From Blood Count Parameters to ROP Risk: Early Hematological Predictors in Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
- Demographic data: gestational age in weeks (GA), birth weight in grams (BW), and sex;
- Hematological parameters: hemoglobin level (Hb), RBC count, and platelet count (PLT), collected sequentially on days 1, 3, 5, 7, 14, 21, and 28 of life, depending on the length of hospitalization;
- Transfusions: type of product administered (RBC or PLT concentrate) and number of units transfused;
- Ophthalmologic findings: the presence or absence of ROP, the highest stage observed, the location of retinal changes according to the International Classification of Retinopathy of Prematurity (ICROP), and any possible therapeutic indications.
3. Results
3.1. General Characteristics of the Study Population
3.2. Erythrocyte Profile and the Role of RBC Transfusions in the Development of ROP
3.3. Platelet Profile and the Role of Platelet Transfusions in the Development of ROP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROP | Retinopathy of prematurity |
| PMA | Postmenstrual age |
| VEGF | Vascular Endothelial Growth Factor |
| HIFs | Hypoxia-inducible factors |
| IGF-1 | Insulin-like Growth Factor 1 |
| RBC | Red blood cell |
| NICU | Neonatal intensive care unit |
| HbF | Fetal hemoglobin |
| HbA | Adult hemoglobin |
| GA | Gestational age |
| BW | Birth weight |
| Hb | Hemoglobin |
| PLT | Platelet |
| AP-ROP | Aggressive posterior retinopathy of prematurity |
References
- Rashidian, P.; Karami, S.; Salehi, S.A. A review on retinopathy of prematurity. Med. Hypothesis Discov. Innov. Ophthalmol. 2025, 13, 201–212. [Google Scholar] [CrossRef]
- Shehadeh, W.; Milhem, F.; Hajjeh, O.; AbuZahra, M.; Zahra, A.A.; Etkaidek, Z.; Atawna, A.; Hassoun, J.; Shweiki, S.; Nazzal, Z. Incidence and risk factors of retinopathy of prematurity in Palestine: A retrospective cohort study. BMC Ophthalmol. 2025, 25, 324. [Google Scholar] [CrossRef]
- Filippi, L.; Gulden, S.; Cammalleri, M.; Araimo, G.; Cavallaro, G.; Villamor, E. Retinopathy of prematurity in the era of precision neonatology: From risk stratification to targeted therapies. World J. Pediatr. 2025, 21, 430–435. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, Q.; Chen, C.; Xu, Y.; Ji, X.; Zhao, P. Ranibizumab injection and laser photocoagulation to treat type 1 retinopathy of prematurity after 40 weeks postmenstrual age: A retrospective case series study. BMC Ophthalmol. 2019, 19, 60. [Google Scholar] [CrossRef]
- Prasad, M.; Ingolfsland, E.C.; Christiansen, S.P. Modifiable Risk Factors and Preventative Strategies for Severe Retinopathy of Prematurity. Life 2023, 13, 1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Anti-VEGF therapy in the management of retinopathy of prematurity: What we learn from representative animal models of oxygen-induced retinopathy. Eye Brain 2016, 8, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, R.R.; McEvoy, C.T. Oxygen in the neonatal ICU: A complicated history and where are we now? Front. Pediatr. 2024, 12, 1371710. [Google Scholar] [CrossRef] [PubMed]
- Van’t Westende, W.; de Boer, L.; Labuschagne, M. Considerations for future studies on the effect of late phase oxygen strategies in preterm infants to prevent development and treatment for ROP. Front. Pediatr. 2022, 10, 975613. [Google Scholar] [CrossRef]
