Proton Pump Inhibitors (PPIs)—An Evidence-Based Review of Indications, Efficacy, Harms, and Deprescribing
Abstract
1. Introduction
2. Methods
3. Indications of PPIs
3.1. Co-Prescribing of PPIs
3.1.1. Antiplatelet Therapy
3.1.2. Helicobacter Pylori with Antiplatelet Therapy
Practical Take-Aways
3.1.3. Anticoagulants
3.1.4. NSAIDs
3.1.5. Corticosteroids
4. Efficacy of Different PPIs
PPIs Dosage
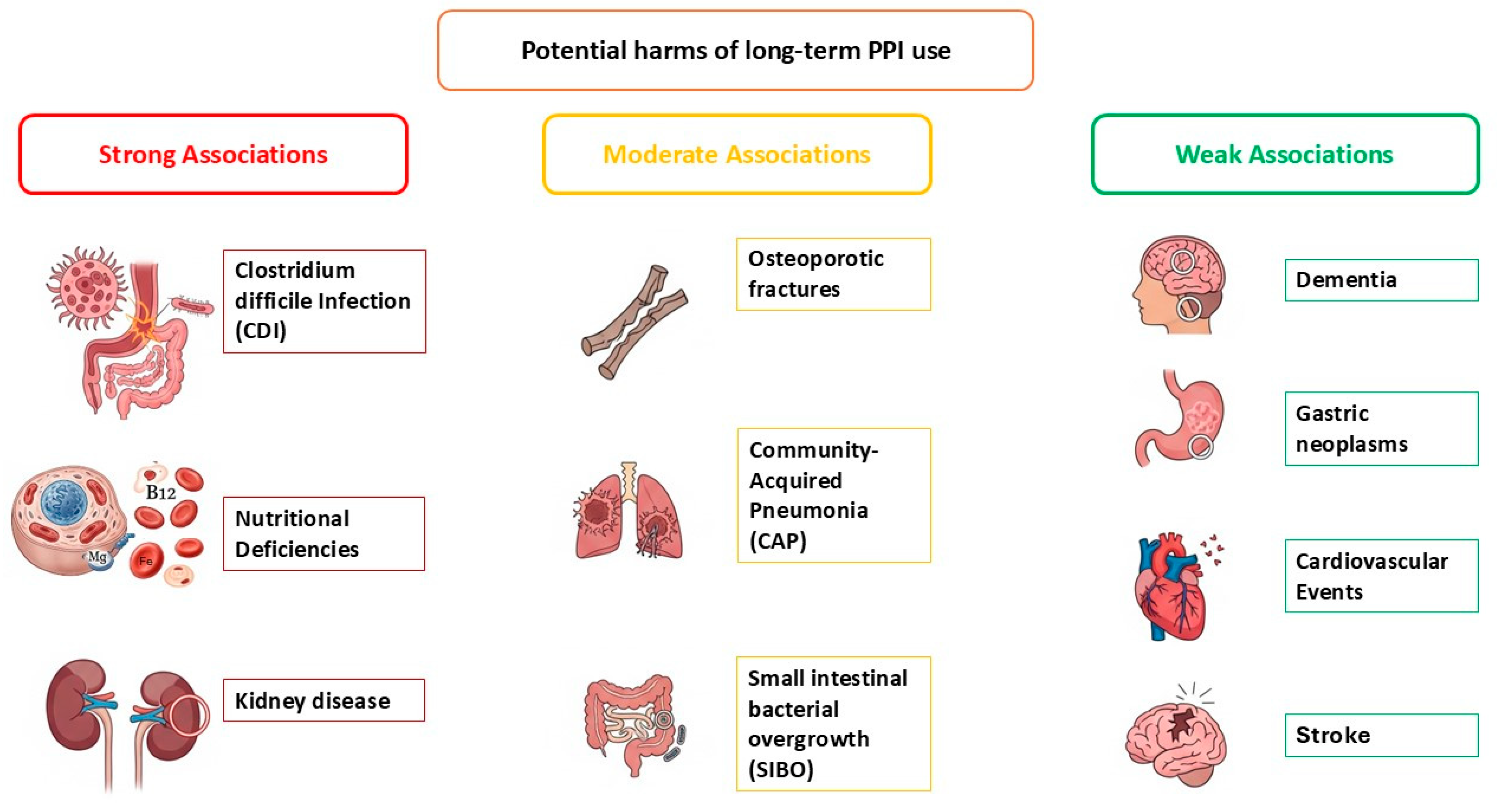
5. Potential Harms of Long-Term PPI Use
5.1. Group 1: Harms with Strong and Consistent Association (Well-Established Risks)
5.1.1. Clostridioide Difficile Infection (CDI) (Short-Term Risk)
5.1.2. Nutritional Deficiencies (Long-Term Risk)
5.1.3. Kidney Disease (Long-Term Risk)
5.2. Group 2: Moderate Association
5.2.1. Osteoporosis and Associated Fractures (Long-Term Risk)
5.2.2. Community-Acquired Pneumonia (CAP) (Short-Term Risk)
5.2.3. Small Intestinal Bacterial Overgrowth (SIBO) (Long-Term Risk)
5.3. Group 3: Potential Risks (Weak/Uncertain Association)
5.3.1. Dementia (Long-Term Risk)
5.3.2. Gastric Neoplasms (Long-Term Risk)
5.3.3. Cardiovascular Outcomes (Long-Term Risk)
5.3.4. Stroke (Long-Term Risk)
6. Overprescribing of PPIs
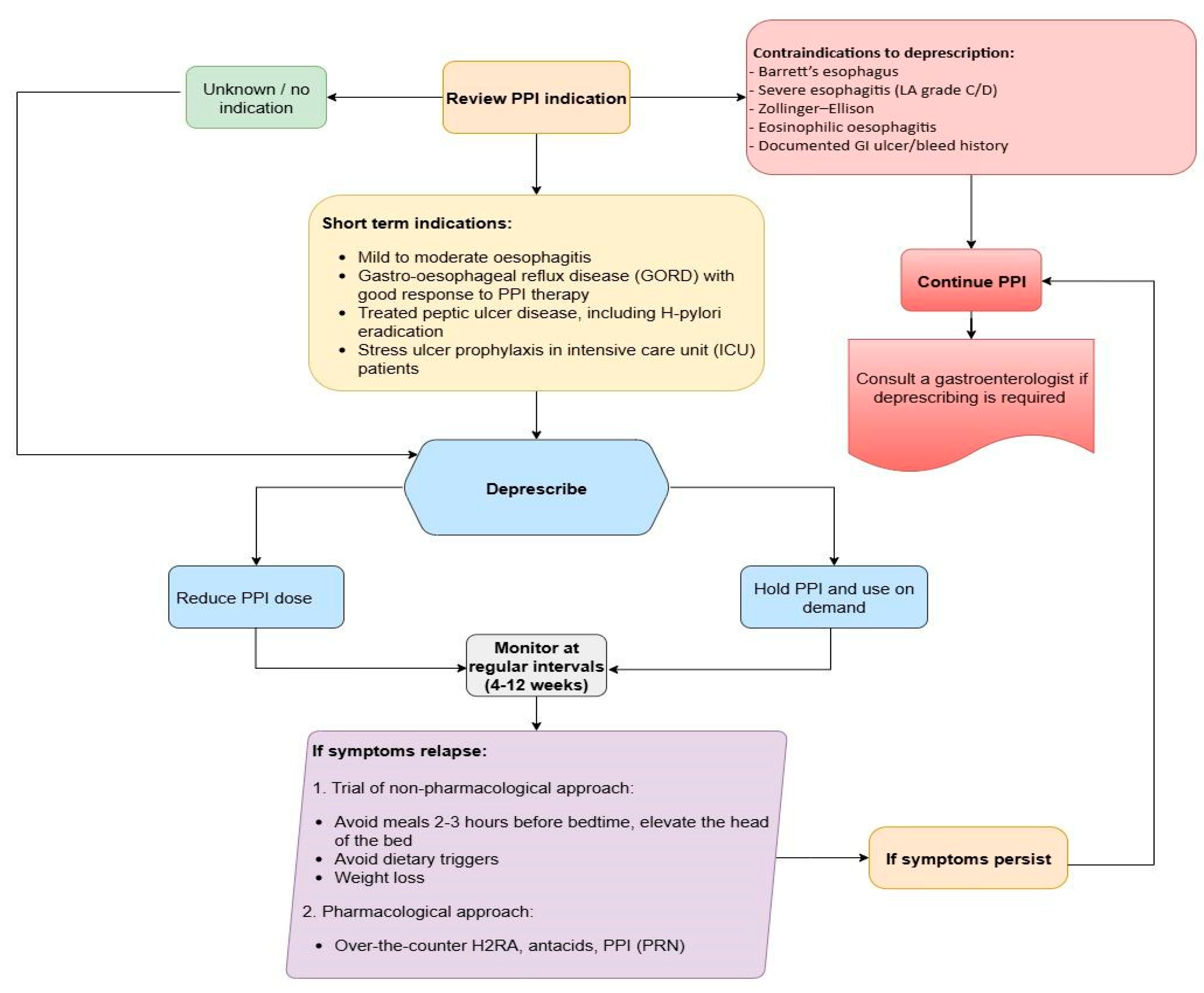
6.1. Evaluation for Discontinuation
Conditions to Avoid Discontinuation
6.2. Different Guideline Recommendations
6.2.1. NICE (UK CG184, 2014/2019)
6.2.2. AGA/ACG (2022)
6.2.3. Canadian (2017)
6.3. Patient Groups and Deprescribing Actions
6.4. Deprescribing Strategies
6.5. Example Scenarios
7. PPIs Versus H2RAs, Gastroprotective Drugs and New Acid Suppressants
8. Clinical Questions, Recommendations and Stepwise Approach to Deprescribing
Stepwise Approach to Deprescribing
9. Limitations
10. Knowledge Gaps
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarpignato, C.; Gatta, L.; Zullo, A.; Blandizzi, C.; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology; The Italian Association of Hospital Gastroenterologists; The Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases—A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016, 14, 179. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.-X. The risks and benefits of long-term use of proton pump inhibitors: Expert review and best practice advice from the American gastroenterological association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef] [PubMed]
- NICE Clinical Guideline CG184. In Gastro-oesophageal Reflux Disease and Dyspepsia in Adults: Investigation and Management; National Institute for Health and Care Excellence (UK): London, UK, 2014.
