracellular vesicles |
| CRC | Colorectal cancer |
Appendix A
Search Strategy and Keywords Used
References
- Senousy, B.E.; Draganov, P.V. Evaluation and management of patients with refractory ascites. World J. Gastroenterol. 2009, 15, 67. [Google Scholar] [CrossRef]
- Biecker, E. Diagnosis and therapy of ascites in liver cirrhosis. World J. Gastroenterol. 2011, 17, 1237. [Google Scholar] [CrossRef]
- Planas, R.; Montoliu, S.; Ballesté, B.; Rivera, M.; Miquel, M.; Masnou, H.; Galeras, J.A.; Giménez, M.D.; Santos, J.; Cirera, I.; et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin. Gastroenterol. Hepatol. 2006, 4, 1385–1394. [Google Scholar] [CrossRef]
- Huang, L.L.; Xia, H.H.X.; Zhu, S.L. Ascitic fluid analysis in the differential diagnosis of ascites: Focus on cirrhotic ascites. J. Clin. Transl. Hepatol. 2014, 2, 58. [Google Scholar] [CrossRef]
- Tarn, A.C.; Lapworth, R. Biochemical analysis of ascitic (peritoneal) fluid: What should we measure? Ann. Clin. Biochem. 2010, 47, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kong, X.; Dou, Q.; Ye, J.; Xu, D.; Shang, H.; Xu, K.; Song, Y. Evaluation of tumor markers for the differential diagnosis of benign and malignant ascites. Ann. Hepatol. 2014, 13, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Berjohn, C.; Tanen, D.A. Ascites as the initial presentation of gastrointestinal carcinoma. J. Emerg. Med. 2013, 44, e195–e198. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Kim, J.W.; Baek, J.H.; Kim, S.H.; Kim, B.G.; Lee, K.L.; Jeong, J.B.; Jung, Y.J.; Kim, J.S.; Jung, H.C.; et al. Role of ascites adenosine deaminase in differentiating between tuberculous peritonitis and peritoneal carcinomatosis. World J. Gastroenterol. 2012, 18, 2837. [Google Scholar] [CrossRef]
- Kaleta, E.J.; Tolan, N.V.; Ness, K.A.; O’Kane, D.; Algeciras-Schimnich, A. CEA, AFP and CA 19-9 analysis in peritoneal fluid to differentiate causes of ascites formation. Clin. Biochem. 2013, 46, 814–818. [Google Scholar] [CrossRef]
- Brain, O.; Brown, L.H.; Suvarna, S.; Chapman, R. Markedly elevated CA19-9 associated with benign ovarian cyst and ascites. Case Rep. 2009, 2009, bcr1120081219. [Google Scholar] [CrossRef]
- Medeiros, L.R.; Rosa, D.D.; da Rosa, M.I.; Bozzetti, M.C. Accuracy of CA 125 in the diagnosis of ovarian tumors: A quantitative systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 142, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, R.; Wei, J.; Ye, J.; He, Q.; Yuan, C.; Ye, J.; Li, Y.; Liu, Z.; Lin, Y. Identification of Candidate Biomarkers in Malignant Ascites from Patients with Hepatocellular Carcinoma by iTRAQ-Based Quantitative Proteomic Analysis. BioMed Res. Int. 2018, 2018, 5484976. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razik, A.; Mousa, N.; Elalfy, H.; Sheta, T.F.; Awad, M.; Abdelsalam, M.; Elhelaly, R.; Elzehery, R.; Gouda, N.S.; Eldars, W. A novel combination of C-reactive protein and vascular endothelial growth factor in differential diagnosis of ascites. J. Gastrointest. Cancer 2017, 48, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 11, 55–61. [Google Scholar] [CrossRef]
- Bérgamo, S.; Trapé, J.; González-García, L.; González-Fernández, C.; Vergara, C.; De-La-Torre, N.; Trujillo, G.; Estivill, D.; Álvarez-González, M.A.; Bosch, L.; et al. Utility of human epididymis protein 4 in the differential diagnosis of ascites. Clin. Biochem. 2023, 120, 110645. [Google Scholar] [CrossRef]
- Castro, C.M.; Im, H.; Le, C.; Lee, H.; Weissleder, R.; Birrer, M.J. Exploring alternative ovarian cancer biomarkers using innovative nanotechnology strategies. Cancer Metast. Rev. 2015, 34, 75–82. [Google Scholar] [CrossRef][Green Version]
- Choi, J.A.; Kim, H.; Kwon, H.; Lee, E.H.; Cho, H.; Chung, J.Y.; Kim, J.H. Ascitic autotaxin as a potential prognostic, diagnostic, and therapeutic target for epithelial ovarian cancer. Br. J. Cancer 2023, 129, 1184–1194. [Google Scholar] [CrossRef]
- Chudecka-Głaz, A.; Cymbaluk-Płoska, A.; Menkiszak, J.; Sompolska-Rzechuła, A.; Byra, E.; Rzepka-Górska, I. HE4 tumor marker concentration in neoplastic peritoneal effusion and in peritoneal fluid associated with benign gynecological diseases. J. Ovarian Res. 2014, 7, 22. [Google Scholar] [CrossRef]
