Leukocyte-Based Inflammatory Profiles Across Dyslipidemia Phenotypes: Patterns of Eosinophil-Related Indices
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Study Design
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Studied Population
3.2. Patterns of Leukocyte-Derived Inflammatory Ratios Across Dyslipidemia Phenotypes
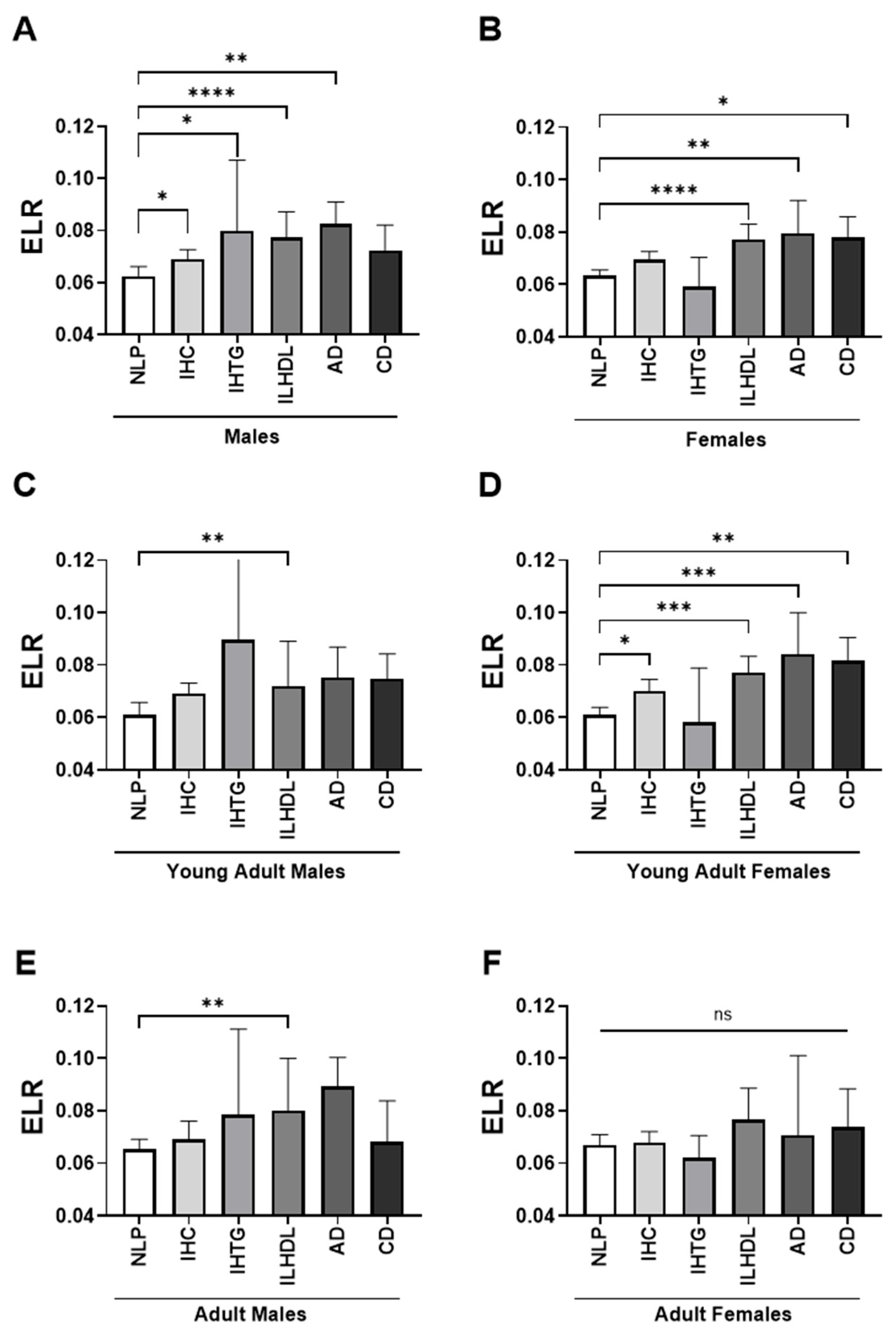
3.3. Sex- and Age-Stratified Differences in Eosinophil-to-Lymphocyte Ratio (ELR) Across Dyslipidemia Phenotypes
3.4. Sex- and Age-Stratified Differences in EA-SIRI Across Dyslipidemia Phenotypes
3.5. ELR Stratification Reveals Trends in Lipid Abnormalities and Dyslipidemia Types
3.6. Distribution of Lipid Parameters and Dyslipidemia Phenotypes by EA-SIRI Stratification
3.7. Frequency Distribution of Dyslipidemia Phenotypes in Relation to Elevated ELR and EA-SIRI Levels
3.8. Risk Estimates of Dyslipidemia Phenotypes According to Elevated ELR and EA-SIRI Levels
3.9. Discriminatory Performance of ELR and EA-SIRI in Identifying Dyslipidemia Phenotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef]
- Hegele, R.A. Combined Lipid Disturbances: More Than the Sum of Their Parts? Circ. Res. 2024, 135, 277–279. [Google Scholar] [CrossRef]
- Klobučar Majanović, S.; Cvijanović Peloza, O.; Detel, D.; Kenđel Jovanović, G.; Bakula, M.; Rahelić, D. Dyslipidemia: Current Perspectives and Implications for Clinical Practice. Manag. Dyslipidemia IntechOpen 2021, 3, 148–158. [Google Scholar] [CrossRef]
- Du, Z.; Qin, Y. Dyslipidemia and Cardiovascular Disease: Current Knowledge, Existing Challenges, and New Opportunities for Management Strategies. J. Clin. Med. 2023, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Shapiro, M.D. Immune-Mediated Inflammatory Diseases, Dyslipidemia, and Cardiovascular Risk: A Complex Interplay. Arter. Thromb. Vasc. Biol. 2024, 44, 2396–2406. [Google Scholar] [CrossRef]
- Alshuweishi, Y.; Almufarrih, A.A.; Abudawood, A.; Alfayez, D.; Alkhowaiter, A.Y.; AlSudais, H.; Almuqrin, A.M. Patterns of Lipid Abnormalities in Obesity: A Comparative Analysis in Normoglycemic and Prediabetic Obese Individuals. J. Pers. Med. 2024, 14, 980. [Google Scholar] [CrossRef]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Aimaiti, X.; Zheng, Y.-Y.; Zhi, X.-Y.; Wang, Z.-L.; Yin, X.; Pan, Y.; Wu, T.-T.; Xie, X. Epidemic trends of dyslipidemia in young adults: A real-world study including more than 20,000 samples. Lipids Health Dis. 2023, 22, 108. [Google Scholar] [CrossRef]
- D’aDamo, E.; Guardamagna, O.; Chiarelli, F.; Bartuli, A.; Liccardo, D.; Ferrari, F.; Nobili, V. Atherogenic Dyslipidemia and Cardiovascular Risk Factors in Obese Children. Int. J. Endocrinol. 2015, 2015, 912047. [Google Scholar] [CrossRef]
- Al-Shehri, S.N.; Saleh, Z.A.; Salama, M.M.; Hassan, Y.M. Prevalence of hyperlipidemia among Saudi school children in Riyadh. Ann. Saudi Med. 2004, 24, 6–8. [Google Scholar] [CrossRef]