- Yumani, D.F.J.; Calor, A.K.; van Weissenbruch, M.M. The Course Of IGF-1 Levels and Nutrient Intake in Extremely and Very Preterm Infants During Hospitalisation. Nutrients 2020, 12, 675. [Google Scholar] [CrossRef]
- Gudu, R.K.; Sahoo, S.; Jena, P.; Behura, S.S.; Priyadarshini, S.; Panda, S.K. Lower Hemoglobin Levels as a Risk Factor for the Development of Retinopathy of Prematurity. Cureus 2024, 16, e64264. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Zhang, H.; Li, K.; Zhao, Z.; Ma, H.; Jiang, X.; Jia, Z.; Ma, Q. Progress in the study of association between hematological indicators and retinopathy of prematurity (Review). Biomed. Rep. 2024, 21, 111. [Google Scholar] [CrossRef]
- Maeda, H.; Go, H.; Iwasa, H.; Hiruta, S.; Ichikawa, H.; Sugano, Y.; Ogasawara, K.; Momoi, N.; Sekiryu, T.; Hosoya, M. Red blood cell parameters as biomarkers of retinopathy of prematurity in preterm infants born before 30 weeks of gestation. Sci. Rep. 2025, 15, 264. [Google Scholar] [CrossRef]
- Prasad, M.; Dombrovsky, D.; Christiansen, S.P.; Ingolfsland, E.C. Anemia and retinopathy of prematurity: A narrative review. Pediatr. Investig. 2025, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tavares, P.; Marques Neves, C.; GenE ROP Study Group. Erythrocyte parameters and retinopathy of prematurity: Genetic and epigenetic insights. Int. J. Mol. Sci. 2023, 24, 11817. [Google Scholar] [CrossRef]
- Chawla, D.; Agarwal, R.; Deorari, A.; Paul, V.; Chandra, P.; Azad, R. Retinopathy of prematurity. Indian J. Pediatr. 2010, 79, 501–509. [Google Scholar] [CrossRef]
- Pheng, E.; Lim, Z.D.; Tai Li Min, E.; Rostenberghe, H.V.; Shatriah, I. Haemoglobin Levels in Early Life among Infants with and without Retinopathy of Prematurity. Int. J. Environ. Res. Public Health 2021, 18, 7054. [Google Scholar] [CrossRef]
- Wang, X.; Rao, R.; Li, H.; Lei, X.; Dong, W. Red Blood Cell Transfusion for Incidence of Retinopathy of Prematurity. JMIR Pediatr. Parent. 2024, 7, e60330. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, T.; Adams, M.; Pfister, R.E.; Snyers, D.; McDougall, J.; Waldvogel, S.; Held-Egli, K.; Spring, L.; Rogdo, B.; Riedel, T.; et al. Associations Between Red Blood Cell and Platelet Transfusions and Retinopathy of Prematurity. Neonatology 2020, 117, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, D. The Role of Thrombocyte Parameters in Retinopathy of Prematurity Development. Int. J. Clin. Pract. 2022, 2022, 7518533. [Google Scholar] [CrossRef]
- Almutairi, M.F.; Gulden, S.; Hundscheid, T.M.; Bartoš, F.; Cavallaro, G.; Villamor, E. Platelet Counts and Risk of Severe Retinopathy of Prematurity: A Bayesian Model-Averaged Meta-Analysis. Children 2023, 10, 1903. [Google Scholar] [CrossRef]
- Ilieva, A.; Manolova, Y. Incidence of retinopathy of prematurity in Varna region, Bulgaria, and evaluation of perinatal risk factors. Scr. Sci. Med. 2022, 54, 36. [Google Scholar] [CrossRef]