- DeVault, K.R. Review article: The role of acid suppression in patients with non-erosive reflux disease or functional heartburn. Aliment. Pharmacol. Ther. 2006, 23 (Suppl. S1), 33–39. [Google Scholar] [CrossRef]
- Forgacs, I.; Loganayagam, A. Overprescribing proton pump inhibitors. BMJ 2008, 336, 2–3. [Google Scholar] [CrossRef]
- Abrahami, D.; McDonald, E.G.; Schnitzer, M.; Azoulay, L. Trends in acid suppressant drug prescriptions in primary care in the UK: A population-based cross-sectional study. BMJ Open 2020, 10, e041529. [Google Scholar] [CrossRef]
- Bergin, E.; Zylberberg, H.M.; Lebwohl, B.; Freedberg, D.E. Trends in use of proton pump inhibitors among adults in the United States from 1999 to 2018. Pharmacoepidemiol. Drug Saf. 2023, 32, 1406–1410. [Google Scholar] [CrossRef]
- Torres-Bondia, F.; de Batlle, J.; Galván, L.; Buti, M.; Barbé, F.; Piñol-Ripoll, G. Evolution of the consumption trend of proton pump inhibitors in the Lleida Health Region between 2002 and 2015. BMC Public Health 2022, 22, 818. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; Chen, T.; Zhang, S.; Luo, H. Evolution of proton pump inhibitor prescribing from 2017 to 2021 at 14 secondary and tertiary hospitals in China: A multicentre cross-sectional study. BMJ Open 2023, 13, e072793. [Google Scholar] [CrossRef]
- Yamamichi, N.; Shimamoto, T.; Takahashi, Y.; Takahashi, M.; Takeuchi, C.; Wada, R.; Fujishiro, M.; Zarnegar, R. Trends in proton pump inhibitor use, reflux esophagitis, and various upper gastrointestinal symptoms from 2010 to 2019 in Japan. PLoS ONE 2022, 17, e0270252. [Google Scholar] [CrossRef] [PubMed]
- Plehhova, K.; Wray, J.; Aluko, P.; Sutton, S.; McArdle, J.; Dawson, A.; Coyle, C.; Stevens, R.M. Prescribing practices for proton pump inhibitors among primary care physicians in England: An evaluation. BJGP Open 2025, 9, BJGPO.2024.0059. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.P.; Kim, S.; Park, J.S.; Kim, M.-S.; Choi, N.-K.; Shin, C.M.; Lee, J. Utilization of acid suppressants after withdrawal of ranitidine in Korea: An interrupted time series analysis. J. Prev. Med. Public Health 2025, 58, 21–30. [Google Scholar] [CrossRef]
- Dutta, A.K.; Jain, A.; Jearth, V.; Mahajan, R.; Panigrahi, M.K.; Sharma, V.; Goenka, M.K.; Kochhar, R.; Makharia, G.; Reddy, D.N.; et al. Guidelines on optimizing the use of proton pump inhibitors: PPI stewardship. Indian J. Gastroenterol. 2023, 42, 601–628. [Google Scholar] [CrossRef]
- Dutta, A.K.; Sharma, V.; Jain, A.; Elhence, A.; Panigrahi, M.K.; Mohta, S.; Kirubakaran, R.; Philip, M.; Goenka, M.; Bhatia, S.; et al. Inappropriate use of proton pump inhibitors in clinical practice globally: A systematic review and meta-analysis. Gut 2024, 74, e5. [Google Scholar] [CrossRef]
- Othman, F.; Card, T.R.; Crooks, C.J. Proton pump inhibitor prescribing patterns in the UK: A primary care database study. Pharmacoepidemiol. Drug Saf. 2016, 25, 1079–1087. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Drug Safety and Availability. 20 February 2025. Available online: https://www.fda.gov/drugs/drug-safety-and-availability (accessed on 20 August 2025).
- Homepage. European Medicines Agency (EMA). 30 March 2023. Available online: https://www.ema.europa.eu (accessed on 20 August 2025).
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Omahony, D.; Sullivan, O.; Byrne, D.; Connor, O.; Ryan, M.N.; Gallagher, C. STOPP/START criteria for potentially inap-propriate prescribing in older people: Version 2. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef]
- Choosing Wisely: An initiative of the ABIM Foundation. Available online: https://www.choosingwisely.org (accessed on 20 August 2025).
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, S.J. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am. J. Gastroenterol. 2022, 117, 27–56. [Google Scholar] [CrossRef]
- Targownik, L.E.; Fisher, D.A.; Saini, S.D. AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review Targownik. Gastroenterology 2022, 162, 1334–1342. [Google Scholar] [CrossRef]
- Peyton-Navarrete, A.; Nguyen, M.H.C.; FakhriRavari, A. Prescribing responsibly: Navigating the Tides of deprescribing in proton pump inhibitor stewardship. Pharmacoepidemiology 2025, 4, 15. [Google Scholar] [CrossRef]
- Watson, G.; O’hAra, J.; Carding, P.; Lecouturier, J.; Stocken, D.; Fouweather, T.; Wilson, J. TOPPITS: Trial Of Proton Pump Inhibitors in Throat Symptoms. Study protocol for a randomised controlled trial. Trials 2016, 17, 175. [Google Scholar] [CrossRef]
- Hooper, L.; Brown, T.J.; Elliott, R.; Payne, K.; Roberts, C.; Symmons, D. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: Systematic review. BMJ 2004, 329, 948. [Google Scholar] [CrossRef]
- Masclee, G.M.; Valkhoff, V.E.; Coloma, P.M.; de Ridder, M.; Romio, S.; Schuemie, M.J.; Herings, R.; Gini, R.; Mazzaglia, G.; Picelli, G.; et al. Risk of Upper Gastrointestinal Bleeding From Different Drug Combinations. Gastroenterology 2014, 147, 784–792.e9, quiz e13-4. [Google Scholar] [CrossRef]
- Loke, Y.K.; Trivedi, A.N.; Singh, S. Meta-analysis: Gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment. Pharmacol. Ther. 2008, 27, 31–40. [Google Scholar] [CrossRef]
