Effect of Chinese Herbal Medicine on Pregnancy Outcomes in IVF Patients with Low Ovarian Reserve: A Predictive Model
Abstract
1. Introduction
2. Materials and Methods
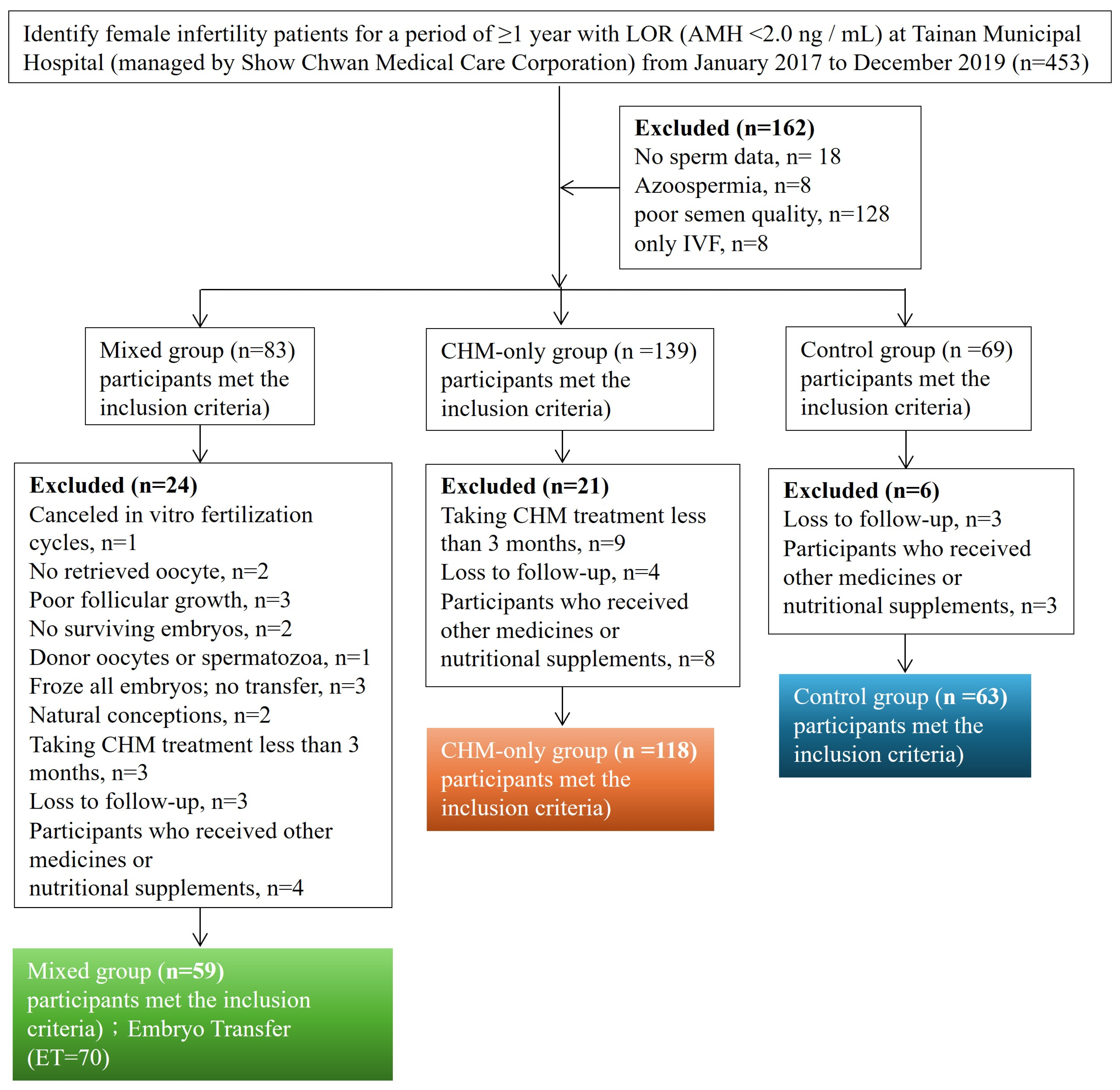
2.1. Data Source and Study Design
2.2. Interventions
2.3. Evaluation Indexes
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Comparison of Baseline Characteristics Among Patients
3.2. Univariate Logistic Regression Analysis of Pregnancy Outcomes Among Patients
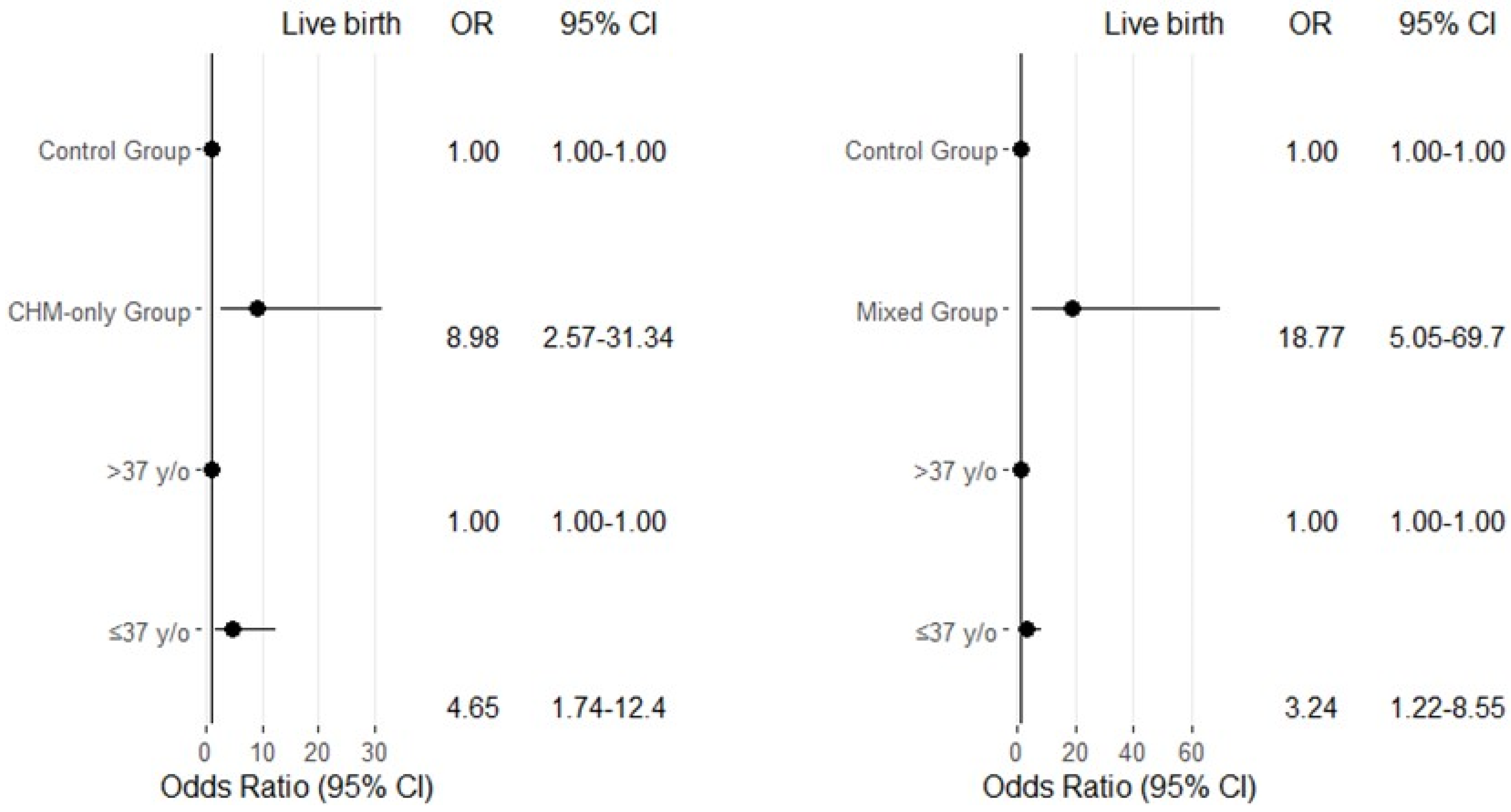
3.3. Multivariate Logistic Regression Analysis of Pregnancy Outcomes Among Control Group and CHM-Only Group Patients
3.4. Multivariate Logistic Regression Analysis of Pregnancy Outcomes Among Control Group and Mixed Group Patients
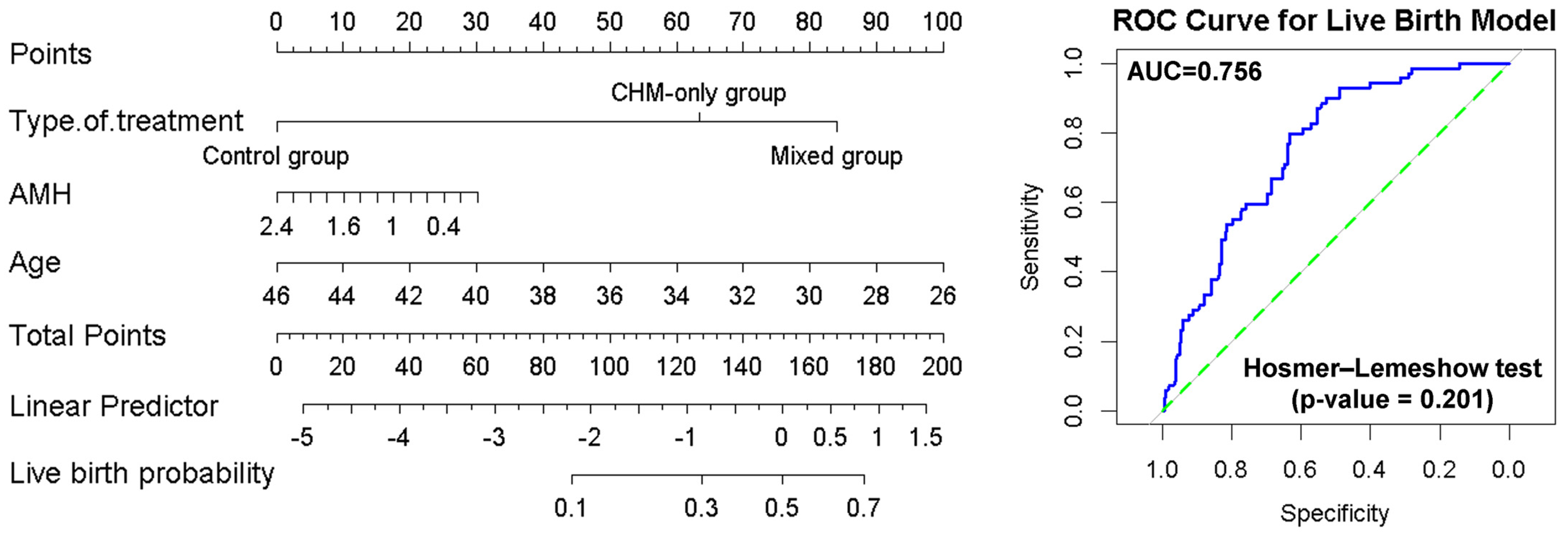
3.5. Prediction of the Live Birth Outcomes Using Nomograms
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOR | Diminished ovarian reserve |
| IVF | In vitro fertilization |
| AMH | Anti-mullerian hormone |
| CHM | Chinese herbal medicine |
| ET | Embryo transfer |
References
- Zhu, Q.; Li, Y.; Ma, J.; Ma, H.; Liang, X. Potential factors result in diminished ovarian reserve: A comprehensive review. J. Ovarian Res. 2023, 16, 208. [Google Scholar] [CrossRef] [PubMed]
- Park, S.U.; Walsh, L.; Berkowitz, K.M. Mechanisms of ovarian aging. Reproduction 2021, 162, R19–R33. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, N.D.; Marsh, E.E. Ovarian Reserve Testing: A Review of the Options, Their Applications, and Their Limitations. Clin. Obstet. Gynecol. 2019, 62, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Wang, Y.; Zheng, L.; Lu, D.; Wu, J.; Wu, B.; Wu, Z.; Jiang, Z. Effect of folic acid supplementation on diminished ovarian reserve: Study protocol of a single-centre, open-label, randomised, placebo-controlled clinical trial. BMJ Open 2022, 12, e057689. [Google Scholar] [CrossRef]
- Silva, M.S.B.; Giacobini, P. New insights into anti-Mullerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell. Mol. Life Sci. 2021, 78, 1–16. [Google Scholar] [CrossRef]