- Hellgren, G.; Lundgren, P.; Pivodic, A.; Löfqvist, C.; Nilsson, A.K.; Ley, D.; Sävman, K.; Smith, L.E.; Hellström, A. Decreased platelet counts and serum levels of VEGF-A, PDGF-BB, and BDNF in extremely preterm infants developing severe ROP. Neonatology 2021, 118, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.; Chechalk, K.; Deane, E.; Fox, R.; Janes, A.; Maguire-Henry, T.; McCabe, D.; O’Connor, C.; Quirk, J.; Swan, E.; et al. Biomarkers in retinopathy of prematurity: A systematic review and meta-analysis. Front. Pediatr. 2024, 12, 1371776. [Google Scholar] [CrossRef]
- Fevereiro-Martins, M.; Santos, A.C.; Marques-Neves, C.; Guimarães, H.; Bicho, M.; GenE-ROP Study Group. Complete blood count parameters as biomarkers of retinopathy of prematurity: A Portuguese multicenter study. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 2997–3006. [Google Scholar] [CrossRef]
- Arcagök, B.C.; Özkan, B. Effective risk factors in the development of retinopathy of prematurity, screening results and intravitreal ranibizumab treatment. Haydarpasa Numune Med. J. 2021, 61, 264–271. [Google Scholar] [CrossRef]
- Molomjamts, M.; Ingolfsland, E.C. Identification of reference genes for the normalization of retinal mRNA expression by RT-qPCR in oxygen induced retinopathy, anemia, and erythropoietin administration. PLoS ONE 2023, 18, e0284764. [Google Scholar] [CrossRef]
- Hirata, M.; Kusakawa, I.; Ohde, S.; Yamanaka, M.; Yoda, H. Risk factors of infant anemia in the perinatal period. Pediatr. Int. 2017, 59, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Modrzejewska, M.; Zdanowska, O.; Potubiński, P. The Role of HIF-1α in Retinopathy of Prematurity: A Review of Current Literature. J. Clin. Med. 2024, 13, 4034. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cheng, Z.; Qiao, H.; Wang, Y. Relationship of retinopathy of prematurity with trajectory of hemoglobin, anemia and blood transfusion in very premature/very low birth weight neonates: A repeated measurement analysis. Res. Square 2022, preprint. [Google Scholar] [CrossRef]
- Pagana, K.D.; Pagana, T.J.; Pagana, T.N. Mosby’s Diagnostic and Laboratory Test Reference, 17th ed.; Elsevier: St. Louis, MO, USA, 2024. [Google Scholar]
- Holzapfel, L.F.; Rysavy, M.A.; Bell, E.F. Red Blood Cell Transfusion Thresholds for Anemia of Prematurity. NeoReviews 2023, 24, e370–e376. [Google Scholar] [CrossRef]
- Pai, H.S.; Joy, R.; Cherian, V.; Peter, P. Anemia in relation to severity of retinopathy of prematurity in preterm babies born in tertiary care centre in South India. Int. J. Contemp. Pediatr. 2020, 7, 2005–2009. [Google Scholar] [CrossRef]
- Christensen, R.D.; Henry, E.; Jopling, J.; Wiedmeier, S.E. The CBC: Reference Ranges for Neonates. Semin. Perinatol. 2009, 33, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Del Vecchio, A.; Henry, E. Expected erythrocyte, platelet and neutrophil values for term and preterm neonates. J. Matern. Fetal Neonatal Med. 2012, 25, 77–79. [Google Scholar] [CrossRef]
- Ochiai, M.; Matsushita, Y.; Inoue, H.; Kusuda, S.; Kang, D.; Ichihara, K.; Nakashima, N.; Ihara, K.; Ohga, S.; Hara, T. Blood Reference Intervals for Preterm Low-Birth-Weight Infants: A Multicenter Cohort Study in Japan. PLoS ONE 2016, 11, e0161439. [Google Scholar] [CrossRef] [PubMed]
- Akyüz Ünsal, A.İ.; Key, Ö.; Güler, D.; Kurt Omurlu, İ.; Anık, A.; Demirci, B.; Dündar, S. Can Complete Blood Count Parameters Predict Retinopathy of Prematurity? Turk. J. Ophthalmol. 2020, 50, 87–93. [Google Scholar] [CrossRef]