- AlMuhaidib, S.; AlBuhairan, F.; Tamimi, W.; AlDubayee, M.; AlAqeel, A.; Babiker, A.; AlFaraidi, H.; AlJuraibah, F.; Badri, M.; Al Alwan, I. Prevalence and factors associated with dyslipidemia among adolescents in Saudi Arabia. Sci. Rep. 2022, 12, 16888. [Google Scholar] [CrossRef] [PubMed]
- Enani, S.; Bahijri, S.; Malibary, M.; Jambi, H.; Eldakhakhny, B.; Al-Ahmadi, J.; Al Raddadi, R.; Ajabnoor, G.; Boraie, A.; Tuomilehto, J. The Association between Dyslipidemia, Dietary Habits and Other Lifestyle Indicators among Non-Diabetic Attendees of Primary Health Care Centers in Jeddah, Saudi Arabia. Nutrients 2020, 12, 2441. [Google Scholar] [CrossRef] [PubMed]
- Bin Saleh, F.S.; Alharbi, W.S.; Alanazi, G.B.; Aldughaither, A. Prevalence and Regulation of Dyslipidemia Among Adults With Type 2 Diabetes From Three Primary Health Care Centers in Riyadh. Cureus 2022, 14, e27573. [Google Scholar] [CrossRef]
- AlTalhi, K.; Otaywi, S.; Alotaibi, M.; Alhamzi, H.A.; Alrashedi, S.; Makkawy, M.; Alokaily, F.; Alotaiwi, S. Prevalence of hyperlipidemia in psoriatic arthritis patients in Riyadh, Saudi Arabia. Saudi Med. J. 2024, 45, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, J.A.; Berbée, J.F.; Havekes, L.M.; Rensen, P.C. Interactions between inflammation and lipid metabolism: Relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis 2013, 228, 306–315. [Google Scholar] [CrossRef]
- Sukhorukov, V.N.; Orekhov, A.N. Molecular Aspects of Inflammation and Lipid Metabolism in Health and Disease: The Role of the Mitochondria. Int. J. Mol. Sci. 2024, 25, 6299. [Google Scholar] [CrossRef]
- Kumar, N.G.; Contaifer, D.; Madurantakam, P.; Carbone, S.; Price, E.T.; Van Tassell, B.; Brophy, D.F.; Wijesinghe, D.S. Dietary Bioactive Fatty Acids as Modulators of Immune Function: Implications on Human Health. Nutrients 2019, 11, 2974. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, C.-Y.; Deng, W.-M. The role of pro-inflammatory cytokines in lipid metabolism of metabolic diseases. Int. Rev. Immunol. 2019, 38, 249–266. [Google Scholar] [CrossRef]
- Seo, I.-H.; Lee, Y.-J. Usefulness of Complete Blood Count (CBC) to Assess Cardiovascular and Metabolic Diseases in Clinical Settings: A Comprehensive Literature Review. Biomedicines 2022, 10, 2697. [Google Scholar] [CrossRef]
- Liu, H.; Dong, H.; Guo, M.; Cheng, H. Association between inflammation indicators (MLR, NLR, SII, SIRI, and AISI) and erectile dysfunction in US adults: NHANES 2001–2004. J. Health Popul. Nutr. 2024, 43, 169. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, Y.A. Effectiveness of Systemic Inflammation Response Index (SIRI) Neutrophil–Lymphocyte Ratio (NLR), Derived Neutrophil–Lymphocyte Ratio (dNLR), and Systemic Immune Inflammation Index (SII) for predicting prognosis of acute diverticulitis. Updat. Surg. 2025, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, H.K.; Sathyabal, V.C.; Vasanthan, M.; Dasarathan, R. The predictive role of Systemic Inflammation