- Du, L.; Wei, X.; Xiao, Z.; Wang, H.; Song, Y. Utility of ascitic tumor markers and adenosine deaminase for differential diagnosis of tuberculous peritonitis and peritoneal carcinomatosis. BMC Gastroenterol. 2022, 22, 423. [Google Scholar] [CrossRef]
- Faria, D.K.; Faria, C.S.; Doi, D.; Acencio, M.M.P.; Antonangelo, L. Hybrid panel of biomarkers can be useful in the diagnosis of pleural and peritoneal effusions. Clin. Chim. Acta 2019, 497, 48–53. [Google Scholar] [CrossRef]
- Han, N.; Sun, X.; Qin, C.; Bakari, K.H.; Wu, Z.; Zhang, Y.; Lan, X. Value of 18F-FDG PET/CT combined with tumor markers in the evaluation of ascites. AJR Am. J. Roentgenol. 2018, 210, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Hrvat, A.; Benders, S.; Kimmig, R.; Brandau, S.; Mallmann-Gottschalk, N. Immunoglobulins and serum proteins impair anti-tumor NK cell effector functions in malignant ascites. Front. Immunol. 2024, 15, 1360615. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Ram, S.; Kumar, H.; Saroch, A.; Sharma, V.; Singh, H. Ascitic and serum levels of tumor biomarkers (CA 72-4, CA 19-9, CEA and CA 125) in discrimination of cause of ascites: A prospective study. Arq. Gastroenterol. 2022, 59, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Janež, J.; Horvat, G.; Jerin, A.; Grosek, J. The significance of blood and peritoneal fluid biochemical markers in identifying early anastomotic leak following colorectal resection—Findings from a single-center study. Medicina 2022, 58, 1253. [Google Scholar] [CrossRef]
- Jerman, K.G.; Kobal, B.; Jakimovska, M.; Verdenik, I.; Černe, K. Control values of ovarian cancer tumor markers and standardisation of a protocol for sampling peritoneal fluid and performing washing during laparoscopy. World J. Surg. Oncol. 2014, 12, 278. [Google Scholar] [CrossRef][Green Version]
- Jin, J.; Son, M.; Kim, H.; Kong, S.-H.; Kim, H.K.; Kim, Y.; Han, D. Comparative proteomic analysis of human malignant ascitic fluids for the development of gastric cancer biomarkers. Clin. Biochem. 2018, 56, 55–61. [Google Scholar] [CrossRef]
- Kim, B.C.; Bae, J.H.; Park, S.M.; Won, D.Y.; Lee, I.K. Is ascites CEA a risk factor for peritoneal carcinomatosis in colorectal cancer?: A long-term follow-up study. Int. J. Color. Dis. 2020, 35, 147–155. [Google Scholar] [CrossRef]
- Kocbek, V.; Vouk, K.; Bersinger, N.A.; Mueller, M.D.; Rižner, T.L. Panels of cytokines and other secretory proteins as potential biomarkers of ovarian endometriosis. J. Mol. Diagn. 2015, 17, 325–334. [Google Scholar] [CrossRef]
- Kolomeyevskaya, N.; Eng, K.H.; Khan, A.N.H.; Grzankowski, K.S.; Singel, K.L.; Moysich, K.; Segal, B.H. Cytokine profiling of ascites at primary surgery identifies an interaction of tumor necrosis factor-α and interleukin-6 in predicting reduced progression-free survival in epithelial ovarian cancer. Gynecol. Oncol. 2015, 138, 352–357. [Google Scholar] [CrossRef]
- Liang, L.; Fang, J.; Han, X.; Zhai, X.; Song, Y.; Lu, Y.; Zhang, Q.; Ma, R. Prognostic value of CEA, CA19-9, CA125, CA724, and CA242 in serum and ascites in pseudomyxoma peritonei. Front. Oncol. 2021, 11, 594763. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, N.; Wei, X.; Xiao, Z.; Song, Y.; Du, L. Diagnostic algorithm based on ratio of ascites-serum tumor markers is superior to tumor markers in the differentiation of benign ascites from malignant ascites. Am. J. Med. Sci. 2024, 368, 361–368. [Google Scholar] [CrossRef]
- Masoumi-Moghaddam, S.; Amini, A.; Wei, A.Q.; Robertson, G.; Morris, D.L. Intratumoral interleukin-6 predicts ascites formation in patients with epithelial ovarian cancer: A potential tool for close monitoring. J. Ovarian Res. 2015, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Mužina Mišić, D.; Zovak, M.; Kopljar, M.; Čiček, S.; Bilić, Z. Comparison of C-reactive protein levels in serum and peritoneal fluid in early diagnosis of anastomotic leakage after colorectal surgery. Acta Clin. Croat. 2023, 62, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kadam, N.; Acharya, S.; Shukla, S.; Gupta, K. Ascitic fluid high sensitive C-reactive protein (hs-CRP): A prognostic marker in cirrhosis with spontaneous bacterial peritonitis. J. Clin. Diagn. Res. 2016, 10, OC20–OC24. [Google Scholar] [CrossRef] [PubMed]