- Dayal, M.; Sagar, S.; Chaurasia, A.; Singh, U. Anti-mullerian hormone: A new marker of ovarian function. J. Obstet. Gynaecol. India 2014, 64, 130–133. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Mullerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- Freour, T.; Miossec, C.; Bach-Ngohou, K.; Dejoie, T.; Flamant, M.; Maillard, O.; Denis, M.G.; Barriere, P.; des Varannes, S.B.; Bourreille, A.; et al. Ovarian reserve in young women of reproductive age with Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 1515–1522. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; He, Y.; Zhang, S.; Qiu, Y.; Feng, R.; Yang, H.; Zeng, Z.; Ben-Horin, S.; Chen, M.; et al. Risk Factors Associated with Impaired Ovarian Reserve in Young Women of Reproductive Age with Crohn’s Disease. Intest. Res. 2020, 18, 200–209. [Google Scholar] [CrossRef]
- Cohen, J.; Chabbert-Buffet, N.; Darai, E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder—A plea for universal definitions. J. Assist. Reprod. Genet. 2015, 32, 1709–1712. [Google Scholar] [CrossRef]
- Rasool, S.; Shah, D. Fertility with early reduction of ovarian reserve: The last straw that breaks the Camel’s back. Fertil. Res. Pract. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Mirmiran, P.; Tehrani, F.R.; Azizi, F. Current Evidence on Associations of Nutritional Factors with Ovarian Reserve and Timing of Menopause: A Systematic Review. Adv. Nutr. 2017, 8, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Sunderam, S.; Kissin, D.M.; Zhang, Y.; Jewett, A.; Boulet, S.L.; Warner, L.; Kroelinger, C.D.; Barfield, W.D. Assisted Reproductive Technology Surveillance—United States, 2017. MMWR Surveill. Summ. 2020, 69, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; van der Poel, S.; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum. Reprod. 2009, 24, 2683–2687. [Google Scholar] [CrossRef]
- Gleicher, N.; Kushnir, V.A.; Barad, D.H. Worldwide decline of IVF birth rates and its probable causes. Hum. Reprod. Open 2019, 2019, hoz017. [Google Scholar] [CrossRef]