Response Index (SIRI), Neutrophil-Lymphocyte Ratio (NLR), and Platelet-Lymphocyte Ratio (PLR) in the prognosis of acute coronary syndrome in a tertiary care hospital. Heliyon 2024, 10, e39029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, X.; Xu, Y.; Wang, K.; Xu, T.; Han, T.; Hu, C.; Li, R.; Lin, X.; Jin, L. Association between inflammatory biomarkers and mortality in individuals with type 2 diabetes: NHANES 2005–2018. Diabetes Res. Clin. Pr. 2024, 209, 111575. [Google Scholar] [CrossRef]
- Si, Y.; Chen, Q.; Xiong, X.; Zheng, M. The association of inflammatory biomarkers with clinical outcomes in diabetic retinopathy participants: Data from NHANES 2009–2018. Diabetol. Metab. Syndr. 2024, 16, 181. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Liu, M.; Zhou, H.; Xu, H. Association between neutrophil-to-lymphocyte ratio and diabetic kidney disease in type 2 diabetes mellitus patients: A cross-sectional study. Front. Endocrinol. 2024, 14, 1285509. [Google Scholar] [CrossRef]
- Konishi, T.; Funayama, N.; Yamamoto, T.; Morita, T.; Hotta, D.; Nishihara, H.; Tanaka, S. Prognostic Value of Eosinophil to Leukocyte Ratio in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J. Atheroscler. Thromb. 2017, 24, 827–840. [Google Scholar] [CrossRef]
- Tosu, A.R.; Kalyoncuoğlu, M.; Biter, H.I.; Çakal, S.; Çakal, B.; Selçuk, M.; Çinar, T. Association of eosinophil-to-lymphocyte ratio with coronary slow-flow phenomenon in patients undergoing coronary angiography. Arch. Med. Sci.—Atheroscler. Dis. 2022, 7, 29–35. [Google Scholar] [CrossRef]
- Lee, Y.; Siddiqui, W.J. Cholesterol Levels; StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Hyassat, D.; Al-Saeksaek, S.; Naji, D.; Mahasneh, A.; Khader, Y.; Abujbara, M.; El-Khateeb, M.; Ajlouni, K. Dyslipidemia among patients with type 2 diabetes in Jordan: Prevalence, pattern, and associated factors. Front. Public. Health 2022, 10, 1002466. [Google Scholar] [CrossRef]
- Ali, N.; Kathak, R.R.; Fariha, K.A.; Taher, A.; Islam, F. Prevalence of dyslipidemia and its associated factors among university academic staff and students in Bangladesh. BMC Cardiovasc. Disord. 2023, 23, 366. [Google Scholar] [CrossRef]
- Wang, P.; Guo, X.; Zhou, Y.; Li, Z.; Yu, S.; Sun, Y.; Hua, Y. Monocyte-to-high-density lipoprotein ratio and systemic inflammation response index are associated with the risk of metabolic disorders and cardiovascular diseases in general rural population. Front. Endocrinol. 2022, 13, 944991. [Google Scholar] [CrossRef]
- Amini, M.; Bashirova, D.; Prins, B.P.; Corpeleijn, E.; LifeLines Cohort Study; Bruinenberg, M.; Franke, L.; van der Harst, P.; Navis, G.; Wolffenbuttel, B.H.R.; et al. Eosinophil Count Is a Common Factor for Complex Metabolic and Pulmonary Traits and Diseases: The LifeLines Cohort Study. PLoS ONE 2016, 11, e0168480. [Google Scholar] [CrossRef]