- Fevereiro-Martins, M.; Aguiar, L.; Inácio, Â.; Cardoso, C.; Santos, A.C.; Marques-Neves, C.; Guimarães, H.; Pinto, R.; Bicho, M. Fetal Hemoglobin as a Predictive Biomarker for Retinopathy of Prematurity: A Prospective Multicenter Cohort Study in Portugal. Biomedicines 2025, 13, 110. [Google Scholar] [CrossRef]
- Tiruneh, T.; Kiros, T.; Getu, S. Hematological reference intervals among full-term newborns in Ethiopia: A cross-sectional study. BMC Pediatr. 2020, 20, 417. [Google Scholar] [CrossRef]
- Teofili, L.; Papacci, P.; Bartolo, M.; Molisso, A.; Orlando, N.; Pane, L.C.; Giannantonio, C.; Serrao, F.; Bianchi, M.; Valentini, C.G.; et al. Transfusion Free Survival Predicts Severe Retinopathy in Preterm Neonates. Front. Pediatr. 2022, 10, 814194. [Google Scholar] [CrossRef]
- Pritišanac, E.; Urlesberger, B.; Schwaberger, B.; Pichler, G. Fetal Hemoglobin and Tissue Oxygenation Measured with Near Infrared Spectroscopy—A Systematic Qualitative Review. Front. Pediatr. 2021, 9, 710465. [Google Scholar] [CrossRef]
- Simons, M.; Gretton, S.; Silkstone, G.G.A.; Rajagopal, B.S.; Allen-Baume, V.; Syrett, N.; Shaik, T.; Leiva-Eriksson, N.; Ronda, L.; Mozzarelli, A.; et al. Comparison of the oxidative reactivity of recombinant fetal and adult human hemoglobin: Implications for the design of hemoglobin-based oxygen carriers. Biosci. Rep. 2018, 38, BSR20180370. [Google Scholar] [CrossRef]
- Slidsborg, C.; Jensen, A.; Forman, J.L.; Rasmussen, S.; Bangsgaard, R.; Fledelius, H.C.; Greisen, G.; la Cour, M. Neonatal Risk Factors for Treatment-Demanding Retinopathy of Prematurity: A Danish National Study. Ophthalmology 2016, 123, 796–803. [Google Scholar] [CrossRef]
- Stutchfield, C.J.; Jain, A.; Odd, D.; Williams, C.; Markham, R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: A pilot prospective cohort study. Eye 2017, 31, 1451–1455. [Google Scholar] [CrossRef]
- Lust, C.; Vesoulis, Z.; Jackups, R.; Liao, S.; Rao, R.; Mathur, A.M. Early red cell transfusion is associated with development of severe retinopathy of prematurity. J. Perinatol. 2019, 39, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hua, X.; Xu, Y.; Zhu, P.; Hong, K.; Ke, Y. Effect of red blood cell transfusion on the development of retinopathy of prematurity: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0234266. [Google Scholar] [CrossRef] [PubMed]
- El Emrani, S.; Derks, L.A.; Tijam, A.M.; van Boheemen, M.M.; Termote, J.U.M.; van der Meeren, L.E.; Reiss, I.K.M.; Taal, H.R.; Lopriore, E.; Schalij-Delfos, N.E. The Association Between Red Blood Cell Transfusion Timing and the Development of Retinopathy of Prematurity: Application of the Two-Phase Theory. Acta Ophthalmol. 2025, 103, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.K.; Ying, G.-S.; Huang, J.; Karp, K.; Quinn, G.E.; Binenbaum, G. Thrombocytopenia and retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus (JAAPOS) 2011, 15, e3–e4. [Google Scholar] [CrossRef]
- Roberts, I.; Murray, N.A. Neonatal thrombocytopenia: Causes and management. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F490–F495. [Google Scholar] [CrossRef]