- Ballester, M.; Oppenheimer, A.; d’Argent, E.M.; Touboul, C.; Antoine, J.M.; Coutant, C.; Daraï, E. Nomogram to predict pregnancy rate after ICSI-IVF cycle in patients with endometriosis. Hum. Reprod. 2012, 27, 451–456. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Zhang, Y.; Lu, H.; Yu, Y. Nomogram prediction for the prediction of clinical pregnancy in Freeze-thawed Embryo Transfer. BMC Pregnancy Childbirth 2022, 22, 629. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, W.; Sun, Y.; Chen, L.; Li, R.; Chen, X.; Zheng, B. Nomogram to predict the probability of clinical pregnancy in women with poor ovarian response undergoing in vitro fertilization/intracytoplasmic sperm injection cycles. Arch. Gynecol. Obstet. 2024, 310, 1697–1707. [Google Scholar] [CrossRef]
- Vaegter, K.K.; Lakic, T.G.; Olovsson, M.; Berglund, L.; Brodin, T.; Holte, J. Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments? Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertil. Steril. 2017, 107, 641–648.e2. [Google Scholar] [CrossRef]
- Dhillon, R.K.; McLernon, D.J.; Smith, P.P.; Fishel, S.; Dowell, K.; Deeks, J.J.; Bhattacharya, S.; Coomarasamy, A. Predicting the chance of live birth for women undergoing IVF: A novel pretreatment counselling tool. Hum. Reprod. 2016, 31, 84–92. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Seifer, D.B. Ethnicity/Race and Age-Specific Variations of Serum AMH in Women—A Review. Front. Endocrinol. 2020, 11, 593216. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Xiang, W. The impact of mitochondrial dysfunction on ovarian aging. J. Transl. Med. 2025, 23, 211. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, F.Z.; Huang, K.; Hu, L.L.; Bu, Z.Q.; Sun, J.; Su, Y.C.; Guo, Y.H. Factors predicting clinical pregnancy rate of in vitro fertilization-embryo transfer (a STROBE-compliant article). Medicine 2019, 98, e18246. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Jiang, H.; Zhao, Y.; Qi, X.; Li, R.; Long, X.; Qiao, J. Nomogram for Predicting Live Birth after the First Fresh Embryo Transfer in Patients with PCOS Undergoing IVF/ICSI Treatment with the GnRH-Ant Protocol. Diagnostics 2023, 13, 1901. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Su, P.F.; Chen, K.Y.; Tie, T.H.; Ke, H.C.; Chen, H.; Su, Y.C.; Ou, H.T.; Subramanian, S.K. Heart rate variability among women undergoing in vitro fertilization treatment: Its predictive ability for pregnancy. PLoS ONE 2018, 13, e0193899. [Google Scholar]