- Nishi, K.; Matsumoto, H.; Tashima, N.; Terada, S.; Nomura, N.; Kogo, M.; Morimoto, C.; Sunadome, H.; Nagasaki, T.; Oguma, T.; et al. Impacts of lipid-related metabolites, adiposity, and genetic background on blood eosinophil counts: The Nagahama study. Sci. Rep. 2021, 11, 15373. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Woollard, K.J.; McClelland, R.L.; Allison, M.A.; Rye, K.-A.; Ong, K.L.; Cochran, B.J. The association of plasma lipids with white blood cell counts: Results from the Multi-Ethnic Study of Atherosclerosis. J. Clin. Lipidol. 2019, 13, 812–820. [Google Scholar] [CrossRef]
- Tucker, B.; Sawant, S.; McDonald, H.; Rye, K.-A.; Patel, S.; Ong, K.L.; Cochran, B.J. The association of serum lipid and lipoprotein levels with total and differential leukocyte counts: Results of a cross-sectional and longitudinal analysis of the UK Biobank. Atherosclerosis 2021, 319, 1–9. [Google Scholar] [CrossRef]
- Lee, E.-H.; Itan, M.; Jang, J.; Gu, H.-J.; Rozenberg, P.; Mingler, M.K.; Wen, T.; Yoon, J.; Park, S.-Y.; Roh, J.Y.; et al. Eosinophils support adipocyte maturation and promote glucose tolerance in obesity. Sci. Rep. 2018, 8, 9894. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Silveira, A.L.M.; de Oliveira, A.C.C.; Lana, J.P.; Costa, K.A.; Vieira, É.L.M.; Pinho, V.; Teixeira, M.M.; Merabtene, F.; Marcelin, G.; et al. Eosinophils protect from metabolic alterations triggered by obesity. Metabolism 2023, 146, 155613. [Google Scholar] [CrossRef]
- Na Ge, X.; Bastan, I.; Dileepan, M.; Greenberg, Y.; Gil Ha, S.; Steen, K.A.; Bernlohr, D.A.; Rao, S.P.; Sriramarao, P. FABP4 regulates eosinophil recruitment and activation in allergic airway inflammation. Am. J. Physiol. Cell Mol. Physiol. 2018, 315, L227–L240. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Gao, L.; Li, X.; Bai, W. Body mass index affects the association between plasma lipids and peripheral eosinophils in a general chinese population: A cross-sectional survey. Lipids Health Dis. 2023, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Wang, C.; Zhang, X. Association between eosinophil number and overweight status: A nonlinear, bidirectional study. Lipids Health Dis. 2025, 24, 172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, C.; Tian, S.; Wang, J.; Gai, X.; Zhou, Q.; Chen, Y.; Gao, X.; Sun, Y.; Liang, Y. Differences in the lipid metabolism profile and clinical characteristics between eosinophilic and non-eosinophilic acute exacerbation of chronic obstructive pulmonary disease. Front. Mol. Biosci. 2023, 10, 1204985. [Google Scholar] [CrossRef]
- Hartl, S.; Breyer, M.-K.; Burghuber, O.C.; Ofenheimer, A.; Schrott, A.; Urban, M.H.; Agusti, A.; Studnicka, M.; Wouters, E.F.; Breyer-Kohansal, R. Blood eosinophil count in the general population: Typical values and potential confounders. Eur. Respir. J. 2020, 55, 1901874. [Google Scholar] [CrossRef]