- Lanza, F.L.; Chan, F.K.; Quigley, E.M. Practice Parameters Committee of the American College of Gastroenterolo-gy.Guidelines for prevention of NSAID-related ulcer complications. Am. J. Gastroenterol. 2009, 104, 728–738. [Google Scholar]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines: An update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 2016, 134, e123–e155. [Google Scholar]
- Abraham, N.S.; Antman, E.M.; Fisher, M. 2020 international consensus on the management of antithrombotic therapy in patients at risk of gastrointestinal bleeding. Ann. Intern. Med. 2020, 172, 447–454. [Google Scholar]
- Caterina, D.; Ammentorp, R.; Darius, B. Position paper of the Italian Society of Cardiology and AIGO on gastro-protection in patients on antiplatelet therapy. G. Ital. Cardiol. 2019, 20, 625–639. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli, D.C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Lombardo, A.; Braschi, A.; Renda, N.; Abrignani, V. Proton pump inhibitors and gastroprotection in patients treated with antithrombotic drugs: A cardiologic point of view. World J. Cardiol. 2023, 15, 375–394. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Dumonceau, J.-M.; Kuipers, E.J.; Lanas, A.; Sanders, D.S.; Kurien, M.; Rotondano, G.; Hucl, T.; Dinis-Ribeiro, M.; Marmo, R.; et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, a1–a46. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—The Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Ray, W.A.; Chung, C.P.; Murray, K.T.; Smalley, W.E.; Daugherty, J.R.; Dupont, W.D.; Stein, C.M. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA 2018, 320, 2221–2230. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Lee, S.-R.; Choi, E.-K.; Rhee, T.-M.; Kwon, S.; Oh, S.; Lip, G.Y.H. Protective effect of proton-pump inhibitor against gastrointestinal bleeding in patients receiving oral anticoagulants: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2022, 88, 4676–4687. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 Guidelines for Management of Venous Thromboembolism: Treatment of Deep Vein Thrombosis and Pulmonary Embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef]
- Chan, E.W.; Lau, W.C.; Leung, W.K.; Mok, M.T.; He, Y.; Tong, T.S.; Wong, I.C. Prevention of Dabigatran-Related Gastrointestinal Bleeding with Gastroprotective Agents: A Population-Based Study. Gastroenterology 2015, 149, 586–595.e3. [Google Scholar] [CrossRef]
- Lanas, A.; Ferrandez, A. Inappropriate prevention of NSAID-induced gastrointestinal events among long-term users in the UK primary care setting. Gastroenterol. Res. Pract. 2013. [Google Scholar]
- Schoenfeld, P.; Kimmey, M.B. Review article: The geographic differences in NSAID-induced ulcer rates--does it matter. Aliment. Pharmacol. Ther. 1999, 13, 3–7. [Google Scholar]
- Barkun, A.N.; Bardou, M.; Kuipers, E.J.; Sung, J.; Hunt, R.H.; Martel, M.; Sinclair, P. for the International Consensus Upper Gastrointestinal Bleeding Conference Group* International Consensus Recommendations on the Management of Patients With Nonvariceal Upper Gastrointestinal Bleeding. Ann. Intern. Med. 2010, 152, 101–113. [Google Scholar] [CrossRef]
- Barkun, A.N.; Almadi, M.; Kuipers, E.J.; Laine, L.; Sung, J.; Tse, F.; Leontiadis, G.I.; Abraham, N.S.; Calvet, X.; Chan, F.K.; et al. Management of Nonvariceal Upper Gastrointestinal Bleeding: Guideline Recommendations from the International Consensus Group. Ann. Intern. Med. 2019, 171, 805–822. [Google Scholar] [CrossRef]
- NICE Guideline NG226. In Osteoarthritis in Over 16s: Diagnosis and Management; NICE: London, UK, 2022.
- NICE. Rheumatoid Arthritis in Adults: Management. Available online: https://www.nice.org.uk/guidance/ng100 (accessed on 20 August 2025).
- Yousefi, C. EULAR recommendations for the management of rheumatoid arthritis with synthetic and bio-logical disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar]
- Narum, S.; Westergren, T.; Klemp, M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open 2014, 4, e004587. [Google Scholar] [CrossRef]
- Wang, W.-H.; Huang, J.-Q.; Zheng, G.-F.; Xia, H.H.X.; Wong, W.-M.; Lam, S.-K.; Wong, B.C.Y. Head-to-head comparison of H2-receptor antagonists and proton pump inhibitors in the treatment of erosive esophagitis: A meta-analysis. World J. Gastroenterol. 2005, 11, 4067–4077. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, R.; Fang, Y.; Zhao, R.; Fu, Y.; Ren, P.; Zhan, Q.; Shao, M. P-CAB versus PPI in the eradication of Helicobacter pylori: A systematic review and network meta-analysis. Therap. Adv. Gastroenterol. 2024, 17, 17562848241241224. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Fan, L.; Yang, J.; Wang, J.; Sun, J.; Wang, Z. Proton pump inhibitors therapy and risk of bone diseases: An update meta-analysis. Life Sci. 2019, 218, 213–223. [Google Scholar] [CrossRef]
- Wu, C.-C.; Liao, M.-H.; Kung, W.-M.; Wang, Y.-C. Proton pump inhibitors and risk of chronic kidney disease: Evidence from observational studies. J. Clin. Med. 2023, 12, 2262. [Google Scholar] [CrossRef]
- Lazarus, B.; Chen, Y.; Wilson, F.P.; Sang, Y.; Chang, A.R.; Coresh, J.; Grams, M.E. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern. Med. 2016, 176, 238. [Google Scholar] [CrossRef]