- Okła, K.; Surówka, J.; Frąszczak, K.; Czerwonka, A.; Kaławaj, K.; Wawruszak, A.; Kotarski, J.; Wertel, I. Assessment of the clinicopathological relevance of mesothelin level in plasma, peritoneal fluid, and tumor tissue of epithelial ovarian cancer patients. Tumour Biol. 2018, 40, 1010428318804937. [Google Scholar] [CrossRef]
- Prunoiu, V.M.; Marincas, A.M.; Brăţucu, R.; Brăţucu, E.; Ionescu, S.; Răvaş, M.M.; Vasile, I.B. The value of C reactive protein and leukocytes in the peritoneal fluid in predicting postoperative digestive fistulas. Chirurgia 2020, 115, 236–245. [Google Scholar] [CrossRef]
- Schindler, P.; Kupcinskas, J.; Juzenas, S.; Skieceviciene, J.; Salteniene, V.; Schulz, C.; Weigt, J.; Malfertheiner, P.; Link, A. Expression of microRNAs in the ascites of patients with peritoneal carcinomatosis and peritonitis. Cancer Cytopathol. 2018, 126, 353–363. [Google Scholar] [CrossRef]
- Song, S.E.; Choi, P.; Kim, J.H.; Jung, K.; Kim, S.E.; Moon, W.; Park, M.I.; Park, S.J. Diagnostic value of carcinoembryonic antigen in ascites for colorectal cancer with peritoneal carcinomatosis. Korean J. Gastroenterol. 2018, 71, 332–337. [Google Scholar] [CrossRef]
- Sparreboom, C.L.; Komen, N.; Rizopoulos, D.; Verhaar, A.P.; Dik, W.A.; Wu, Z.; van Westreenen, H.L.; Doornebosch, P.G.; Dekker, J.W.T.; Menon, A.G.; et al. A multicentre cohort study of serum and peritoneal biomarkers to predict anastomotic leakage after rectal cancer resection. Color. Dis. 2020, 22, 36–45. [Google Scholar] [CrossRef]
- Sun, J.; Chang, Y.X.; Niu, C.Y. Evaluation of ascitic soluble human leukocyte antigen-G for distinguishing malignant ascites from benign ascites. Tumour Biol. 2017, 39, 1010428317726840. [Google Scholar] [CrossRef]
- Sun, X.; Gui, Y.; Yang, T.; Chen, L.; Zhang, Y.; Yan, L.; Chen, W.; Wang, B. PD-L1+ neutrophils induced NETs in malignant ascites is a potential biomarker in HCC. Cancer Immunol. Immunother. 2024, 73, 2863–2875. [Google Scholar] [CrossRef]
- Tong, H.; Tai, Y.; Ye, C.; Wu, H.; Zhang, L.-H.; Gao, J.-H.; Yan, Z.-P.; Huang, Z.-Y.; Tang, C.-W. Carbohydrate antigen 125 and carcinoembryonic antigen in the differentiation of tuberculous peritonitis and peritonitis carcinomatosa. Oncotarget 2017, 8, 78068–78075. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trapé, J.; Sant, F.; Montesinos, J.; Arnau, A.; Sala, M.; Figols, C.; Franquesa, J.; Esteve-Valverde, E.; Pérez, R.; Aligué, J.; et al. Comparative assessment of two strategies for interpreting tumor markers in ascitic effusions. In Vivo 2020, 34, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Yoshida, H.; Fukuhara, M.; Yoshida, M.; Motoi, N.; Sugawara, S.; Sone, M.; Arai, Y.; Tamura, K.; Uno, M.; et al. Efficacy of ascitic fluid cell block for diagnosing primary ovarian, peritoneal, and tubal cancer in patients with peritoneal carcinomatosis with ascites. Gynecol. Oncol. 2020, 157, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Dalal, V.; Kumar, R.; Kumar, S.; Sharma, A.; Kumar, L.; Sharma, J.B.; Roy, K.K.; Singh, N.; Vanamail, P. Biomarker potential of IL-6 and VEGF-A in ascitic fluid of epithelial ovarian cancer patients. Clin. Chim. Acta 2018, 482, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, R.; Rao, B.; Xu, H. Serum and ascites tumor markers in the diagnostic and prognostic prediction for appendiceal pseudomyxoma peritonei. BMC Cancer 2023, 23, 1104. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yoshinaga, K.; Matsuyama, A.; Tsutsui, S.; Ishida, T. CEA/CA72-4 levels in peritoneal lavage fluid are predictive factors in patients with gastric carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 607–612. [Google Scholar] [CrossRef]
- Yamamoto, C.M.; Oakes, M.L.; Murakami, T.; Muto, M.G.; Berkowitz, R.S.; Ng, S.W. Comparison of benign peritoneal fluid- and ovarian cancer ascites-derived extracellular vesicle RNA biomarkers. J. Ovarian Res. 2018, 11, 20. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J. Ascites-derived hsa-miR-181a-5p serves as a prognostic marker for gastric cancer-associated malignant ascites. BMC Genom. 2024, 25, 10359. [Google Scholar] [CrossRef]