- Benson, V.S.; Hartl, S.; Barnes, N.; Galwey, N.; Van Dyke, M.K.; Kwon, N. Blood eosinophil counts in the general population and airways disease: A comprehensive review and meta-analysis. Eur. Respir. J. 2021, 59, 2004590. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, N.; Berdnikovs, S.; Schroeder, H.; Zalewski, A.; Bryce, P.J. Gender-specific differences in the molecular signatures of adult Eosinophilic Oesophagitis. Clin. Exp. Allergy 2017, 47, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Brigger, D.; Riether, C.; van Brummelen, R.; Mosher, K.I.; Shiu, A.; Ding, Z.; Zbären, N.; Gasser, P.; Guntern, P.; Yousef, H.; et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat. Metab. 2020, 2, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-H.; Kim, H.-S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]
- Toth, P.P.; Phan, B.A.P. Dyslipidemia in women: Etiology and management. Int. J. Women’s Health 2014, 6, 185–194. [Google Scholar] [CrossRef]
- Wu, B.; Fan, B.; Qu, Y.; Li, C.; Chen, J.; Liu, Y.; Wang, J.; Zhang, T.; Chen, Y. Trajectories of Blood Lipids Profile in Midlife Women: Does Menopause Matter? J. Am. Hearth Assoc. 2023, 12, e030388. [Google Scholar] [CrossRef]
- Patel, N.; Mittal, N.; Wilkinson, M.J.; Taub, P.R. Unique features of dyslipidemia in women across a lifetime and a tailored approach to management. Am. J. Prev. Cardiol. 2024, 18, 100666. [Google Scholar] [CrossRef]
- Ichikawa, T.; Okada, H.; Hamaguchi, M.; Hara, M.; Takashima, N.; Suzuki, S.; Tanoue, S.; Kato, Y.; Nagayoshi, M.; Tamura, T.; et al. Association of sex hormone-binding globulin and dyslipidemia with Japanese postmenopausal women: A cross-sectional study. Lipids Health Dis. 2025, 24, 212. [Google Scholar] [CrossRef]
- van Oortmerssen, J.A.; Mulder, J.W.; Kavousi, M.; van Lennep, J.E.R. Lipid metabolism in women: A review. Atherosclerosis 2025, 405, 119213. [Google Scholar] [CrossRef]
- Demir; Demir, M.; Keceoglu, S.; Melek, M. The Relationship Between Plasma Eosinophil Count and Coronary Artery Ectasia. Cardiol. Res. 2013, 4, 159–164. [Google Scholar] [CrossRef][Green Version]
- Meng, Z.; Zhang, S.; Li, W.; Wang, Y.; Wang, M.; Liu, X.; Liu, C.-L.; Liao, S.; Liu, T.; Yang, C.; et al. Cationic proteins from eosinophils bind bone morphogenetic protein receptors promoting vascular calcification and atherogenesis. Eur. Heart J. 2023, 44, 2763–2783. [Google Scholar] [CrossRef]
- Biener, L.; Frisch, B.C.; Skowasch, D.; Pizarro, C.; Budimovska, A.; Nickenig, G.; Stumpf, M.J.; Schahab, N.; Schaefer, C. Blood eosinophil count is associated with early atherosclerotic artery changes in asthma. BMC Pulm. Med. 2024, 24, 509. [Google Scholar] [CrossRef]
- Gao, S.; Deng, Y.; Wu, J.; Zhang, L.; Deng, F.; Zhou, J.; Yuan, Z.; Wang, L. Eosinophils count in peripheral circulation is associated with coronary artery disease. Atherosclerosis 2019, 286, 128–134. [Google Scholar] [CrossRef] [PubMed]
- G GI for A. Global Initiative for Asthma—Global Initiative for Asthma—GINA. 2024. Available online: https://ginasthma.org/ (accessed on 27 July 2025).