- Zhang, C.; Xu, X. Advancement in the treatment of diminished ovarian reserve by traditional Chinese and Western medicine. Exp. Ther. Med. 2016, 11, 1173–1176. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, W.; Zhang, J.; Wang, C.; Bi, Y.; Li, P.; Yang, S.; Li, J.; Xu, Y.T.; Wang, T. Protective Effects and Possible Mechanisms of Actions of Bushen Cuyun Recipe on Diminished Ovarian Reserve Induced by Cyclophosphamide in Rats. Front. Pharmacol. 2020, 11, 546. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Dong, Z.; Sun, H.; Diao, Z.; Li, Y.; Du, D.; Ma, Y. The role of Chinese herbal medicine in diminished ovarian reserve management. J. Ovarian Res. 2025, 18, 90. [Google Scholar] [CrossRef]
- Cao, H.; Han, M.; Ng, E.H.; Wu, X.; Flower, A.; Lewith, G.; Liu, J.P.; Walker, N. Can Chinese herbal medicine improve outcomes of in vitro fertilization? A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e81650. [Google Scholar] [CrossRef]
- Hullender Rubin, L.E.; Opsahl, M.S.; Wiemer, K.E.; Mist, S.D.; Caughey, A.B. Impact of whole systems traditional Chinese medicine on in-vitro fertilization outcomes. Reprod. Biomed. Online 2015, 30, 602–612. [Google Scholar] [CrossRef]
- Chang, H.; Yeung, T.C.; Yang, X.; Gao, J.; Wu, X.; Wang, C.C. Chinese herbal medicines as complementary therapy to in vitro fertilization-embryo transfer in women with infertility: Protocols and applications. Hum. Fertil. 2023, 26, 845–863. [Google Scholar] [CrossRef]

| Variable | Control Group | CHM-Only Group | Mixed Group | p-Value |
|---|---|---|---|---|
| (n = 63) | (n = 118) | (n = 59) ET = 70 | ||
| Age, years (SD) | 37.63 (4.00) | 36.69 (3.69) | 38.39 (4.00) | 0.250 |
| Married year, year (SD) | 4.34 (3.12) | 4.08 (2.91) | 4.38 (2.73) | 0.764 |
| BMI, mean (SD) | 22.11 (3.22) | 22.51 (3.71) | 22.54 (3.81) | 0.734 |
| AMH levels (ng/mL) | 0.94 (0.57) | 1.03 (0.55) | 0.93 (0.58) | 0.410 |
| Smokers | ||||
| No | 62 (98%) | 118 (100%) | 59 (100%) | 0.244 |
| Yes | 1 (2%) | 0 (0%) | 0 (0%) | |
| Alcohol use | ||||
| No | 60 (95%) | 115 (97%) | 58 (98%) | 0.569 |
| Yes | 3 (5%) | 3 (3%) | 1 (2%) | |
| Infertility type | ||||
| Primary | 40 (63%) | 78 (66%) | 40 (68%) | 0.879 |