- Yu, T.; Shu, L.; Chen, Y.; Zhu, Y.; Lu, N.; Lai, Y.; Huang, T.; Shu, X. Diagnosis of malignant versus tuberculous ascites using tumor markers and globulin ratios in serum and ascites: A Fisher discriminant model. Arab J. Gastroenterol. 2021, 22, 93–98. [Google Scholar] [CrossRef]
- Yuksel, I.; Karaahmet, F.; Coskun, Y.; Kılıncalp, S.; Hamamci, M.; Akinci, H.; Ustun, Y.; Simsek, Z.; Erarslan, E.; Coban, S. Significance of serum and ascitic fluid C-reactive protein in differential diagnosis of benign and malignant ascites. Dig. Dis. Sci. 2014, 59, 2588–2593. [Google Scholar] [CrossRef]
- Záveský, L.; Jandáková, E.; Weinberger, V.; Minář, L.; Hanzíková, V.; Dušková, D.; Drábková, L.Z.; Svobodová, I.; Hořínek, A. Ascites-derived extracellular microRNAs as potential biomarkers for ovarian cancer. Reprod. Sci. 2019, 26, 510–522. [Google Scholar] [CrossRef]
- Zhan, N.; Dong, W.G.; Wang, J. The clinical significance of vascular endothelial growth factor in malignant ascites. Tumour Biol. 2016, 37, 3719–3725. [Google Scholar] [CrossRef]
- Zhu, F.L.; Ling, A.S.; Wei, Q.; Ma, J.; Lu, G. Tumor markers in serum and ascites in the diagnosis of benign and malignant ascites. Asian Pac. J. Cancer Prev. 2015, 16, 719–722. [Google Scholar] [CrossRef]
| Study | Sample Size | Age | Study Duration | Study Center | Study Type |
|---|---|---|---|---|---|
| Abdel-Razik et al., 2016 [13] | 315 | Benign: 55.4 ± 15; Malignant: 53.9 ± 16 | May 2013–July 2015 | Mansoura University, Egypt | Prospective |
| Bérgamo et al., 2023 [16] | 118 | Mean 68 years (42–92) | Not specified | Althaia Xarxa Assistencial Universitària de Manresa, Manresa, Catalonia, Spain | Retrospective observational |
| Castro et al., 2015 [17] | 30 ascites samples (20 cancer, 10 benign) | Not specified | Not specified | Massachusetts General Hospital Cancer Center, USA | Proof-of-concept nanotechnology platform |
| Choi et al., 2023 [18] | 138 | Not specified | Not specified | Gangnam Severance Hospital, Yonsei University, South Korea | Prospective observational |
| Chudecka-Głaz et al., 2014 [19] | 139 | 24–88 years | 2012–2013 | Central Laboratory of the Independent Public Hospital, Poland | Prospective observational |
| Du et al., 2022 [20] | 169 | TBP: Median 44; PC: Median 60 | May 2015–January 2022 | Union Hospital, Tongji Med. College, China | Retrospective cohort |
| Faria et al., 2019 [21] | 120 (33 peritoneal, 87 pleural) | Mean 57.7 ± 16.6 | Not specified | Hospital das Clínicas, Univ. of São Paulo, Brazil | Prospective observational |
| Han et al., 2018 [22] | 177 (104 malignant, 73 benign) | Mean 56 ± 13 (20–80) | January 2010–December 2014 | Union Hospital, Tongji Med. College, China | Retrospective observational |
| Hrvat et al., 2024 [23] | 28 malignant, 1 benign ascites; healthy serum | Not specified | Not specified | Univ. Hospitals Essen & Cologne, Germany | In vitro mechanistic coculture study |
| Jain et al., 2022 [24] | 93 | Mean 47 (14–81) | July 2018–December 2019 | PGI Medical Education & Research, Chandigarh, India | Prospective observational |
| Janež et al., 2022 [25] | 43 | Mean 69.9 ± 13.6 | Not specified | Univ. Medical Centre Ljubljana, Slovenia | Prospective pilot cohort |
| Jerman et al., 2014 [26] | 33 | Mean 43 ± 1.82 | December 2011–September 2013 | University Medical Centre Ljubljana, Slovenia | Prospective protocol development |
| Jin et al., 2018 [27] | 85 GC, 27 benign; ELISA: 57 GC + 27 benign | Not specified | Not specified | Seoul National Univ. College of Medicine & Hospital, South Korea | Proteomics discovery + ELISA validation |
| Kim et al., 2019 [28] | 495 | Median 65 (24–93) | January 2006–November 2014 | St. Mary’s Hospital, Catholic Univ. of Korea, South Korea | Retrospective cohort |
| Kocbek et al., 2015 [29] | 98 (58 endo, 40 controls) | Not stated | Not specified | Univ. of Ljubljana & Berne, Slovenia and Switzerland | Prospective |
| Kolomeyevskaya et al., 2015 [30] | 39 | Median 61 (range 44–82) | Not specified | Roswell Park Cancer Institute, Buffalo, NY, USA | Prospective observational cohort |
| Liang et al., 2021 [31] | 512 | Median 58 (IQR 49–64.5) | May 2009–October 2019 | Aerospace Center Hospital, Beijing, China | Prospective observational |