- Italiano, J.E., Jr.; Richardson, J.L.; Patel Hett, S.; Battinelli, E.; Zaslavsky, A.; Short, S.; Ryeom, S.; Folkman, J.; Klement, G.L. Angiogenesis is regulated by a novel mechanism: Pro and anti angiogenic proteins are organized into separate platelet α granules and differentially released. Blood 2008, 111, 1227–1233. [Google Scholar] [CrossRef]
- Tao, Y.; Dong, Y.; Lu, C.; Yang, W.; Yang, J.; Li, Q. Relationship between mean platelet volume and retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1791–1794. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, L.; Li, Y.; Yue, Y.; Lv, X. Platelet count and retinopathy of prematurity: A systematic review and meta-analysis. Front. Pediatr. 2025, 13, 1413271. [Google Scholar] [CrossRef]
- Şahinoğlu Keşkek, N.; Gülcan, H.; Yılmaz, G.; Akkoyun, İ. Impact of platelet count in retinopathy of prematurity. Turk. J. Ophthalmol. 2020, 50, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Choręziak, A.; Szpecht, D.; Chmielarz Czarnocińska, A.; Pawłowska, I.; Gotz Wiątkowska, A. The association of platelet counts with development and treatment for retinopathy of prematurity—Is thrombocytopenia a risk factor? Arch. Med. Sci. 2019, 18, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Sola-Visner, M.; Bercovitz, R.S. Neonatal Platelet Transfusions and Future Areas of Research. Transfus. Med. Rev. 2016, 30, 183–188. [Google Scholar] [CrossRef]
- Lundgren, P.; Lundberg, L.; Hellgren, G.; Holmström, G.; Hård, A.-L.; Smith, L.E.; Wallin, A.; Hallberg, B.; Hellström, A. Aggressive posterior retinopathy of prematurity is associated with multiple infectious episodes and thrombocytopenia. Neonatology 2016, 111, 79–85. [Google Scholar] [CrossRef]
- Cakir, B.; Liegl, R.; Hellgren, G.; Lundgren, P.; Sun, Y.; Klevebro, S.; Löfqvist, C.; Mannheimer, C.; Cho, S.; Poblete, A.; et al. Thrombocytopenia is associated with severe retinopathy of prematurity. JCI Insight 2018, 3, e99448. [Google Scholar] [CrossRef]
- Elgendy, M.M.; Durham, R.; Othman, H.F.; Heis, F.; Abu-Shaweesh, G.; Saker, F.; Karnati, S.; Aly, H. Platelet transfusion and outcomes of preterm infants: A cross-sectional study. Neonatology 2021, 118, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Rocha, G.; Flôr-de-Lima, F.; Ferreras, C.; Moita, R.; Silva, R.; Azevedo, I. Thrombocytopenia requiring platelet transfusions is predictive of retinopathy of prematurity in preterm infants. Minerva Pediatr. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]

| ROP Status | Pearson Chi-Squared Test | |||||
|---|---|---|---|---|---|---|
| ROP | Non-ROP | |||||
| n | % | n | % | |||
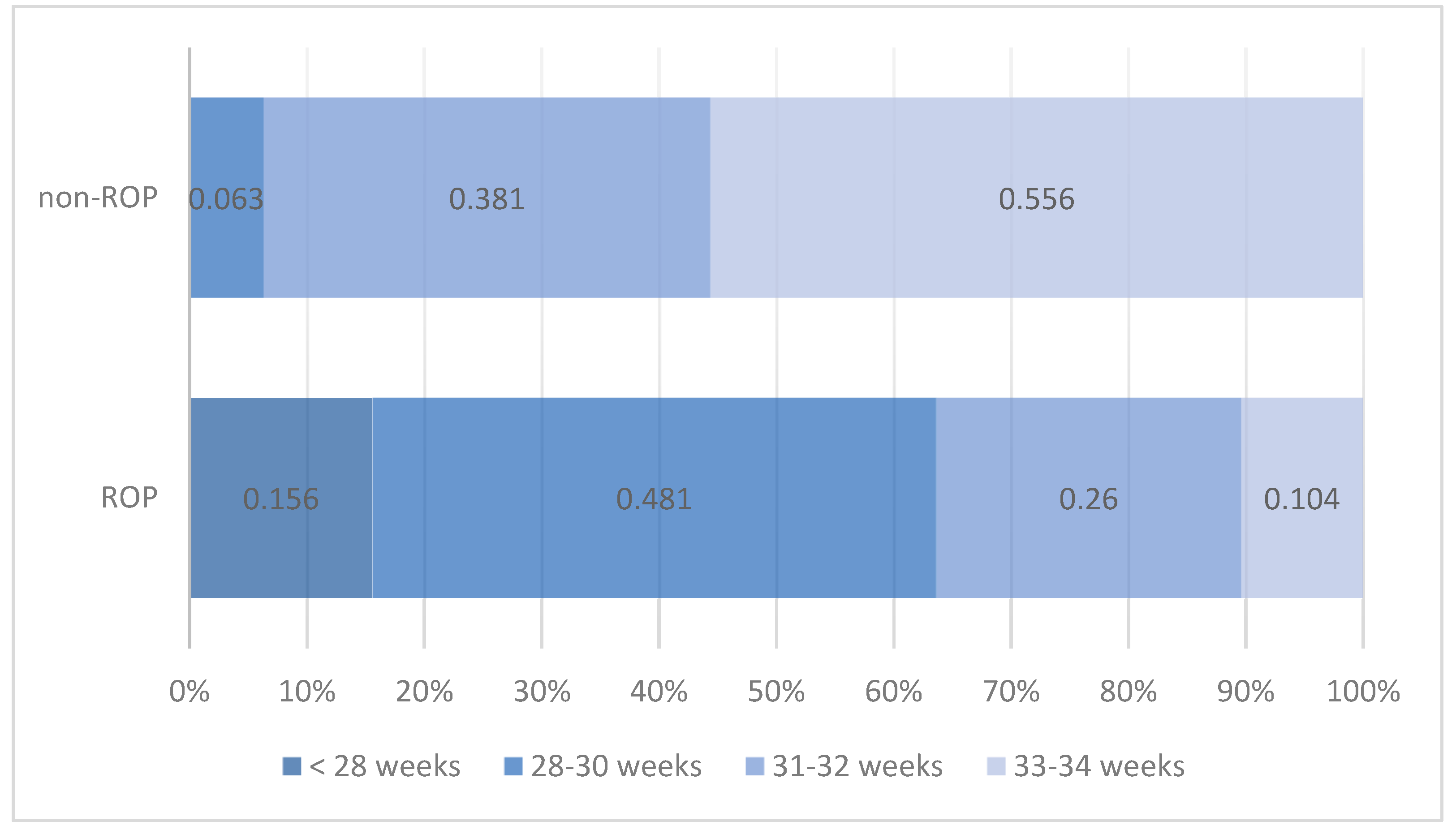
| Gestational age | <28 weeks | 12 | 15.6% | Chi2 = 55.028 | ||
| 28–30 weeks | 37 | 48.1% | 4 | 6.3% | p < 0.001 | |
| 31–32 weeks | 20 | 26.0% | 24 | 38.1% | ||
| 33–34 weeks | 8 | 10.4% | 35 | 55.6% | ||
| Total | 77 | 100.0% | 63 | 100.0% | ||
| HGB | n | Mean | Standard Deviation | Min | Max | Median | IQR | t-Student/ Mann–Whitney/ANOVA/Kruskal–Wallis Test | |
|---|---|---|---|---|---|---|---|---|---|
| 25th | 75th | ||||||||
| TOTAL | |||||||||
| HGB 1 | 140 | 17.2094 | 4.13786 | 7.10 | 57.60 | 16.8000 | 15.4250 | 18.5000 | |
| HGB 3 | 140 | 16.5614 | 3.42766 | 9.00 | 42.10 | 16.4500 | 14.4250 | 18.2750 | |
| HGB 5 | 140 | 15.7271 | 2.54148 | 8.30 | 22.10 | 15.6000 | 14.0000 | 17.6750 | |
| HGB 7 | 140 | 14.6964 | 2.55538 | 9.50 | 20.80 | 14.8000 | 12.6000 | 16.6750 | |