| Secondary | 23 (37%) | 40 (34%) | 19 (32%) | |
| Causes of infertility | ||||
| LOR | 63 (100%) | 118 (100%) | 59 (100%) | ND |
| PCOS | 1 (1%) | 7 (5%) | 4 (6%) | 0.341 |
| Endometriosis | 11 (17%) | 24 (20%) | 13 (22%) | 0.813 |
| Uterine myoma | 9 (14%) | 20 (17%) | 6 (10%) | 0.483 |
| Endocrine disorders | 4 (6%) | 11 (9%) | 7 (11%) | 0.571 |
| Immune disorder | 2 (3%) | 4 (3%) | 3 (5%) | 0.822 |
| High prolactin levels | 6 (10%) | 11 (9%) | 7 (11%) | 0.859 |
| Clinical Pregnancy Rate, n (%) | Live Birth Rate, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Treatment | No | Yes | OR, 95% CI | p Value | No | Yes | OR, 95% CI | p Value | |
| Control group | 63 | 56 (88) | 7 (12) | Reference | 60 (95) | 3 (5) | Reference | ||
| CHM-only group | 118 | 73 (62) | 45 (38) | 4.93 (2.06–11.76) | <0.001 | 81 (68) | 37 (32) | 9.13 (2.68–31.03) | <0.001 |
| Mixed group (ET) | 70 | 34 (48) | 36 (52) | 8.47 (3.39–21.14) | <0.001 | 41 (58) | 29 (42) | 14.14 (4.04–49.53) | <0.001 |
| Age, years | |||||||||
| >37 (medium) | 114 | 80 (70) | 34 (30) | Reference | 95 (83) | 19 (17) | Reference | ||
| ≤37 (medium) | 137 | 83 (60) | 54 (40) | 1.53 (0.90–2.59) | 0.114 | 87 (63) | 50 (37) | 2.87 (1.57–5.25) | 0.001 |
| BMI, kg/m2 | |||||||||
| ≤21.78 (medium) | 126 | 84 (66) | 42 (34) | Reference | 91 (72) | 35 (28) | Reference | ||
| >21.78 (medium) | 125 | 79 (63) | 46 (37) | 1.16 (0.69–1.65) | 0.565 | 91 (72) | 34 (28) | 0.97 (0.55–1.69) | 0.918 |
| AMH levels (ng/mL) | |||||||||
| ≤1.01 (medium) | 126 | 80 (63) | 46 (37) | Reference | 86 (68) | 40 (32) | Reference | ||
| >1.01 (medium) | 125 | 83 (66) | 42 (34) | 0.88 (0.52–1.47) | 0.629 | 96 (76) | 29 (24) | 0.64 (0.37–1.13) | 0.131 |
| Infertility type | |||||||||
| Primary | 205 | 138 (67) | 67 (23) | Reference | 150 (73) | 55 (27) | Reference | ||
| Secondary | 46 | 25 (54) | 21 (46) | 1.73 (0.90–3.31) | 0.098 | 32 (69) | 14 (31) | 1.19 (0.59–2.40) | 0.621 |
| Type of Treatment* Age | |||||||||
| Control group (>37) | 31 | 27 (87) | 4 (13) | Reference | 30 (96) | 1 (4) | Reference | ||
| Control group (≤37) | 32 | 29 (90) | 3 (10) | 0.69 (0.14–3.41) | 0.657 | 30 (93) | 2 (7) | 2.00 (0.17–23.25) | 0.580 |