| Liu et al., 2014 [6] | 437 | Not specified | 2007–2012 | Union & Tongji Hospitals + Qingdao Univ., China | Retrospective observational |
| Liu et al., 2024 [32] | 418 | Not specified | June 2018–October 2023 | Union Hospital, Tongji Med. College, Wuhan, China | Retrospective cohort |
| Masoumi-Moghaddam et al., 2015 [33] | 98 | Median 62 (35–84) | 2001–2012 | St. George Hospital, Sydney, Australia | Retrospective |
| Mužina Mišić et al., 2023 [34] | 59 | Median 68 (range 25–91) | January–October 2019 | Sestre Milosrdnice University Hospital Center, Croatia | Prospective observational |
| Nakul-Kadam et al., 2016 [35] | 100 (50 SBP, 50 sterile ascites) | Mean 46.84 ± 12.46 | September 2013–October 2015 | Jawaharlal Nehru Medical College, Wardha, India | Prospective case-control |
| Okła et al., 2018 [36] | 101 | Median 56 (20–89) | 2012–2016 | Medical University of Lublin, Poland | Prospective observational |
| Prunoiu et al., 2020 [37] | 100 | Adults (not specified) | May 2016–December 2017 | Bucharest Oncology Institute, Romania | Prospective observational |
| Schindler et al., 2018 [38] | 45 patients (15 PCA, 15 SBP, 15 PH) | Mean 61.6 ± 10.4 years | Not specified | Univ. of Magdeburg; Lithuanian Univ. of Health Sciences, Germany, Lithuania | Prospective molecular profiling |
| Song et al., 2018 [39] | 259 (82 CRC, 177 benign) | Median 60 (range 17–87) | January 2000–May 2013 | Kosin University, Busan, South Korea | Retrospective observational |
| Sparreboom et al., 2020 [40] | 292 | Median 63 (IQR 57–71) | August 2015–October 2017 | 10 hospitals in Netherlands and Belgium | Prospective multicenter cohort |
| Sun et al., 2017 [41] | 64 malignant, 30 benign ascites | Mean 56.04 ± 14.53 years | January–December 2016 | First Affiliated Hospital of Xi’an Medical University, China | Prospective diagnostic biomarker study |
| Sun et al., 2024 [42] | 35 HCC ascites, 35 benign | HCC: 62.3 ± 9.1; Benign 55.3 ± 9.8 | September 2022–March 2024 | Second Affiliated Hospital of Chongqing Med. Univ., China | Prospective diagnostic & mechanistic study |
| Tong et al., 2017 [43] | 292 (TBP: 101; non-OCA: 120; OCA: 71) | TBP: 40.8 ± 18.4; non-OCA: 61.9 ± 12.8; OCA: 56.3 ± 12.4 | January 2009–December 2013 | West China Hospital, Sichuan University, China | Retrospective observational |
| Trapé et al., 2020 [44] | 157 | Mean 67.7 (35–96) | January 2008–December 2017 | Althaia Xarxa Assistencial Universitària de Manresa, Manresa, Catalonia, Spain | Retrospective observational |
| Uehara et al., 2020 [45] | 81 patients with PC and ascites | Median 64 (range 28–87) | 2010–2018 | National Cancer Center Hospital, Tokyo, Japan | Retrospective diagnostic accuracy study |
| V. Dalal et al., 2018 [46] | 45 (30 epithelial ovarian cancer (EOC) patients; 15 benign controls) | Patients: Mean 51.63 ± 12 years; Controls: Mean 48.40 ± 5.05 years | Not specified | All India Institute of Medical Sciences (AIIMS), New Delhi, India | Prospective observational |
| Wang et al., 2023 [47] | 281 (183 PMP + 98 controls) | Median 56 (19–77) | May 2012–June 2020 | Aerospace Center Hospital, Beijing, China | Retrospective cohort |
| Yamamoto et al., 2014 [48] | 193 | Mean 68.9 ± SD | October 2006–March 2011 | Hiroshima Atomic Bomb Survivors Hospital, Japan | Prospective observational |
| Yamamoto et al., 2018 [49] | 8 OC ascites; 10 benign peritoneal fluids | OC: Mean 64 ± 12 years | Not specified | Brigham & Women’s Hospital; Tufts Medical Center, USA | Exploratory observational with qPCR & RNA-seq |
| Yang and Zhang, 2024 [50] | 22 (12 GC malignant, 10 benign ascites) | Matched (not numerically specified) | Not specified | Sunshine Union Hospital, Weifang, China | Bioinformatic analysis using GEO/TCGA |
| Yu et al., 2021 [51] | 63 | TB: Mean 43.6 ± 18.5; Malignant: 60.3 ± 12.3 | November 2013–December 2017 | First Affiliated Hospital of Nanchang Univ., China | Prospective observational |
| Yüksel et al., 2014 [52] | 91 | Adults with ascites | February–November 2013 | Dıskapı Yıldırım Beyazıt Hospital, Turkey | Prospective |
| Záveský et al., 2019 [53] | 60 (26 OC patients, 34 controls) | Median 60 (ascites), 62 (lavage) | Not specified | General Univ. Hospital Prague; Univ. Hospital Brno, Czech Republic | Prospective observational |