| HGB 14 | 131 | 13.5031 | 3.67421 | 7.80 | 44.70 | 13.3000 | 11.7000 | 14.9000 | |
| HGB 21 | 124 | 12.0242 | 3.12663 | 7.70 | 35.00 | 11.6000 | 10.1250 | 13.1000 | |
| HGB 28 | 111 | 10.7946 | 2.77842 | 1.60 | 28.30 | 10.5000 | 9.2000 | 12.0000 | |
| HGB 1 | |||||||||
| ROP | 77 | 17.4625 | 5.23090 | 7.10 | 57.60 | 16.8000 | 15.7000 | 18.6000 | U = 2309.500 |
| non-ROP | 63 | 16.9000 | 2.16065 | 13.00 | 23.30 | 16.9000 | 15.2000 | 18.5000 | p = 0.627 |
| HGB 3 | |||||||||
| ROP | 77 | 16.4182 | 3.99818 | 9.00 | 42.10 | 15.9000 | 14.4000 | 17.8000 | U = 2038.500 |
| non-ROP | 63 | 16.7365 | 2.58621 | 11.70 | 21.30 | 17.0000 | 15.3000 | 18.5000 | p = 0.105 |
| HGB 5 | |||||||||
| ROP | 77 | 15.4506 | 2.67467 | 8.30 | 22.10 | 15.2000 | 13.8500 | 16.9500 | t = −1.428 |
| non-ROP | 63 | 16.0651 | 2.34536 | 11.70 | 20.10 | 16.5000 | 14.1000 | 18.0000 | p = 0.155 |
| HGB 7 | |||||||||
| ROP | 77 | 14.3104 | 2.68179 | 9.50 | 20.80 | 14.3000 | 12.2500 | 15.8000 | t = −1.997 |
| non-ROP | 63 | 15.1683 | 2.32648 | 10.80 | 19.80 | 15.2000 | 13.3000 | 16.9000 | p = 0.048 |
| HGB 14 | |||||||||
| ROP | 77 | 13.3506 | 4.43861 | 7.80 | 44.70 | 12.8000 | 11.0000 | 14.4500 | U = 1674.000 |
| non-ROP | 54 | 13.7204 | 2.18635 | 9.40 | 18.60 | 13.4500 | 12.1500 | 15.2000 | p = 0.058 |
| HGB 21 | |||||||||
| ROP | 75 | 11.8893 | 3.64241 | 7.70 | 35.00 | 11.6000 | 9.3000 | 13.1000 | U = 1560.500 |
| non-ROP | 49 | 12.2306 | 2.12722 | 8.30 | 17.40 | 11.7000 | 10.9000 | 13.2000 | p = 0.157 |
| HGB 28 | |||||||||
| ROP | 74 | 10.8649 | 3.12583 | 1.60 | 28.30 | 10.5500 | 9.3000 | 12.0000 | U = 1302.500 |
| non-ROP | 37 | 10.6541 | 1.93500 | 7.90 | 15.40 | 10.2000 | 9.2000 | 12.0000 | p = 0.677 |
| RBC | n | Mean | Standard Deviation | Min | Max | Median | IQR | t-Student/Mann–Whitney/ANOVA/Kruskal–Wallis Test | |
|---|---|---|---|---|---|---|---|---|---|
| 25th | 75th | ||||||||
| TOTAL | |||||||||
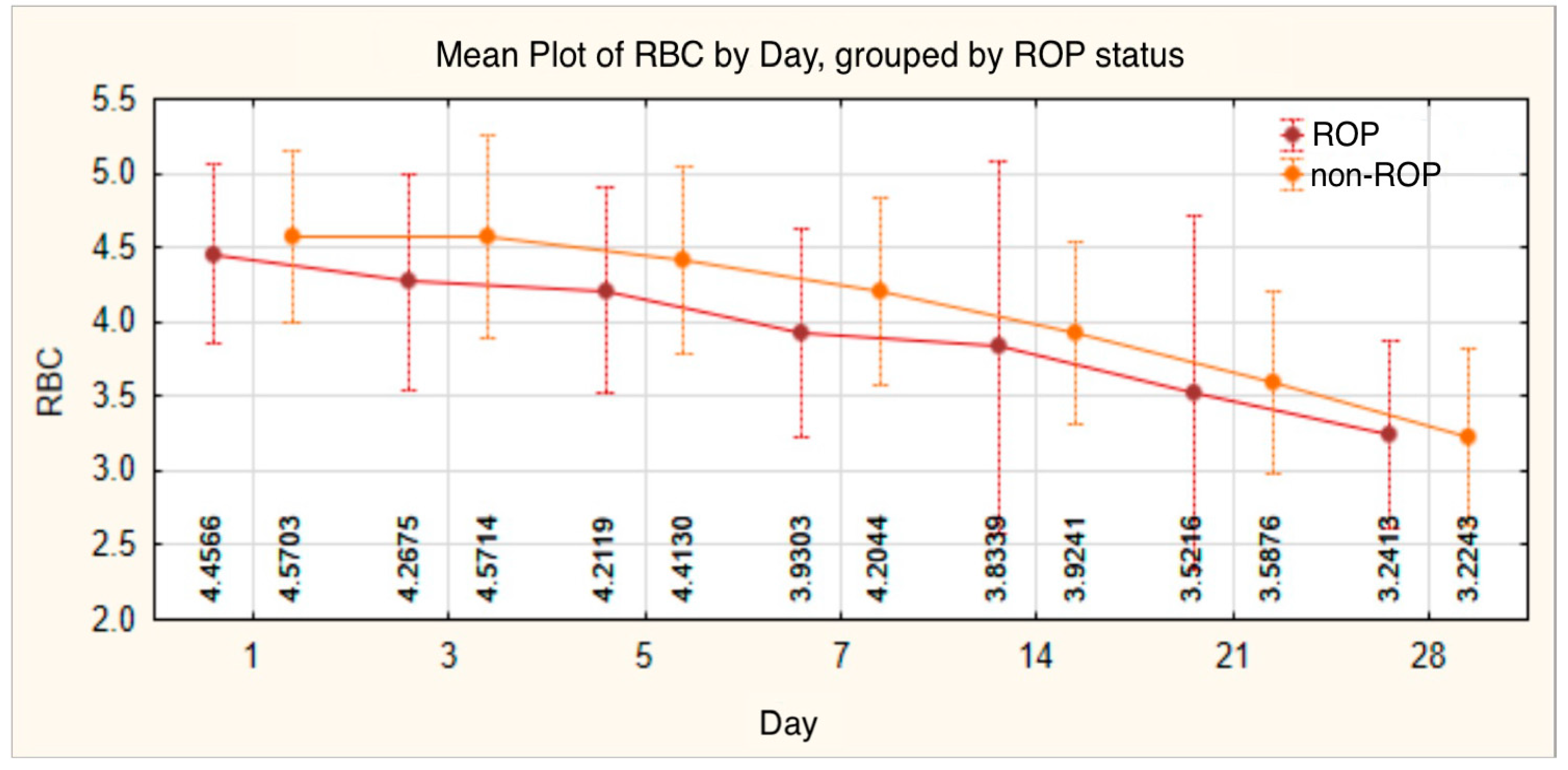
| RBC 1 | 140 | 4.5078 | 0.59300 | 1.98 | 6.17 | 4.4800 | 4.1075 | 4.8975 | |
| RBC 3 | 140 | 4.4043 | 0.71631 | 2.38 | 6.42 | 4.4200 | 3.8475 | 4.8500 | |
| RBC 5 | 140 | 4.3024 | 0.67117 | 2.79 | 5.82 | 4.3050 | 3.8700 | 4.7900 | |
| RBC 7 | 140 | 4.0536 | 0.67613 | 2.46 | 5.71 | 4.0500 | 3.5625 | 4.5675 | |
| RBC 14 | 131 | 3.8711 | 1.03540 | 2.22 | 12.80 | 3.7600 | 3.4100 | 4.2500 | |
| RBC 21 | 125 | 3.5480 | 1.00112 | 2.14 | 11.80 | 3.4600 | 3.0000 | 3.9000 | |
| RBC 28 | 109 | 3.2355 | 0.62008 | 1.79 | 5.00 | 3.1100 | 2.7850 | 3.6600 | |
| RBC 1 | |||||||||
| ROP | 77 | 4.4566 | 0.60374 | 1.98 | 5.58 | 4.4700 | 4.0000 | 4.8700 | t = −1.130 |
| non-ROP | 63 | 4.5703 | 0.57822 | 3.26 | 6.17 | 4.5600 | 4.2300 | 4.9000 | p = 0.261 |
| RBC 3 | |||||||||
| ROP | 77 | 4.2675 | 0.72217 | 2.38 | 6.42 | 4.2700 | 3.7800 | 4.6000 | t = −2.546 |
| non-ROP | 63 | 4.5714 | 0.67781 | 3.14 | 5.81 | 4.5900 | 4.1000 | 5.1000 | p = 0.012 |
| RBC 5 | |||||||||
| ROP | 77 | 4.2119 | 0.68960 | 2.79 | 5.82 | 4.2600 | 3.8350 | 4.5900 | t = −1.777 |
| non-ROP | 63 | 4.4130 | 0.63591 | 3.29 | 5.54 | 4.4800 | 3.8700 | 4.8900 | p = 0.078 |
| RBC 7 | |||||||||
| ROP | 77 | 3.9303 | 0.69662 | 2.46 | 5.71 | 3.9500 | 3.5050 | 4.3650 | t = −2.429 |
| non-ROP | 63 | 4.2044 | 0.62292 | 2.98 | 5.54 | 4.2200 | 3.6900 | 4.7200 | p = 0.016 |