- Hu, X.; Wang, Y.; Hao, L.-Y.; Liu, X.; Lesch, C.; Sanchez, B.M.; Wendling, J.M.; Morgan, R.W.; Aicher, T.D.; Carter, L.L.; et al. Sterol metabolism controls TH17 differentiation by generating endogenous RORγ agonists. Nat. Chem. Biol. 2015, 11, 141–147. [Google Scholar] [CrossRef]
- Sheha, D.; El-Korashi, L.; AbdAllah, A.M.; El Begermy, M.M.; Elzoghby, D.M.; Elmahdi, A. Lipid Profile and IL-17A in Allergic Rhinitis: Correlation With Disease Severity and Quality of Life. J. Asthma Allergy 2021, ume 14, 109–117. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, I.; Andaloro, C.; Albanese, P.G.; Varricchio, A. Blood lipid levels related to allergic rhinitis: A significant association? EuroMediterranean Biomed. J. 2017, 12, 144–147. [Google Scholar] [CrossRef]
- Lim, J.E.; Kim, H.M.; Kim, J.H.; Baek, H.S.; Han, M.Y. Association between dyslipidemia and asthma in children: A systematic review and multicenter cohort study using a common data model. Clin. Exp. Pediatr. 2023, 66, 357–365. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Zhang, X.; Yuan, Y.L.; Chen, Z.H.; Hsu, A.C.-Y.; Oliver, B.G.; Xie, M.; Qin, L.; Li, W.M.; et al. Dyslipidemia Is Associated With Worse Asthma Clinical Outcomes: A Prospective Cohort Study. J. Allergy Clin. Immunol. Pr. 2022, 11, 863–872.e8. [Google Scholar] [CrossRef]
- Li, W.; Marx, N.; Yang, Q.; Fang, D.; Zhang, Y. Obesity: Next game changer of allergic airway diseases? Clin. Transl. Med. 2025, 15, e70316. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.H.; Payne, S.C.; Fermin, C.R.; Churnin, I.; Qazi, J.; Mattos, J.L. Statin use protective for chronic rhinosinusitis in a nationally representative sample of the United States. Laryngoscope 2020, 130, 848–851. [Google Scholar] [CrossRef] [PubMed]


| Variable | NLP | IHC | IHTG | ILHDL | AD | CD | p-Value |
|---|---|---|---|---|---|---|---|
| Age (yrs) | 45.0 (37.0–53.0) | 46.0 (38.0–54.0) | 47 (39–55) | 45.0 (37.0–54.0) | 48.0 (38.0–56.0) | 45.0 (37.0–53.0) | 0.1035 |
| Sex (Female%) | 62.26% | 60.73% | 64.00% | 59.97% | 53.08% | 55.68% | --- |
| WBC (×109/μL) | 5.55 (4.48–6.87) | 5.51 (4.48–6.82) | 6.22 (4.89–7.48) | 5.68 (4.58–7.01) | 6.09 (4.99–7.67) | 5.79 (4.71–7.26) | <0.001 |
| NEU (×109/μL) | 2.55 (1.69–3.55) | 2.48 (1.67–3.52) | 2.96 (1.91–4.08) | 2.54 (1.78–3.67) | 2.74 (2.00–3.72) | 3.04 (1.96–3.83) | <0.001 |
| MON (×109/μL) | 0.44 (0.36–0.54) | 0.43 (0.35–0.53) | 0.46 (0.37–0.57) | 0.47 (0.38–0.57) | 0.50 (0.41–0.61) | 0.49 (0.39–0.60) | <0.001 |