| Zhan et al., 2016 [54] | 1012 | Mean ~52 | 2007–2012 | Renmin Hospital of Wuhan University, China | Retrospective observational |
| Zhu et al., 2015 [55] | 76 (45 malignant, 31 benign) | Malignant: 54.4 ± 11.8; Benign: 42.7 ± 16.4 | June 2012–June 2014 | People’s Hospital Anqing & Zhejiang Prov. Hospital, China | Prospective observational |
| Marker(s) | Fluid(s) Measured | Cancer(s) | Marker Used Alone or in Panel | Diagnostic and Prognostic Value |
|---|---|---|---|---|
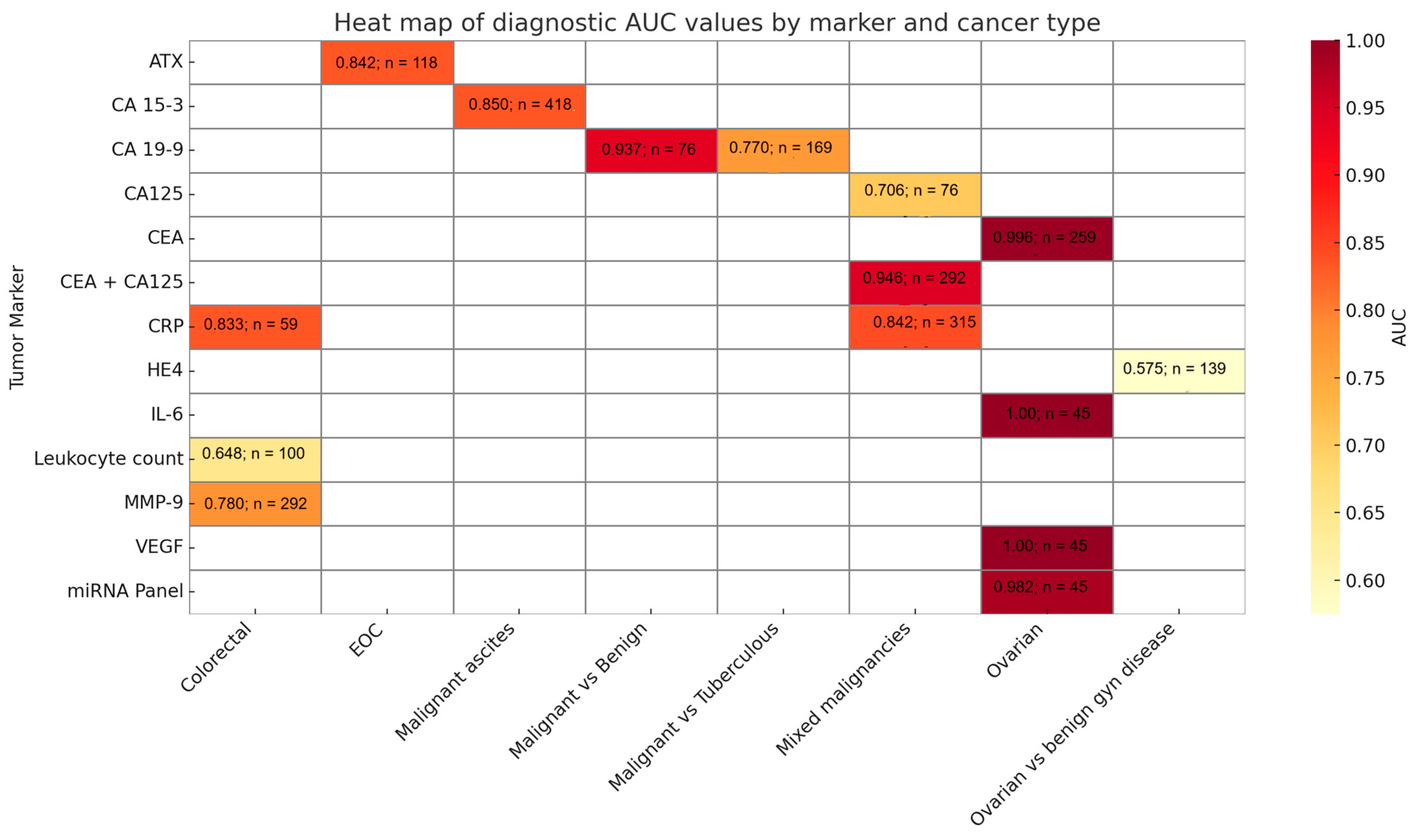
| CEA | Ascitic fluid | Ovarian/Mixed malignancies | CEA + CA125 [43]; CEA + CA125 ± PET/CT [22] | Diagnostic Value: composite index (CEA × CA125) AUC = 0.946, higher than either marker alone, distinguishing OCA from TBP/non-OCA [43]. PET/CT combined with ascitic CEA + CA125 improved diagnostic accuracy [22]. Prognostic Value: Not specified. |
| Pseudomyxoma peritonei (PMP) | CEA in marker count [31]; CEA + CA125 + CA19-9 [47] | Diagnostic Value: No AUC reported. Both studies used multi-marker counts/panels rather than single-marker diagnostics. Prognostic value: Higher number of positive markers associated with higher pathology grade and poorer survival [31]. Multi-marker positivity stratified survival [47]. | ||
| Colorectal | CEA alone [28,39] | Diagnostic Value: ascitic CEA had high accuracy (AUC 0.996) and was the best-performing single marker [39]. CEA ≥ 5 ng/mL indicated higher risk factors [28]. Prognostic Value: Yes [28]. | ||
| Ascitic + serum | Mixed malignancies/Multicancer cohorts [44,51,55] | CEA + CA125 + CA19-9 [51]; CEA + CA15-3 + CA72-4 + CA19-9 [44]; CEA + CA125 + CA19-9 [55] | Diagnostic Value: Fisher model with ascites/serum ratios achieved AUC = 0.908, outperforming single markers [51]. F/S strategy improved accuracy (sensitivity ~76%, specificity ~95–99%) [44]. CEA in ascites (AUC = 0.859) and CEA ascites/serum ratio (AUC = 0.879) showed high specificity; panels increased sensitivity [55]. Prognostic Value: not specified. | |
| Peritoneal/Pleural | Mixed malignancies [21,48]; Non-malignant (AL context) [25] | CY-CEA + CY-CA 72-4 [48]; CEA ± NGAL [21]; CEA ± Lactate [25] | Diagnostic Value: CY-CEA/CY-CA72-4 positivity predicted peritoneal dissemination [48]. CEA + NGAL improved diagnostic performance (accuracy 79.2%) for malignant effusions vs. single markers [21] Not cancer-related, used perioperatively to detect anastomotic leakage [25]. Prognostic Value: combined positivity associated with worse 5-year survival [48]. | |
| CA 125 | Serum + Ascitic fluid | Mixed malignancies | Alone and in panel [55]; alone and in ascitic-to-serum ratio [51] | Diagnostic Value: ascitic CA125 AUC = 0.706; ascitic-to-serum ratio AUC = 0.726 [55]; AUC = 0.823 [51]. Limited standalone value; ratios and panels improved discrimination. Prognostic Value: not specified. |
| CA 19-9 | Ascitic fluid | Peritoneal carcinomatosis (PC) vs. tuberculous peritonitis (TBP) | Alone and in panel [24] | Diagnostic Value: In combination with CEA and CA72-4, achieved sensitivity ~86% and specificity ~55% [24]. Prognostic Value: not specified. |
| Ascitic + Serum | Mixed malignancies | Alone and in panel [6,55] | Diagnostic Value: ascitic CA19-9 AUC = 0.697, Fisher model combining CEA + CA125 + CA19-9 achieved AUC = 0.984 [55]. The combination panel (CEA + CA19-9 + CA15-3) improved to sensitivity 85%, specificity 97% [6]. Prognostic Value: not specified. | |
| Malignant vs. benign ascites | Alone and in ascitic-to-serum ratio [55] | Diagnostic Value: ascites AUC 0.697, the ascitic-to-serum ratio clearly improved discrimination, being highly diagnostic. Prognostic Value: not specified. | ||
| Malignant vs. tuberculous | Alone and in combination [20] | Diagnostic Value: ascitic CA19-9 sensitivity = 56% and specificity = 88% [20]. Prognostic Value: not specified. | ||
| HE4 | Ascitic fluid | Malignant vs. benign ascites | Single marker [16] | Diagnostic Value: sensitivity (21.2%) and high specificity (100%) for malignancy. When combined with adjusted clinical parameters (ADA, CRP, PMN, eGFR), sensitivity improved modestly. Prognostic Value: not specified [16]. |