- Finke, M.; Boven, A.; Vlieghe, E.; Engstrand, L.; Orsini, N.; Brusselaers, N. Proton pump inhibitors and the risk of Clostridioides difficile infection: A systematic review and dose-response meta-analysis. J. Infect. 2025, 90, 106488. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Ungprasert, P.; Erickson, S.B.; Kittanamongkolchai, W.; Srivali, N.; Edmonds, P.J.; O’Corragain, O.A.; Korpaisarn, S. Proton pump inhibitors linked to hypomagnesemia: A systematic review and meta-analysis of observational studies. Ren. Fail. 2015, 37, 1237–1241. [Google Scholar] [CrossRef]
- Lambert, A.A.; Lam, J.O.; Paik, J.J.; Ugarte-Gil, C.; Drummond, M.B.; Crowell, T.A. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0128004. [Google Scholar] [CrossRef]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013, 310, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Sarzynski, E.; Puttarajappa, C.; Xie, Y.; Grover, M.; Laird-Fick, H. Association between proton pump inhibitor use and anemia: A retrospective cohort study. Dig. Dis. Sci. 2011, 56, 2349–2353. [Google Scholar] [CrossRef]
- Howell, M.D.; Novack, V.; Grgurich, P.; Soulliard, D.; Novack, L.; Pencina, M.; Talmor, D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch. Intern. Med. 2010, 170, 784–790. [Google Scholar] [CrossRef]
- Nalabothula, S.; Chava, S.; Doddapaneni, N.S.P.; Gottimukkala, H.S.K.; Durga, D. Proton pump inhibitor use and risk of Clostridioides difficile infection: An umbrella review of 11 meta-analyses. Cureus 2025, 17, e87383. [Google Scholar] [CrossRef] [PubMed]
- Ozdil, K.; Kahraman, R.; Sahin, A.; Calhan, T.; Gozden, E.H.; Akyuz, U.; Erer, B.; Sokmen, M.H. Bone density in proton pump inhibitors users: A prospective study. Rheumatol. Int. 2013, 33, 2255–2260. [Google Scholar] [CrossRef]
- Gray, S.L.; LaCroix, A.Z.; Larson, J.; Robbins, J.; Cauley, J.A.; Manson, J.E.; Chen, Z. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: Results from the Women’s Health Initiative. Arch. Intern. Med. 2010, 170, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.F.; Yang, Y.-X.; Howden, C.W. Complications of proton pump inhibitor therapy. Gastroenterology 2017, 153, 35–48. [Google Scholar] [CrossRef]
- Targownik, L.E.; Leslie, W.D.; Davison, K.S.; Goltzman, D.; Jamal, S.A.; Kreiger, N.; Josse, R.G.; Kaiser, S.M.; Kovacs, C.S.; Prior, J.C.; et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: A population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos). Am. J. Gastroenterol. 2012, 107, 1361–1369. [Google Scholar] [CrossRef]
- Targownik, L.E.; Goertzen, A.L.; Luo, Y.; Leslie, W.D. Long-term proton pump inhibitor use is not associated with changes in bone strength and structure. Am. J. Gastroenterol. 2017, 112, 95–101. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Mishiro, T.; Oka, K.; Kuroki, Y.; Takahashi, M.; Tatsumi, K.; Saitoh, T.; Tobita, H.; Ishimura, N.; Sato, S.; Ishihara, S.; et al. Oral microbiome alterations of healthy volunteers with proton pump inhibitor. J. Gastroenterol. Hepatol. 2018, 33, 1059–1066. [Google Scholar] [CrossRef]
- Su, T.; Lai, S.; Lee, A.; He, X.; Chen, S. Meta-analysis: Proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J. Gastroenterol. 2018, 53, 27–36. [Google Scholar] [CrossRef]
- Lo, W.-K.; Chan, W.W. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: A meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 483–490. [Google Scholar] [CrossRef]
- Mehta, R.S.; Kochar, B.; Zhou, Z.; Broder, J.C.; Chung, P.; Yang, K.; Lockery, J.; Fravel, M.; Ryan, J.; Mahady, S.; et al. Association of proton pump inhibitor use with incident dementia and cognitive decline in older adults: A prospective cohort study. Gastroenterology 2023, 165, 564–572.e1. [Google Scholar] [CrossRef]
- Xie, K.; Li, J.; Tang, C.; Huang, Z.; Chen, M. Association between proton pump inhibitors and dementia risk: A Mendelian randomization study. Sci. Rep. 2024, 14, 28624. [Google Scholar] [CrossRef]
- Gomm, W.; von Holt, K.; Thomé, F.; Broich, K.; Maier, W.; Fink, A.; Doblhammer, G.; Haenisch, B. Association of proton pump inhibitors with risk of dementia. JAMA Neurol. 2016, 73, 410. [Google Scholar] [CrossRef]
- Li, M.; Luo, Z.; Yu, S.; Tang, Z. Proton pump inhibitor use and risk of dementia: Systematic review and meta-analysis. Medicine 2019, 98, e14422. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2018, 67, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Cryer, B.L.; Contant, C.F.; Cohen, M.; Lanas, A.; Schnitzer, T.J.; Shook, T.L.; Lapuerta, P.; Goldsmith, M.A.; Laine, L.; et al. Clopidogrel with or without omeprazole in coronary artery disease. N. Engl. J. Med. 2010, 363, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Sehested, T.S.G.; Gerds, T.A.; Fosbøl, E.L.; Hansen, P.W.; Charlot, M.G.; Carlson, N.; Hlatky, M.A.; Torp-Pedersen, C.; Gislason, G.H. Long-term use of proton pump inhibitors, dose–response relationship and associated risk of ischemic stroke and myocardial infarction. J. Intern. Med. 2018, 283, 268–281. [Google Scholar] [CrossRef]
- Shabil, M.; Padhi, B.K.; Khatib, M.N.; Menon, S.V.; Kaur, M.; Kumari, M.; Sudan, P.; Naidu, K.S.; Zahiruddin, Q.S.; Rustagi, S.; et al. Risk of stroke associated with proton pump inhibitor use among individuals with and without pre-existing cardiovascular diseases: A systematic review and meta-analysis. J. Cardiothorac. Surg. 2025, 20, 107. [Google Scholar] [CrossRef]