| LYM (×109/μL) | 2.32 (1.91–2.77) | 2.31 (1.92–2.75) | 2.60 (2.12–2.94) | 2.35 (1.94–2.79) | 2.48 (2.06–3.14) | 2.6 (2.16–3.10) | <0.001 |
| EOS (×109/μL) | 0.15 (0.09–0.23) | 0.16 (0.10–0.24) | 0.18 (0.11–0.26) | 0.19 (0.11–0.28) | 0.21 (0.14–0.30) | 0.20 (0.13–0.30) | <0.001 |
| Hb (g/dL) | 14.5 (13.3–15.6) | 14.7 (13.6–15.8) | 14.8 (13.7–15.8) | 14.9 (13.8–15.8) | 14.8 (13.8–15.8) | 14.8 (13.6–15.8) | <0.001 |
| RBC (×1012/L) | 5.53 (5.12–5.94) | 5.66 (5.23–6.09) | 5.76 (5.24–6.20) | 5.75 (5.37–6.20) | 5.85 (5.51–6.30) | 5.84 (5.36–6.30) | <0.001 |
| PLT (×109/L) | 326 (276–384) | 322 (271–376) | 334 (281–387) | 314 (266–374) | 299 (248–349) | 314 (268–368) | <0.001 |
| CRP (mg/L) | 1.10 (1.01–1.20) | 1.08 (1.00–1.20) | 1.07 (0.98–1.20) | 1.10 (1.02–1.20) | 1.07 (0.98–1.20) | 1.07 (0.98–1.20) | <0.001 |
| FBG (mg/dL) | 100 (93–109) | 105 (95–114) | 100 (93–109) | 105 (96–115) | 103 (94–113) | 100 (93–109) | <0.001 |
| Variable | T1 | T2 | T3 | p-Value |
|---|---|---|---|---|
| Lipid Parameters | ||||
| TG (mg/dL) | 83 (63–113) | 90 (68–121) | 94 (71–123) | <0.001 |
| HDL-C (mg/dL) | 50 (44–58) | 49 (43–57) | 47 (42–55) | <0.001 |
| TC (mg/dL) | 180 (161–205) | 185 (164–208) | 183 (161–207) | 0.003 |
| LDL-C (mg/dL) | 113 (95–134) | 117 (99–137) | 117 (97–138) | < 0.001 |
| DLD Phenotypes (%) | ||||
| NLP | 52.91 | 47.05 | 43.86 | – |
| IHC | 28.95 | 32.23 | 30.76 | – |
| IHTG | 2.63 | 2.50 | 2.41 | – |
| ILHDL | 9.13 | 9.22 | 13.31 | – |
| AD | 1.85 | 3.40 | 3.83 | – |
| CD | 4.52 | 5.60 | 5.82 | – |
| Variable | T1 | T2 | T3 | p Value |
|---|---|---|---|---|
| Lipid Parameters | ||||
| TG (mg/dL) | 82 (63–110) | 89 (68–118) | 96 (73–127) | <0.001 |
| HDL-C (mg/dL) | 50 (44–59) | 49 (43–56) | 47 (42–55) | <0.001 |
| TC (mg/dL) | 183 (161–206) | 183 (163–207) | 182 (161–206) | 0.515 |
| LDL-C (mg/dL) | 115 (96–135) | 115 (98–137) | 116 (97–137) | 0.209 |
| DLD Phenotypes (%) | ||||
| NLP | 51.27 | 49.03 | 43.52 | – |
| IHC | 31.45 | 31.24 | 29.25 | – |
| IHTG | 2.24 | 2.33 | 2.97 | – |
| ILHDL | 8.79 | 9.91 | 12.97 | – |
| AD | 1.81 | 2.67 | 4.61 | – |
| CD | 4.44 | 4.83 | 6.68 | – |
| DLD Phenotype | N—ELR (%) | H—ELR (%) | N—EA-SIRI (%) | H—EA-SIRI (%) |
|---|---|---|---|---|
| NLP | 51.52 | 43.52 | 49.99 | 35.32 |
| IHC | 29.86 | 31.62 | 31.97 | 22.57 |
| IHTG | 2.57 | 2.44 | 2.25 | 2.22 |
| ILHDL | 9.02 | 12.44 | 8.97 | 9.81 |
| AD | 2.29 | 3.94 | 2.22 | 3.17 |
| CD | 4.73 | 6.03 | 4.60 | 4.86 |
| ELR | EA-SIR | |||||
|---|---|---|---|---|---|---|
| DLD Phenotype | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| IHC | 0.999 | 0.89–1.12 | p = 0.986 | 1.25 | 1.12–1.40 | p < 0.001 |