| CHM-only group (>37) | 43 | 30 (69) | 13 (31) | 2.92 (0.85–10.06) | 0.089 | 37 (86) | 6 (14) | 4.86 (0.55–42.65) | 0.153 |
| CHM-only group (≤37) | 75 | 43 (57) | 32 (43) | 5.02 (1.59–15.79) | 0.006 | 44 (58) | 31 (42) | 21.13 (2.73–163.31) | 0.003 |
| Mixed group (>37) | 40 | 23 (57) | 17 (43) | 4.98 (1.46–16.94) | 0.010 | 28 (70) | 12 (30) | 12.85 (1.56–105.41) | 0.017 |
| Mixed group (≤37) | 30 | 11 (36) | 19 (64) | 11.65 (3.22–42.19) | <0.001 | 13 (43) | 17 (57) | 39.23 (4.71–326.57) | 0.001 |
| Clinical Pregnancy Rate, n (%) | Live Birth Rate, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Treatment | No | Yes | OR, 95% CI | a p Value | No | Yes | OR, 95% CI | a p Value | |
| Control group | 63 | 56 (88) | 7 (12) | Reference | 60 (95) | 3 (5) | Reference | ||
| CHM-only group | 118 | 73 (62) | 45 (38) | 5.04 (2.06–12.31) | <0.001 | 81 (68) | 37 (32) | 8.98 (2.57–31.34) | 0.001 |
| Age, years | |||||||||
| >37 (medium) | 74 | 57 (77) | 17 (23) | Reference | 67 (90) | 7 (10) | Reference | ||
| ≤37 (medium) | 107 | 72 (67) | 35 (33) | 1.69 (0.79–3.62) | 0.172 | 74 (69) | 33 (31) | 4.65 (1.74–12.40) | 0.002 |
| BMI, kg/m2 | |||||||||
| ≤21.78 (medium) | 93 | 66 (70) | 27 (30) | Reference | 71 (76) | 22 (24) | Reference | ||
| >21.78 (medium) | 88 | 63 (71) | 25 (29) | 0.90 (0.44–1.83) | 0.787 | 70 (79) | 18 (21) | 0.89 (0.40–1.98) | 0.784 |
| AMH levels (ng/mL) | |||||||||
| ≤1.01 (medium) | 93 | 64 (68) | 29 (32) | Reference | 69 (74) | 24 (26) | Reference | ||
| >1.01 (medium) | 88 | 65 (73) | 23 (25) | 0.80 (0.39–1.69) | 0.532 | 72 (81) | 16 (19) | 0.62 (0.28–1.37) | 0.238 |
| Infertility type | |||||||||
| Primary | 145 | 108 (74) | 37 (26) | Reference | 115 (79) | 30 (21) | Reference | ||
| Secondary | 36 | 21 (58) | 15 (42) | 2.59 (1.09–6.15) | 0.030 | 26 (72) | 10 (28) | 2.27 (0.82–6.28) | 0.112 |
| Clinical Pregnancy Rate, n (%) | Live Birth Rate, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Treatment | No | Yes | OR, 95% CI | a p Value | No | Yes | OR, 95% CI | a p Value | |
| Control group | 63 | 56 (88) | 7 (12) | Reference | 60 (95) | 3 (5) | Reference | ||
| Mixed group (ET) | 70 | 34 (48) | 36 (52) | 10.61 (3.96–28.43) | <0.001 | 41 (58) | 29 (42) | 18.77 (5.05–69.70) | <0.001 |