- Rossi, A.; Perrella, L.; Scotti, S.; Olmastroni, E.; Galimberti, F.; Ardoino, I.; Orlando, V.; Menditto, E.; Franchi, C.; Casula, M. Approaches to deprescribing proton pump inhibitors in clinical practice: A systematic review. J. Clin. Med. 2024, 13, 6283. [Google Scholar] [CrossRef]
- Onge, E.S.; Phillips, B. Vonoprazan: A new potassium-competitive acid blocker. J. Pharm. Technol. 2023, 39, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Miwa, H. Potent potassium-competitive acid blockers: A New Era for the treatment of acid-related diseases. J. Neurogastroenterol. Motil. 2018, 24, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.; Pottie, K.; Thompson, W.; Boghossian, T.; Pizzola, L.; Rashid, F.J.; Rojas-Fernandez, C.; Walsh, K.; Welch, V.; Moayyedi, P. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can. Fam. Physician 2017, 63, 354–364. [Google Scholar] [PubMed]
- Inatomi, N.; Matsukawa, J.; Sakurai, Y.; Otake, K. Potassium-competitive acid blockers: Advanced therapeutic option for acid-related diseases. Pharmacol. Ther. 2016, 168, 12–22. [Google Scholar] [CrossRef]
- Scarpignato, C.; Howden, C.W.; Leifke, E.; Mulford, D.J.; Lahu, G.; Facius, A.; Hunt, R. A translational pharmacokinetic/pharmacodynamic approach supports optimal vonoprazan dosing for erosive oesophagitis and Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2023, 58, 16–25. [Google Scholar] [CrossRef]
- Frech, E.J.; Go, M.F. Treatment and chemoprevention of NSAID-associated gastrointestinal complications. Ther. Clin. Risk Manag. 2009, 5, 65–73. [Google Scholar]
- Kudaravalli, P.; Patel, P.; John, S. Sucralfate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cao, Y.; Zhang, J.; Liu, Y.; Zhang, L.; Wang, L.; Wang, J.; Qi, Y.; Lv, H.; Liu, J.; Huo, L.; et al. The efficacy and safety of different bismuth agents in Helicobacter pylori first-line eradication: A multicenter, randomized, controlled clinical trial. Medicine 2021, 100, e27923. [Google Scholar] [CrossRef]
- Gotoh, Y.; Ishibashi, E.; Honda, S.; Nakaya, T.; Noguchi, C.; Kagawa, K.; Murakami, K. Efficacy of vonoprazan for initial and maintenance therapy in reflux esophagitis, nonerosive esophagitis, and proton pump inhibitor-resistant gastroesophageal reflux disease. Medicine 2020, 99, e19520. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, H.; Zhang, Z.; Zhou, Z.; Zhang, Q. Comparative efficiency and safety of potassium competitive acid blockers versus Lansoprazole in peptic ulcer: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1304552. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Watanabe, T.; Tanigawa, T.; Tominaga, K.; Fujiwara, Y.; Morimoto, K. Quality of ulcer healing in gastrointestinal tract: Its pathophysiology and clinical relevance. World J. Gastroenterol. 2012, 18, 4811–4822. [Google Scholar] [CrossRef]
- Wedemeyer, R.-S.; Blume, H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: An update. Drug Saf. 2014, 37, 201–211. [Google Scholar] [CrossRef]
- Reimer, C.; Søndergaard, B.; Hilsted, L.; Bytzer, P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology 2009, 137, 80–87.e1. [Google Scholar] [CrossRef]
- Alonso, N.; Zappia, C.D.; Cabrera, M.; Davio, C.A.; Shayo, C.; Monczor, F.; Fernã¡Ndez, N.C. Physiological implications of biased signaling at histamine H2 receptors. Front. pharmacology 2015, 6, 45. [Google Scholar] [CrossRef]
- Fuchs, K.H.; Babic, B.; Breithaupt, W.; Dallemagne, B.; Fingerhut, A.; Furnee, E.; Granderath, F.; Horvath, P.; Kardos, P.; Pointner, R.; et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg. Endosc. 2014, 28, 1753–1773. [Google Scholar] [CrossRef]
- Lanas, A.; Chan, F.K. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Taha, A.S.; Mccloskey, C.; Prasad, R.; Bezlyak, V. Famotidine for the prevention of peptic ulcers and oe-sophagitis in patients taking low-dose aspirin (FAMOUS): A phase III, randomised, double-blind, placebo-controlled trial. Lancet 2009, 374, 119–125. [Google Scholar] [CrossRef]
- Scarpignato, C.; Lanas, A.; Blandizzi, C.; Lems, W.F.; Hermann, M.; Hunt, R.H. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis--an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. Correction: ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2018, 113, 1102. [Google Scholar] [CrossRef] [PubMed]
- Odenthal, D.R.; Philbrick, A.M.; Harris, I.M. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J. Am. Pharm. Assoc. (2003) 2020, 60, 100–104. [Google Scholar] [CrossRef] [PubMed]
| Type of Indication | Long-Term PPI (>8 Weeks) | Short-Term PPI (<8 Weeks) | ||
|---|---|---|---|---|
| Definite | Conditional | Definite | Conditional | |
| Therapeutic | GORD with erosive oesophagitis (LA C+D) Peptic stricture Eosinophilic oesophagitis with histological response Barrett’s oesophagus Zollinger-Ellison syndrome | GORD with incomplete response to short-term PPI GORD with recurrence of symptoms on PPI cessation Eosinophilic oesophagitis (maintenance) Idiopathic chronic cough (GORD-confirmed) | GORD H. pylori eradication (combination therapy) Non-erosive GORD (symptom relief) Peptic ulcer disease Mild peptic inflammation | Functional dyspepsia (trial) Laryngopharyngeal reflux (LPR) (trial and review) Mild gastritis |