| IHTG | 1.40 | 1.03–1.90 | p = 0.030 | 1.12 | 0.82–1.52 | p = 0.467 |
| ILHDL | 1.55 | 1.32–1.82 | p < 0.001 | 1.63 | 1.39–1.92 | p < 0.001 |
| AD | 2.02 | 1.52–2.68 | p < 0.001 | 2.04 | 1.54–2.71 | p < 0.001 |
| CD | 1.49 | 1.20–1.85 | p < 0.001 | 1.51 | 1.22–1.87 | p < 0.001 |
| ELR | EA-SIRI | |||
|---|---|---|---|---|
| DLD Phenotype | AUC (95% CI) | p-Value | AUC (95% CI) | p-Value |
| IHC | 0.53 (0.51–0.55) | <0.001 | 0.51 (0.49–0.52) | 0.340 |
| IHTG | 0.52 (0.48–0.57) | 0.309 | 0.56 (0.52–0.61) | 0.005 |
| ILHDL | 0.57 (0.55–0.60) | <0.001 | 0.57 (0.55–0.60) | <0.001 |
| AD | 0.60 (0.56–0.64) | <0.001 | 0.62 (0.58–0.66) | <0.001 |
| CD | 0.57 (0.53–0.61) | <0.001 | 0.60 (0.57–0.64) | <0.001 |
| Parameter | Criterion | Cut-Off Value | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|
| ELR | Youden (balanced) | >0.073 | 58.8 (52.0–65.2) | 58.7 (57.0–60.4) |
| Rule-out (high sensitivity) | >0.037 | 90.5 (85.8–93.8) | 19.6 (18.3–21.0) | |
| Rule-in (high specificity) | >0.159 | 10.4 (7.0–15.3) | 91.5 (90.5–92.4) | |
| EA-SIRI | Youden (balanced) | >0.088 | 58.8 (52.0–65.2) | 58.6 (56.9–60.3) |
| Rule-out (high sensitivity) | >0.030 | 90.1 (85.3–93.4) | 20.0 (18.6–21.3) | |
| Rule-in (high specificity) | >0.234 | 16.1 (11.8–21.7) | 90.0 (89.0–91.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshuweishi, Y.; Alsaidan, M.; Basudan, A.M.; Aljohani, H.A.; Almutairi, H.S.; Algarni, N. Leukocyte-Based Inflammatory Profiles Across Dyslipidemia Phenotypes: Patterns of Eosinophil-Related Indices. Medicina 2025, 61, 1579. https://doi.org/10.3390/medicina61091579
Alshuweishi Y, Alsaidan M, Basudan AM, Aljohani HA, Almutairi HS, Algarni N. Leukocyte-Based Inflammatory Profiles Across Dyslipidemia Phenotypes: Patterns of Eosinophil-Related Indices. Medicina. 2025; 61(9):1579. https://doi.org/10.3390/medicina61091579
Chicago/Turabian StyleAlshuweishi, Yazeed, Muath Alsaidan, Ahmed M. Basudan, Hussam A. Aljohani, Hamad S. Almutairi, and Nizar Algarni. 2025. "Leukocyte-Based Inflammatory Profiles Across Dyslipidemia Phenotypes: Patterns of Eosinophil-Related Indices" Medicina 61, no. 9: 1579. https://doi.org/10.3390/medicina61091579
APA StyleAlshuweishi, Y., Alsaidan, M., Basudan, A. M., Aljohani, H. A., Almutairi, H. S., & Algarni, N. (2025). Leukocyte-Based Inflammatory Profiles Across Dyslipidemia Phenotypes: Patterns of Eosinophil-Related Indices. Medicina, 61(9), 1579. https://doi.org/10.3390/medicina61091579