| Age, years | |||||||||
| >37 (medium) | 71 | 50 (70) | 21 (30) | Reference | 58 (81) | 13 (19) | Reference | ||
| ≤37 (medium) | 62 | 40 (64) | 22 (36) | 1.91 (0.81–4.54) | 0.138 | 43 (69) | 19 (31) | 3.24 (1.22–8.55) | 0.018 |
| BMI, kg/m2 | |||||||||
| ≤21.78 (medium) | 68 | 48 (70) | 20 (30) | Reference | 53 (77) | 15 (23) | Reference | ||
| >21.78 (medium) | 65 | 42 (64) | 23 (36) | 1.19 (0.50–2.79) | 0.685 | 48 (73) | 17 (27) | 1.34 (0.51–3.52) | 0.542 |
| AMH levels (ng/mL) | |||||||||
| ≤1.01 (medium) | 68 | 47 (69) | 21 (31) | Reference | 50 (73) | 18 (27) | Reference | ||
| >1.01 (medium) | 65 | 43 (66) | 22 (34) | 0.98 (0.42–2.25) | 0.963 | 51 (78) | 14 (22) | 0.55 (0.22–1.41) | 0.216 |
| Infertility type | |||||||||
| Primary | 110 | 77 (70) | 33 (30) | Reference | 84 (76) | 26 (24) | Reference | ||
| Secondary | 23 | 13 (56) | 10 (44) | 3.08 (0.98–9.65) | 0.053 | 17 (73) | 6 (27) | 1.76 (0.50–6.17) | 0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-H.; Wang, S.-C.; Su, P.-F.; Tsai, L.-M.; Chen, P.-M.; Li, C.-J.; Wu, H.-J.; Chang, C.-H. Effect of Chinese Herbal Medicine on Pregnancy Outcomes in IVF Patients with Low Ovarian Reserve: A Predictive Model. Medicina 2025, 61, 1571. https://doi.org/10.3390/medicina61091571
Wu M-H, Wang S-C, Su P-F, Tsai L-M, Chen P-M, Li C-J, Wu H-J, Chang C-H. Effect of Chinese Herbal Medicine on Pregnancy Outcomes in IVF Patients with Low Ovarian Reserve: A Predictive Model. Medicina. 2025; 61(9):1571. https://doi.org/10.3390/medicina61091571
Chicago/Turabian StyleWu, Meng-Hsing, Shu-Chiu Wang, Pei-Fang Su, Liang-Miin Tsai, Po-Ming Chen, Chia-Jung Li, Hsing-Ju Wu, and Chiung-Hung Chang. 2025. "Effect of Chinese Herbal Medicine on Pregnancy Outcomes in IVF Patients with Low Ovarian Reserve: A Predictive Model" Medicina 61, no. 9: 1571. https://doi.org/10.3390/medicina61091571
APA StyleWu, M.-H., Wang, S.-C., Su, P.-F., Tsai, L.-M., Chen, P.-M., Li, C.-J., Wu, H.-J., & Chang, C.-H. (2025). Effect of Chinese Herbal Medicine on Pregnancy Outcomes in IVF Patients with Low Ovarian Reserve: A Predictive Model. Medicina, 61(9), 1571. https://doi.org/10.3390/medicina61091571