| Prophylactic | - Chronic NSAID/aspirin use + high GI risk - Antiplatelet therapy post-bleeding ulcer - Systemic sclerosis with reflux | - Long-term corticosteroids + GI risk factors | - NSAID/aspirin use (short course + risk factors) - Post-endoscopic ulcer therapy - Stress ulcer prophylaxis (ICU only) | - Post-bariatric surgery (short-term) - Post-sclerotherapy and band ligation To cover short-term NSAID/high dose steroid prescription |
| Conditions | Short-Term Use | Long-Term Use |
|---|---|---|
| Therapeutic | Isolated throat symptoms [24] Acute undifferentiated upper GI symptoms (e.g., pain, nausea, vomiting) not seem to be due to GORD or PUD [22] Isolated lower GI symptoms | Non-erosive reflux or functional dyspepsia with no response to high-dose PPI therapy Peptic ulcer disease including gastric or duodenal erosions |
| Prophylactic | Steroid therapy in the absence of concomitant NSAID/antiplatelet therapy [22] Prevention of recurrent GI bleed for reasons other than peptic ulcer disease [22] |
| Organization | Guideline Summary | Notes |
|---|---|---|
| ACCF/ACG/AHA (2010, updated 2016) [29] | PPIs are recommended for high-risk patients only on DAPT only. Routine use is not recommended. | Based on interpretation of COGENT trial results. Focussed approach; avoid blanket PPI use |
| ESC (2018) [32] | Routine PPI use for all patients on DAPT. | Emphasises reducing bleeding risks associated with DAPT. |
| International Consensus (2020) [30] | Conditional recommendation for PPI in patients with previous ulcer bleeding on DAPT. | Based on low-quality evidence, no increased mortality from DAPT and PPI. |
| ANMCO/AIGO (Italian Guidelines) [31] | PPI recommended for patients with GI risk factors, history of PUD, use of NSAIDs or steroids. | Focuses on additional risk factors including age, GORD, and dyspeptic symptoms. |
| PPI | % Time pH > 4 (24 h) | CYP2C19 Metabolism | Bioavailability (%) | Duration of Acid Suppression | References |
|---|---|---|---|---|---|
| Dexlansoprazole | ~70–80% | Low (dual delayed-release bypasses metabolism impact) | ~60–70% | ~24 h (dual release) | [1,2] |
| Rabeprazole | ~60–70% | Low (non-enzymatic metabolism largely) | ~52% | ~16 h | [25,28] |
| Esomeprazole | ~60–70% | Moderate (less than omeprazole; S-isomer) | ~64% | ~16–20 h | [25,29] |
| Lansoprazole | ~50–60% | High (significantly affected by CYP2C19 polymorphism) | ~80–90% | ~12–14 h | [25,29] |
| Pantoprazole | ~45–55% | Moderate (less interaction than omeprazole) | ~77% | ~10–12 h | [28,29] |
| Omeprazole | ~40–50% | High (extensively metabolized by CYP2C19) | ~30–40% | ~10–12 h | [25,28] |
| Study (year) | Condition | Comparison | Key Finding | Reference |
|---|---|---|---|---|
| Wang et al., 2005 (meta-analysis) | Erosive oesophagitis | Standard-dose PPI vs. H2RA | PPIs healed significantly more patients at 2–8 weeks; PPI 2–8 wk healing > H2HRA at 8 wk (63.4% vs. 52.0%) | [49] |
| Jiang et al., 2024 (NMA) | H. pylori eradication | Vonoprazan-based vs. PPI-based | Vonoprazan triple (2 wk) had highest eradication rate; superior to PPI quadruple (RR ≈ 0.90 favoring vonoprazan) | [50] |
| PPI | Standard Dose | Low Dose (On-Demand) | High (Double) Dose |
|---|---|---|---|
| Omeprazole | 20 mg once daily (40 mg once daily if severe oesophagitis) | * 10 mg once daily (20 mg once daily if severe oesophagitis) | 40 mg once daily (40 mg twice daily if severe oesophagitis) |
| Lansoprazole | 30 mg once daily | 15 mg once daily | * 30 mg twice daily |
| Pantoprazole | 40 mg once daily | 20 mg once daily | * 40 mg twice daily |
| Rabeprazole | 20 mg once daily | 10 mg once daily | * 20 mg twice daily |
| Esomeprazole Rabeprazole | † 20 mg once daily (40 mg once daily if severe oesophagitis) | Not available (2010 mg once daily if severe oesophagitis) | ‡ 40 mg once daily (40 mg twice daily if severe oesophagitis) |
| Harm Category | Effect Size (95% CI) | Suggested Monitoring |
|---|---|---|
| Bone fracture (major) | RR ~1.28 (1.22–1.35) [51] RR ~1.28 (1.22–1.35) [52] | Ensure adequate calcium and vitamin D intake; consider bone density assessment in high-risk patients |
| Chronic kidney disease | HR ~1.26 (1.16–1.38) [52,53] HR ~1.26 (1.16–1.38) [54,55] | Monitor renal function (serum creatinine/eGFR) periodically |
| Community-acquired pneumonia | OR ~1.37 (1.22–1.53) [56] OR ~1.37 (1.22–1.53) [57] | Monitor for respiratory infections, especially early in treatment |
| Clostridioides difficile infection | OR ~1.26 (1.12–1.39) [54] OR ~1.26 (1.12–1.39) [58] | Review any new diarrhoeal illness promptly; avoid unnecessary long-term use |
| Hypomagnesaemia | OR ~1.78 (1.08–2.92) [55] OR ~1.78 (1.08–2.92) [59] | Check serum magnesium in long-term users, especially if on diuretics |
| Vitamin B12 deficiency | [57] | Check levels after >2 years’ continuous use or if symptomatic |
| Iron deficiency/anaemia | [58] | Assess iron status if unexplained anaemia develops during therapy |
| Characteristic | PPIs | H2RAs | Cytoprotective Agents | PCABs (e.g., Vonoprazan) |
|---|---|---|---|---|
| Mechanism | Irreversibly inhibit H+/K+ ATPase | Block histamine H2 receptors | Form barriers (sucralfate); prostaglandin analogue (misoprostol); antimicrobial (bismuth) [84,85,86] | Reversibly inhibit H+/K+ ATPase at potassium site [79] |
| Onset of Action | Slower onset | Faster onset [4] | Variable, often local [88] | Rapid [79] |