| Ovarian cancer vs. benign gynecologic diseases | Single marker [19] | Diagnostic Value: AUC = 0.575. No significant difference between cancer and benign states; also elevated in benign effusions. Prognostic Value: Higher ascitic HE4 associated with worse overall survival and poorer platinum response [19]. | ||
| CA 72-4 | Ascitic fluid | Malignant vs. benign ascites | Alone and in panel [24,44] | Diagnostic Value: panel (CEA + CA 19-9 + CA 72-4) sensitivity 86%, specificity 55% [24]. Panel (CEA + CA 15-3 + CA 72-4) diagnostic accuracy ~90% [44]. Prognostic Value: not specified. |
| Gastric cancer | Alone and in combination [48] | Diagnostic value: sensitivity 77%, specificity 84.4%; CA 72-4 and CEA in lavage fluid strongly correlated. Prognostic value: combined positivity predicted significantly poorer survival [48]. | ||
| Ascitic + Serum | Pseudomyxoma peritonei (PMP) | Panel (CEA, CA 125, CA 19-9, CA 72-4, CA 242) [31] | Diagnostic Value: panel evaluated in relation to pathology grade and completeness of cytoreduction. Prognostic Value: high number of elevated markers significantly associated with poorer survival [31]. | |
| Peritoneal lavage | Gastric cancer | Alone, in panel with CEA [48] | Diagnostic Value: combination with CEA in lavage fluid improved accuracy. Prognostic Value: combined CA72-4 and CEA positivity significantly associated with poorer survival [48]. | |
| CYFRA 21-1 | Ascitic fluid | Malignant ascites [16], mixed effusions [44] | Single marker | Diagnostic Value: levels increased in malignant vs. benign ascites [16]. Standalone accuracy not consistently reported [44]. Prognostic Value: not specified. |
| CA 15-3 | Ascitic fluid | Malignant, benign ascites | Alone, in panel and ascitic-to-serum ratio [32,44] | Diagnostic Value: Panel (CEA, CA15-3, CA72-4) specificity 100%, sensitivity of 79.7%. In patients with negative cytology, the sensitivity of three markers was 69.7%, at a specificity of 100% [44]. AUC = 0.850 (ascites); ascitic-to-serum ratio AUC = 0.863 [32]. Prognostic Value: not specified. |
| Peritoneal carcinomatosis (PC) vs. tuberculous peritonitis (TBP) | Alone and in panel [24] | Diagnostic Value: in panel (CEA or CA 15-3 or CA 19-9) there is higher accuracy 94.67%. Prognostic Value: not specified [24]. | ||
| Mixed malignancies | Alone and in panel [6] | Diagnostic Value: for CA 15-3 alone: sensitivity 23%, specificity 100%. Panel (CEA + CA 19-9 + CA 15-3): sensitivity 85.87%, specificity 97.32%. Prognostic Value: not specified | ||
| Ascitic + Serum | Pseudomyxoma peritonei (PMP) | Panel [31,47] | Diagnostic Value: CA 15-3 included in multi-marker count/panel for staging and assessment of completeness of cytoreduction. Prognostic Value: elevated positive markers (including CA 15-3) associated with poorer survival [31,47]. | |
| AFP | Ascitic fluid | Mixed malignancies | Panel [55] | Diagnostic Value: AFP measured in combination with CA125 and CA19-9 in diagnostic panels, including evaluation of ascitic-to-serum ratios. Prognostic Value: not specified [55]. |
| Autotaxin (ATX) | Ascitic fluid | EOC (Epithelial ovarian cancer) | Alone and in panel with CA125 [18] | Diagnostic Value: elevated in malignant ascites compared to benign; promotes cancer invasion and stemness. Prognostic Value: high ATX linked to advanced FICO stage and shorter disease-free survival [18]. |
| miRNA panel | Ascitic fluid | Ovarian cancer | miR-21, miR-23b, miR-29a [53]; miRNA panel in EVs [49]; miR-21/miR-223 ratio [38] | Diagnostic Value: miRNA levels significantly elevated in tumor-cell–enriched effusions compared with benign [53]. EV-derived miRNAs enabled tumor cell classification; provided added discriminatory power [49]. Very high discriminatory power between malignant and benign effusions [38]. Prognostic Value: expression levels of miR-21, miR-23b and miR-29a linked to poor progression-free and overall survival [53]. |
| MSLN | Peritoneal fluid | EOC (epithelial ovarian cancer) | Single marker [36] | Diagnostic Value: PF MSLN correlated with plasma MSLN. Prognostic Value: not specified for peritoneal fluid; only plasma MSLN was associated with prognosis [36]. |
| Marker(s) | Fluid(s) Measured | Cancer(s) | Marker Used Alone or in Panel | Diagnostic and Prognostic Value |