| RBC 14 | |||||||||
| ROP | 77 | 3.8339 | 1.25248 | 2.22 | 12.80 | 3.7300 | 3.3300 | 4.2100 | U = 1740.000 |
| non-ROP | 54 | 3.9241 | 0.61257 | 2.70 | 5.56 | 3.8750 | 3.5425 | 4.4225 | p = 0.113 |
| RBC 21 | |||||||||
| ROP | 75 | 3.5216 | 1.19604 | 2.14 | 11.80 | 3.4000 | 2.8300 | 3.9100 | U = 1588.000 |
| non-ROP | 50 | 3.5876 | 0.61096 | 2.45 | 5.15 | 3.5000 | 3.2625 | 3.7950 | p = 0.148 |
| RBC 28 | |||||||||
| ROP | 72 | 3.2413 | 0.63456 | 1.79 | 5.00 | 3.1050 | 2.8050 | 3.7500 | U = 2001.000 |
| non-ROP | 37 | 3.2243 | 0.59931 | 2.31 | 4.47 | 3.2300 | 2.7800 | 3.4800 | p = 0.828 |
| Anemia | ROP Status | Total | |||||
|---|---|---|---|---|---|---|---|
| ROP | Non-ROP | ||||||
| n | % | n | % | n | % | ||
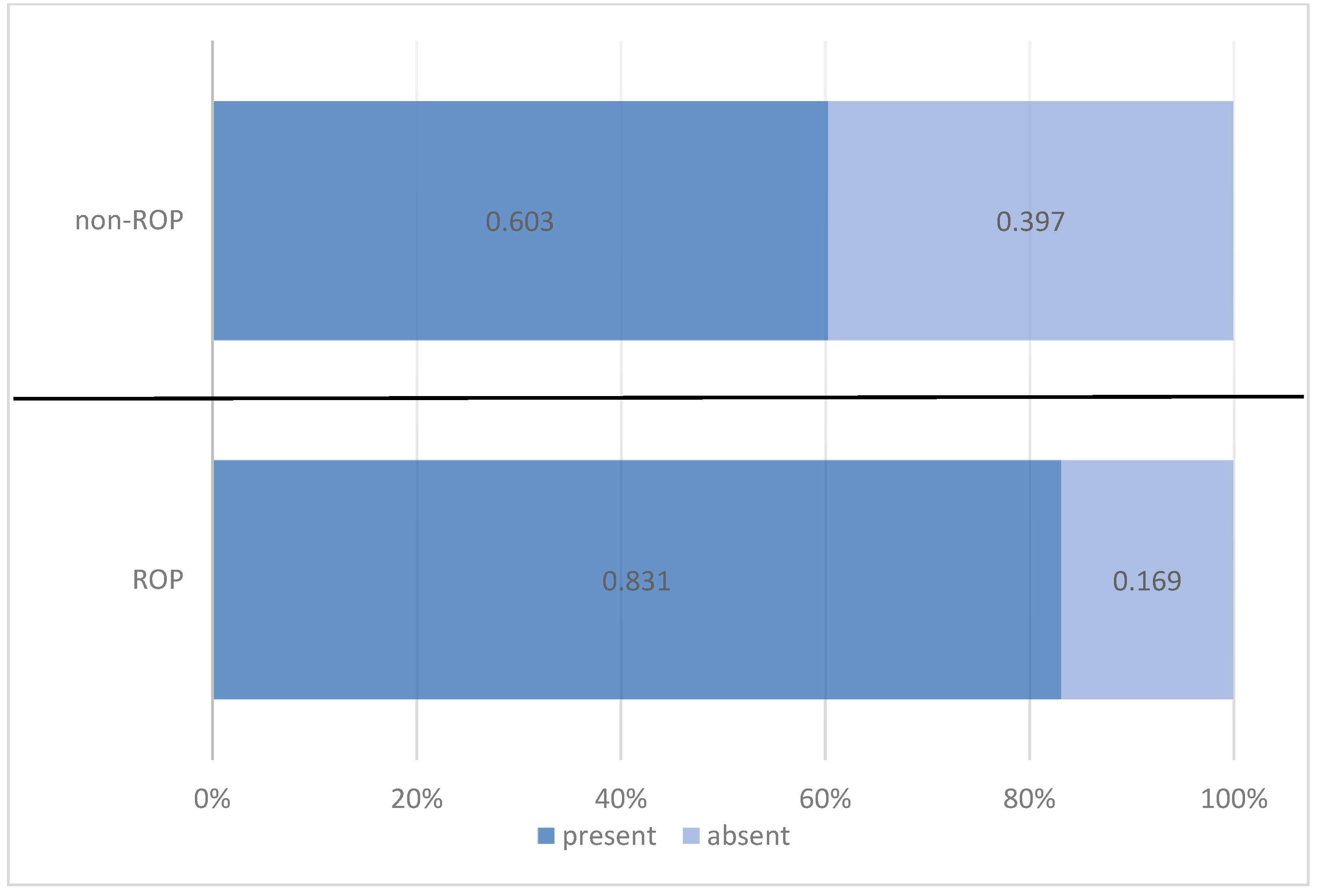
| present | 64 | 83.1% | 38 | 60.3% | 102 | 72.9% | |
| absent | 13 | 16.9% | 25 | 39.7% | 38 | 27.1% | |
| Pearson Chi-squared test: Chi2 = 9.108/p = 0.003 OR = 3.239/95%CI = (1.482 ÷ 7.074) | |||||||
| Total | 77 | 100.0% | 63 | 100.0% | 140 | 100.0% | |
| Day of Anemia Onset | n | Mean | Standard Deviation | Min | Max | Median | IQR | Mann–Whitney/Kruskal–Wallis Test | |
|---|---|---|---|---|---|---|---|---|---|
| 25th | 75th | ||||||||
| TOTAL | 102 | 13.59 | 7.606 | 1 | 28 | 12.50 | 6.00 | 21.00 | |
| ROP status | U = 1083.500 | ||||||||
| ROP | 64 | 13.03 | 7.307 | 1 | 28 | 12.00 | 6.00 | 19.75 | p = 0.358 |
| non-ROP | 38 | 14.53 | 8.097 | 2 | 28 | 15.00 | 6.75 | 21.25 | |
| Red Blood Cell Transfusion | ROP Status | Total | |||||
|---|---|---|---|---|---|---|---|
| ROP | Non-ROP | ||||||
| n | % | n | % | n | % | ||
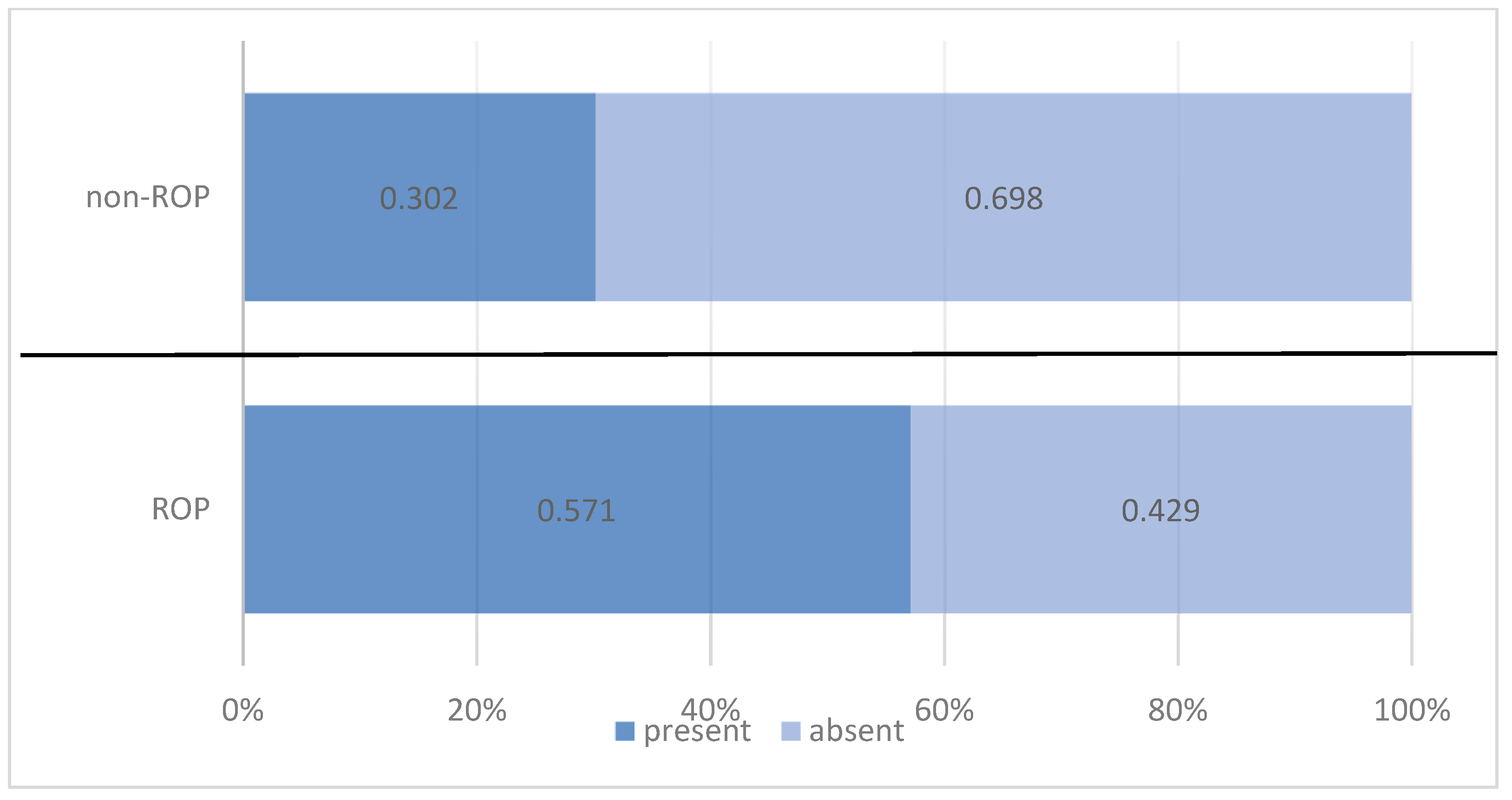
| Present | 44 | 57.1% | 19 | 30.2% | 63 | 45.0% | |
| Absent | 33 | 42.9% | 44 | 69.8% | 77 | 55.0% | |
| Pearson Chi-squared test: Chi2 = 10.194/p = 0.001 OR = 3.088/95%CI = (1.530 ÷ 6.232) | |||||||
| Total | 77 | 100.0% | 63 | 100.0% | 140 | 100.0% | |
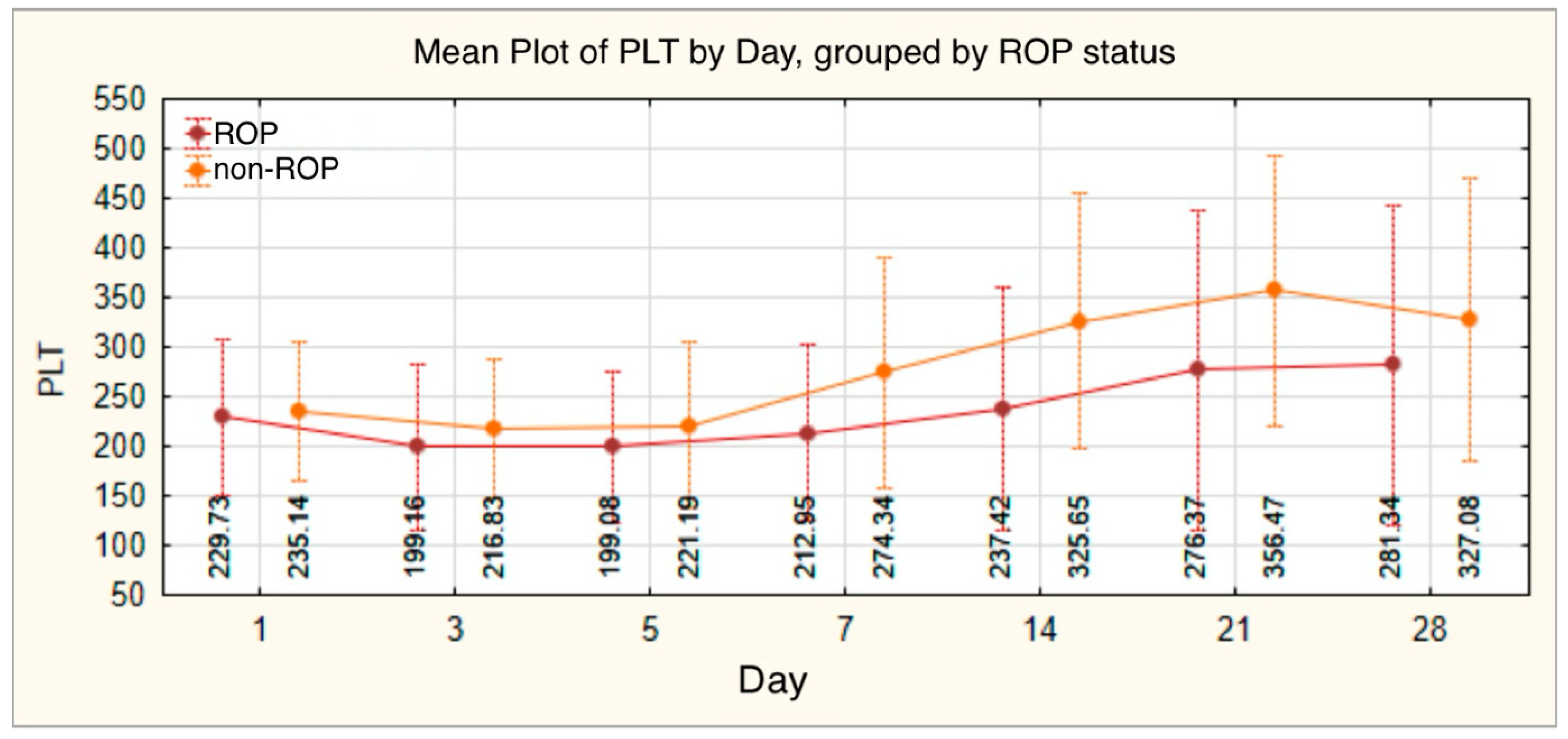
| PLT | n | Mean | Standard Deviation | Min | Max | Median | IQR | t-Student/Mann–Whitney/ANOVA/Kruskal–Wallis Test | |
|---|---|---|---|---|---|---|---|---|---|
| 25th | 75th | ||||||||
| TOTAL | |||||||||
| PLT 1 | 140 | 232.16 | 75.325 | 28 | 479 | 229.50 | 180.75 | 285.25 | |
| PLT 3 | 140 | 207.11 | 78.258 | 21 | 430 | 199.50 | 157.00 | 256.25 | |
| PLT 5 | 140 | 209.03 | 80.006 | 35 | 416 | 206.00 | 155.25 | 258.50 | |
| PLT 7 | 139 | 240.33 | 105.892 | 53 | 721 | 228.00 | 168.00 | 297.00 | |
| PLT 14 | 130 | 274.07 | 132.394 | 47 | 683 | 264.00 | 167.50 | 373.50 | |
| PLT 21 | 124 | 308.02 | 156.271 | 40 | 718 | 300.00 | 170.00 | 429.00 | |
| PLT 28 | 111 | 296.59 | 156.638 | 45 | 847 | 287.00 | 168.00 | 416.00 | |
| PLT 1 | |||||||||
| ROP | 77 | 229.73 | 79.086 | 28 | 479 | 228.00 | 177.00 | 279.00 | t = −0.422 |
| non-ROP | 63 | 235.14 | 70.972 | 42 | 414 | 233.00 | 180.00 | 286.00 | p = 0.674 |
| PLT 3 | |||||||||
| ROP | 77 | 199.16 | 84.140 | 21 | 430 | 188.00 | 149.00 | 252.00 | t = −1.333 |
| non-ROP | 63 | 216.83 | 69.842 | 32 | 416 | 221.00 | 169.00 | 261.00 | p = 0.185 |
| PLT 5 | |||||||||
| ROP | 77 | 199.08 | 76.093 | 35 | 416 | 201.00 | 151.50 | 248.50 | t = −1.637 |
| non-ROP | 63 | 221.19 | 83.544 | 35 | 394 | 221.00 | 168.00 | 277.00 | p = 0.104 |
| PLT 7 | |||||||||