| Duration of Action | Long-lasting [4] | Shorter duration [4] | Requires frequent dosing [84,85] | Sustained, even during night-time [79,87] |
| Mucosal Protection | Indirect via acid reduction [89] | Indirect [89] | Direct mucosal protection [89] | Both acid suppression and mucosal healing |
| Adverse Effects | Long-term risks (e.g., C. difficile, fractures) [63] [64] | Headache, dizziness [2] | Diarrhoea (misoprostol), contraindicated in pregnancy [84] | Similar to PPIs, but with some rare serious AEs [88] |
| Drug Interactions | CYP2C19 interactions (esp. omeprazole) [90] | Fewer interactions [2] | Minimal | Lesser CYP2C19 influence [79,82] |
| Tolerance/Rebound | Rebound acidity after withdrawal [91] | Tolerance with prolonged use [92] | Not common | Minimal data; likely less tolerance development |
| Dosing | Once or twice daily | Once or twice daily | Multiple daily doses [84,85] | Often once daily [83] |
| Indication | Preferred Therapy | Key Points |
|---|---|---|
| GORD | PPIs first line | As per ACG/ESGE, H2RAs used for intermittent symptoms [21,93]. |
| Peptic Ulcer Disease | PPIs preferred; H2RAs still effective | Famotidine prevents ulcers in aspirin users [94,95] |
| NSAID-Induced Ulcers | PPIs and misoprostol | Recommended in high-risk groups [28,96] |
| H. pylori Eradication | PPI-based regimens | Key in triple and bismuth quadruple therapy [35,93,97]. |
| Condition | Comparative Efficacy |
|---|---|
| GORD | Non-inferior/superior to PPIs; effective in erosive, non-erosive, and PPI-resistant disease [87]. |
| H. pylori | Higher eradication in clarithromycin-resistant strains (65.8–69.6% vs. 31.9%) [50] |
| Ulcer Healing | Comparable to PPIs; faster symptom relief reported; rare serious adverse events possible [88] |
| Aspect | UK Guidelines Summary | Notes |
|---|---|---|
| PPI Indications | PPIs are first-line treatment for GORD, peptic ulcer disease, and H. pylori eradication. | NICE and BSG recommend PPIs for GORD and ulcer healing. |
| Dosage and Duration | Standard dosing for GORD and ulcers. Long-term PPI use is common, especially in elderly patients. | Dosing varies based on severity; an annual review is recommended by the NHS to assess necessity. |
| Co-prescription with NSAIDs | NICE and BSG recommend co-prescription of PPIs with NSAIDs in high-risk patients (e.g., age > 65, history of ulcers, or anticoagulant use). | Reduces risk of GI bleeding, especially in patients on long-term NSAID therapy. |
| Co-prescription with Antiplatelet Therapy | PPIs should be prescribed for patients on dual antiplatelet therapy (DAPT) or single therapy with additional GI risk factors (e.g., history of ulcers, H. pylori). | Prevents gastrointestinal bleeding in high-risk groups. |
| Co-prescription with Anticoagulants | PPIs should be considered for patients on warfarin or direct oral anticoagulants (DOACs) at high risk of GI bleeding. Pantoprazole is preferred due to fewer drug interactions. | Particularly important for patients with prior GI bleeding or ulcer history. |
| Co-prescription with Corticosteroids | NICE recommends considering PPIs for high-risk patients on corticosteroids, particularly when combined with NSAIDs or other GI risk factors. | Focuses on reducing risk of peptic ulcers from corticosteroid use. |
| Deprescribing PPIs | NICE and NHS recommend annual reassessment of PPI use to reduce overprescription. PPIs should not be stopped in high-risk patients (GORD, Barrett’s oesophagusesophagus). | Gradual tapering or continued use depending on clinical need. |
| Adverse Effects | Long-term PPI use is associated with risks such as gastrointestinal infections (C. difficile), nutrient deficiencies (B12, magnesium), and bone fractures. | NICE and NHS advise caution with long-term use; alternatives considered when appropriate. |
| Alternative Therapies | Sucralfate and misoprostol are alternatives for ulcer treatment, especially when PPIs are not suitable. | Cytoprotective agents and H2RAs as alternatives. |
| New Acid Suppressants | Potassium-competitive acid blockers (PCABs) like vonoprazan may offer advantages over traditional PPIs, particularly in PPI-resistant GORD and H. pylori eradication. | Emerging therapies are considered, but PPIs remain standard. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrawes, M.; Andrawes, W.; Das, A.; Siau, K. Proton Pump Inhibitors (PPIs)—An Evidence-Based Review of Indications, Efficacy, Harms, and Deprescribing. Medicina 2025, 61, 1569. https://doi.org/10.3390/medicina61091569
Andrawes M, Andrawes W, Das A, Siau K. Proton Pump Inhibitors (PPIs)—An Evidence-Based Review of Indications, Efficacy, Harms, and Deprescribing. Medicina. 2025; 61(9):1569. https://doi.org/10.3390/medicina61091569
Chicago/Turabian StyleAndrawes, Monica, Wessam Andrawes, Abhishek Das, and Keith Siau. 2025. "Proton Pump Inhibitors (PPIs)—An Evidence-Based Review of Indications, Efficacy, Harms, and Deprescribing" Medicina 61, no. 9: 1569. https://doi.org/10.3390/medicina61091569
APA StyleAndrawes, M., Andrawes, W., Das, A., & Siau, K. (2025). Proton Pump Inhibitors (PPIs)—An Evidence-Based Review of Indications, Efficacy, Harms, and Deprescribing. Medicina, 61(9), 1569. https://doi.org/10.3390/medicina61091569