|---|---|---|---|---|
| CRP | Ascitic fluid + Serum | HCC, CRC, Ovarian [13] | Alone and in Panel | Diagnostic Value: ascites AUC = 0.842, serum AUC = 0.821. The combined model yielded best performance. Prognostic Value: VEGF correlated with stage; CRP with severity [13]. |
| Malignant ascites | Alone [16] | Diagnostic Value: ascitic fluid CRP has stronger performance than serum. Prognostic Value: not specified [16]. | ||
| Ascitic fluid | Cirrhosis/Non-malignant | Alone [35] | Diagnostic Value: higher level of hs-CRP before antibiotic therapy of the patients with SBP than that of the patients without SBP. Prognostic Value: greater ascitic fluid hs-CRP levels in SBP poorly correlate with the prognosis of the patients with cirrhosis [35]. | |
| Peritoneal fluid + Serum | Colorectal | Alone [34] | Diagnostic Value: peritoneal fluid AUC = 0.833 (POD4), serum AUC = 0.886 (POD4). Peritoneal fluid slightly less accurate than serum. Prognostic Value: CRP POD4 predicted AL [34]. | |
| IL-6 | Ascitic fluid | Ovarian (EOC) | Alone and in Panel [30,46] | Diagnostic Value: AUC = 1.0 [46]. Diagnostic performance enhanced in combination with TNF-α [30]. Prognostic Value: Shorter PFS and OS [30]. IL-6 and VEGF-A predicted PFS [46]. |
| Panel (IL-6 + TNF-α) [30] | Diagnostic Value: Best recurrence stratification. Prognostic Value: shorter PFS and OS [30]. | |||
| Panel (IL-6 + VEGF-A) [46] | Diagnostic Value: both AUC = 1.000. Combined use showed strongest performance. Prognostic Value: IL-6 and VEGF-A predicted PFS [46]. | |||
| Tumor tissue (IHC) | Ovarian (EOC) | Alone [33] | Diagnostic Value: Tumoral IL-6 predicted post-treatment ascites. Prognostic Value: IL-6 expression was predictive of the occurrence of post-treatment ascites in ovarian cancer patients [33]. | |
| VEGF | Ascitic fluid | Ovarian (EOC), Gastric, Colorectal, HCC | Alone and in Panel [46,54] | Diagnostic Value: Strong prognostic marker for EOC [46]. High VEGF = shorter OS [54]. Prognostic Value: high VEGF predicted shorter OS [54]. |
| Ascitic + Serum | HCC, CRC, Ovarian | Alone and in Panel (VEGF/VEGF-A) [13] | Diagnostic Value: highest discriminative performance. Prognostic Value: VEGF correlated with stage [13]. | |
| sPD-L1 | Ascitic + Serum | Pleural and Peritoneal effusions | Alone [21] | Diagnostic Value: sensitivity 52.2%, specificity 58.5% in the diagnosis of malignant effusions. Prognostic Value: not specified [21]. |
| TNF-α | Ascitic | Epithelial Ovarian Cancer (EOC) | Alone and in Panel [30] | Diagnostic Value: shorter PFS and OS. Prognostic Value: TNF-α levels were predictive of survival outcomes [30]. |
| sOPN, Scd44-V6, sVCAM-1 | PF, Washing fluid | Benign gynecologic disease | Panel [26] | Diagnostic Value: sOPN—reproductible in washing samples; sCD44-v6—strong correlation between fluid types; sVCAM-1—validated washing as PF substitute. Prognostic Value: not specified [26]. |
| MMP-9 | Drain fluid | Colorectal | Alone and in Panel [40] | Diagnostic Value: best predictor of AL when combined with CRP. Prognostic Value: CRP + MMP-9 levels on day 3 predicted AL [40]. |
| Leukocyte count | Peritoneal fluid | Colorectal | Alone [37] | Diagnostic Value: best when used with CRP. Prognostic Value: CRP and leukocyte count predicted AL [37]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratkoceri Hasi, G.; Osredkar, J.; Jerin, A. The Diagnostic Role of Tumor and Inflammatory Biomarkers in Ascitic Fluid: A Systematic Review. Medicina 2025, 61, 1582. https://doi.org/10.3390/medicina61091582
Ratkoceri Hasi G, Osredkar J, Jerin A. The Diagnostic Role of Tumor and Inflammatory Biomarkers in Ascitic Fluid: A Systematic Review. Medicina. 2025; 61(9):1582. https://doi.org/10.3390/medicina61091582
Chicago/Turabian StyleRatkoceri Hasi, Gentiana, Joško Osredkar, and Aleš Jerin. 2025. "The Diagnostic Role of Tumor and Inflammatory Biomarkers in Ascitic Fluid: A Systematic Review" Medicina 61, no. 9: 1582. https://doi.org/10.3390/medicina61091582
APA StyleRatkoceri Hasi, G., Osredkar, J., & Jerin, A. (2025). The Diagnostic Role of Tumor and Inflammatory Biomarkers in Ascitic Fluid: A Systematic Review. Medicina, 61(9), 1582. https://doi.org/10.3390/medicina61091582