| ROP | 77 | 212.95 | 88.906 | 53 | 420 | 202.00 | 160.50 | 261.50 | t = −3.536 |
| non-ROP | 62 | 274.34 | 115.747 | 61 | 721 | 262.50 | 191.75 | 346.00 | p = 0.001 |
| PLT 14 | |||||||||
| ROP | 76 | 237.42 | 122.846 | 47 | 549 | 226.00 | 133.75 | 312.00 | t = −3.951 |
| non-ROP | 54 | 325.65 | 129.117 | 70 | 683 | 347.50 | 230.00 | 394.25 | p < 0.001 |
| PLT 21 | |||||||||
| ROP | 75 | 276.37 | 161.710 | 40 | 622 | 256.00 | 135.00 | 405.00 | t = −2.871 |
| non-ROP | 49 | 356.47 | 135.286 | 112 | 718 | 358.00 | 271.50 | 442.00 | p = 0.005 |
| PLT 28 | |||||||||
| ROP | 74 | 281.34 | 161.911 | 45 | 847 | 288.00 | 143.00 | 398.00 | t = −1.458 |
| non-ROP | 37 | 327.08 | 142.749 | 111 | 607 | 269.00 | 212.00 | 449.50 | p = 0.148 |
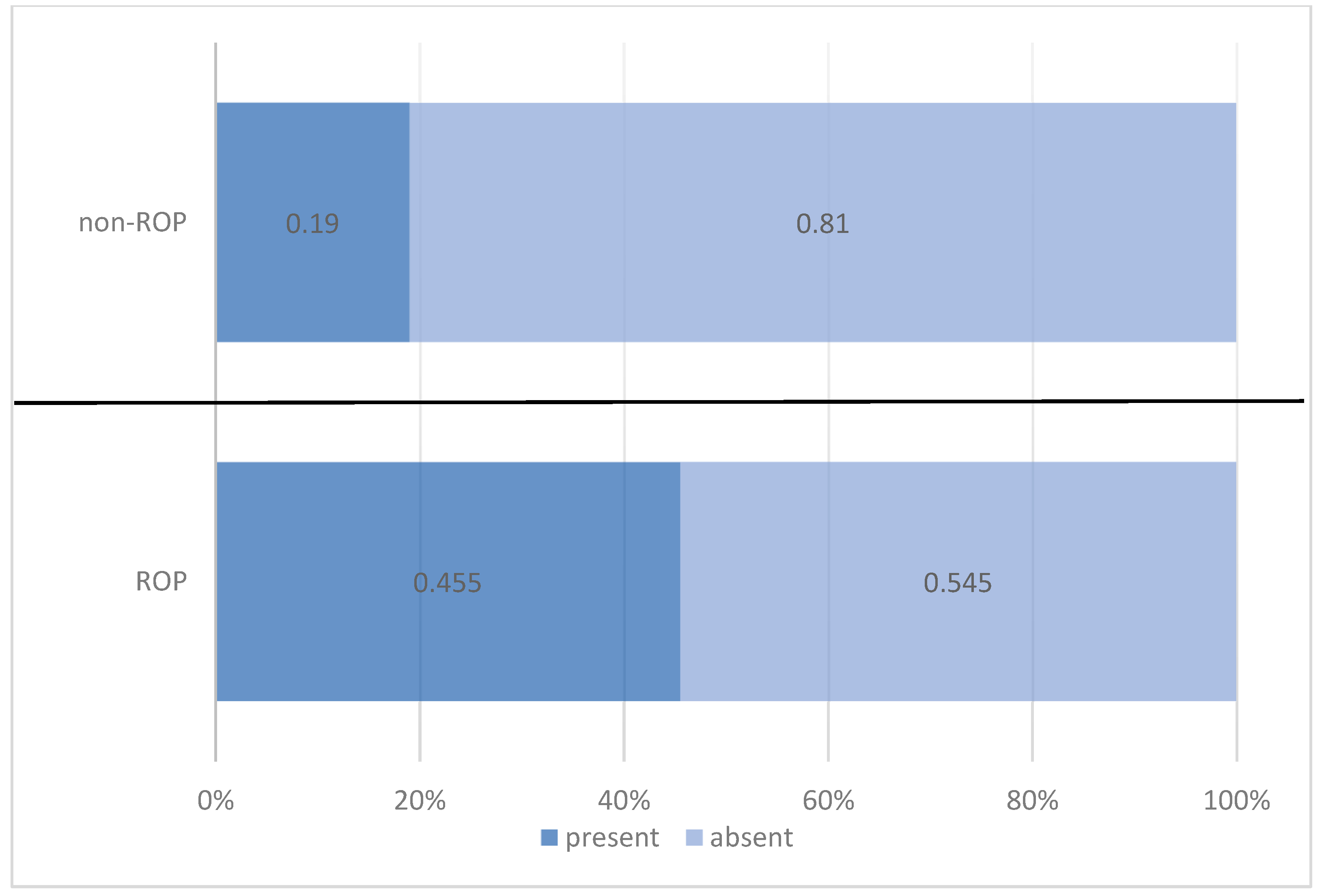
| Thrombocytopenia | ROP Status | Total | |||||
|---|---|---|---|---|---|---|---|
| ROP | Non-ROP | ||||||
| n | % | n | % | n | % | ||
| present | 35 | 45.5% | 12 | 19.0% | 47 | 33.6% | |
| absent | 42 | 54.5% | 51 | 81.0% | 93 | 66.4% | |
| Pearson Chi-squared test: Chi2 = 10.835/p = 0.001 OR = 3.542/95% CI = (1.636 ÷ 7.668) | |||||||
| Total | 77 | 100.0% | 63 | 100.0% | 140 | 100.0% | |
| Day of Thrombocytopenia Onset | n | Mean | Standard Deviation | Min | Max | Median | IQR | Mann–Whitney/Kruskal–Wallis Test | |
|---|---|---|---|---|---|---|---|---|---|
| 25th | 75th | ||||||||
| TOTAL | 47 | 7.49 | 5.830 | 1 | 21 | 5.00 | 3.00 | 14.00 | |
| ROP status | U = 160.000 | ||||||||
| ROP | 35 | 8.06 | 6.053 | 1 | 21 | 6.00 | 3.00 | 14.00 | p = 0.220 |
| non-ROP | 12 | 5.83 | 4.988 | 1 | 14 | 4.50 | 2.00 | 11.25 | |
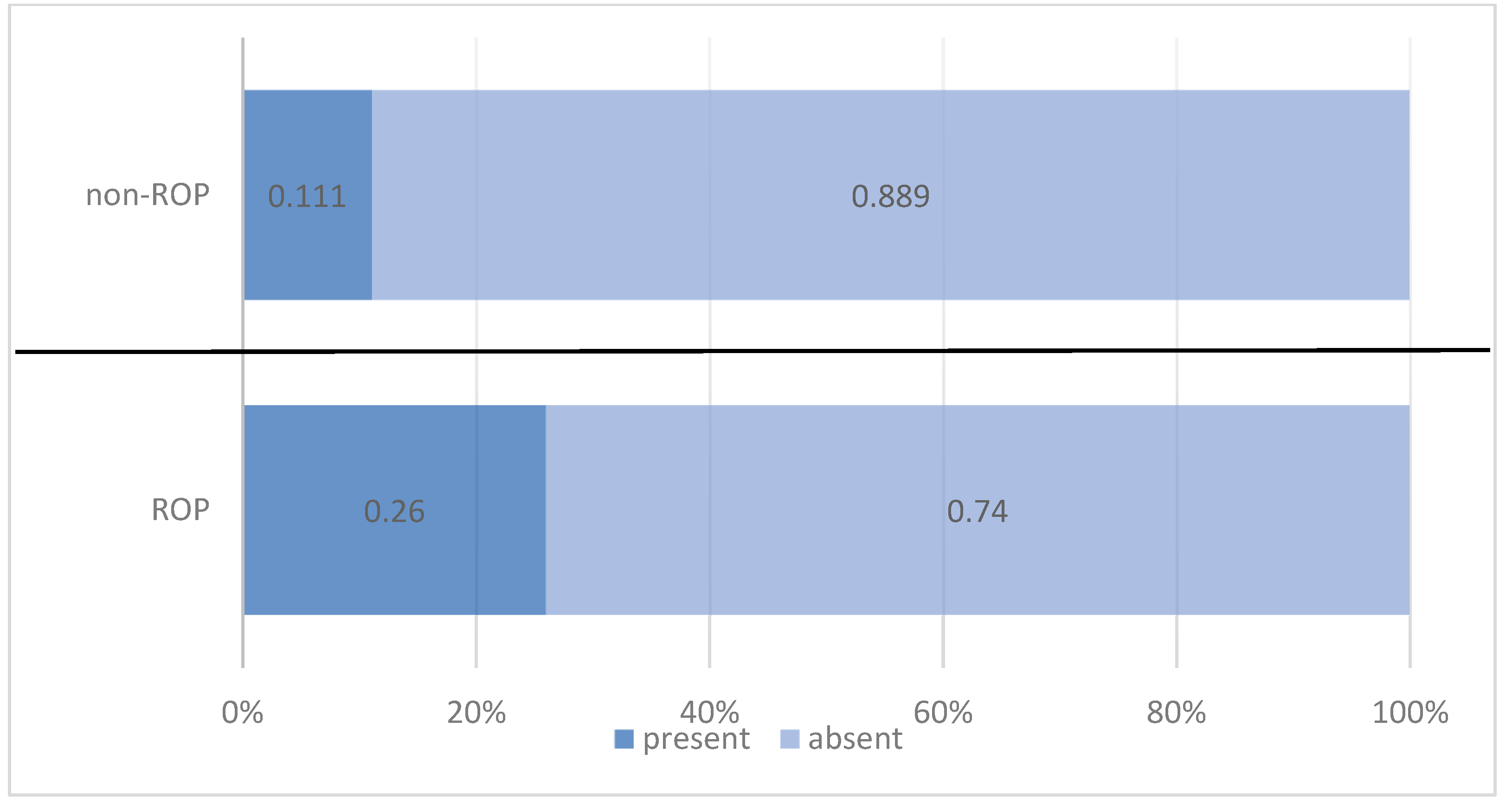
| Platelet Transfusion | ROP Status | Total | |||||
|---|---|---|---|---|---|---|---|
| ROP | Non-ROP | ||||||
| n | % | n | % | n | % | ||
| present | 20 | 26.0% | 7 | 11.1% | 27 | 19.3% | |
| absent | 57 | 74.0% | 56 | 88.9% | 113 | 80.7% | |
| Pearson Chi-squared test: Chi2 = 4.917/p = 0.027 OR = 2.807/95% CI = (1.100 ÷ 7.160) | |||||||
| Total | 77 | 100.0% | 63 | 100.0% | 140 | 100.0% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujoreanu Bezman, L.; Tiutiuca, C.; Bujoreanu, F.C.; Cârneciu, N.; Crăescu, M.; Dimofte, F.; Niculeț, E.; Nechita, A. From Blood Count Parameters to ROP Risk: Early Hematological Predictors in Preterm Infants. Medicina 2025, 61, 1581. https://doi.org/10.3390/medicina61091581
Bujoreanu Bezman L, Tiutiuca C, Bujoreanu FC, Cârneciu N, Crăescu M, Dimofte F, Niculeț E, Nechita A. From Blood Count Parameters to ROP Risk: Early Hematological Predictors in Preterm Infants. Medicina. 2025; 61(9):1581. https://doi.org/10.3390/medicina61091581
Chicago/Turabian StyleBujoreanu Bezman, Laura, Carmen Tiutiuca, Florin Ciprian Bujoreanu, Nicoleta Cârneciu, Mihaela Crăescu, Florentin Dimofte, Elena Niculeț, and Aurel Nechita. 2025. "From Blood Count Parameters to ROP Risk: Early Hematological Predictors in Preterm Infants" Medicina 61, no. 9: 1581. https://doi.org/10.3390/medicina61091581
APA StyleBujoreanu Bezman, L., Tiutiuca, C., Bujoreanu, F. C., Cârneciu, N., Crăescu, M., Dimofte, F., Niculeț, E., & Nechita, A. (2025). From Blood Count Parameters to ROP Risk: Early Hematological Predictors in Preterm Infants. Medicina, 61(9), 1581. https://doi.org/10.3390/medicina61091581







