Comparison of Functional Movement, Balance, Vertical Jumping, Hip Strength and Injury Risk in Adolescent Female Volleyball Players with and Without Chronic Ankle Instability
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Procedure
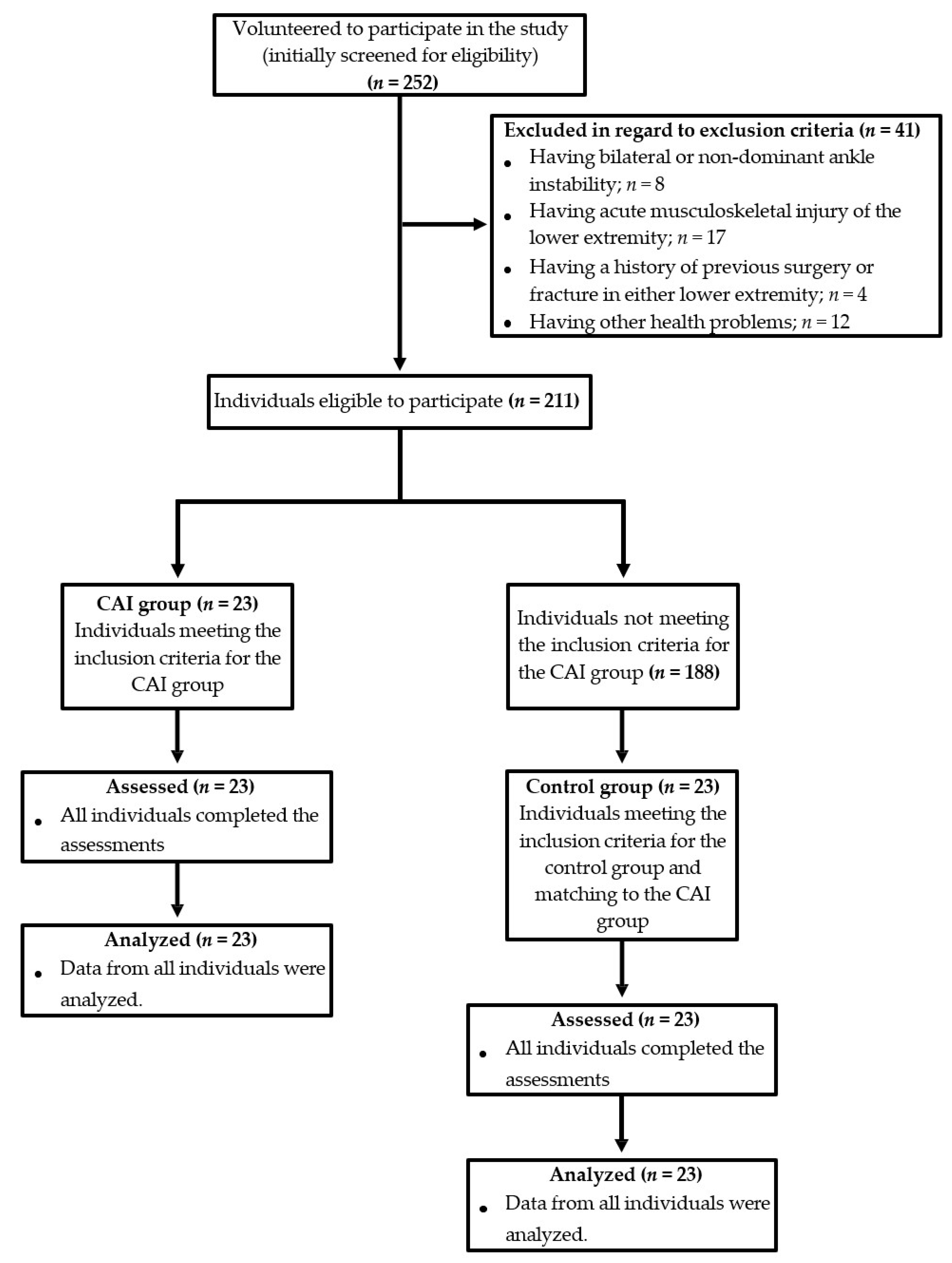
2.2. Individuals
2.3. Assessment Procedures and Measurement Tools
2.3.1. Functional Movement Screen
2.3.2. Y-Balance Test
2.3.3. Vertical Jump Tests
2.3.4. Hip-Muscle Strength Measurements
2.4. Statistical Analysis
The Post Hoc Power Analysis
3. Results
3.1. Characteristics of the Individuals
3.2. Quality of Functional Movements and Injury Risk
3.3. Dynamic Balance and Injury Risk
3.4. Vertical Jumping Performance
3.5. Hip Muscle Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAI | Chronic ankle instability |
| FMS | Functional movement screen |
| YBT | Y-Balance Test |
| IdFAI | Identification of functional ankle instability |
| ANT | Y-Balance Test—anterior direction |
| PM | Y-Balance Test—posteromedial direction |
| PL | Y-Balance Test—posterolateral direction |
| SJwH | Squat jump with hands on the hips |
| CMJwH | Countermovement jump with hands on the hips |
| CMJwA | Countermovement jump with arm swing |
| ASIS | Anterior superior iliac spine |
| SPSS | Statistical Package for the Social Sciences |
| CI | Confidence interval |
| SEBT | Star Excursion Balance Test |
| SLHT | Single-Leg Hop Test |
References
- Milić, V.; Radenković, O.; Čaprić, I.; Mekić, R.; Trajković, N.; Špirtović, O.; Koničanin, A.; Bratić, M.; Mujanović, R.; Preljević, A.; et al. Sports Injuries in Basketball, Handball, and Volleyball Players: Systematic Review. Life 2025, 15, 529. [Google Scholar] [CrossRef]
- Attenborough, A.S.; Hiller, C.E.; Smith, R.M.; Stuelcken, M.; Greene, A.; Sinclair, P.J. Chronic ankle instability in sporting populations. Sports Med. 2014, 44, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Young, W.K.; Briner, W.; Dines, D.M. Epidemiology of Common Injuries in the Volleyball Athlete. Curr. Rev. Musculoskelet. Med. 2023, 16, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.; Delahunt, E.; Caulfield, B.; Hertel, J.; Ryan, J.; Bleakley, C. The incidence and prevalence of ankle sprain injury: A systematic review and meta-analysis of prospective epidemiological studies. Sports Med. 2014, 44, 123–140. [Google Scholar] [CrossRef]
- Root, H.J.; Frank, B.S.; Denegar, C.R.; Casa, D.J.; Gregorio, D.I.; Mazerolle, S.M.; DiStefano, L.J. Application of a Preventive Training Program Implementation Framework to Youth Soccer and Basketball Organizations. J. Athl. Train. 2019, 54, 182–191. [Google Scholar] [CrossRef]
- Feger, M.A.P.; Glaviano, N.R.M.; Donovan, L.P.; Hart, J.M.P.; Saliba, S.A.P.; Park, J.S.; Hertel, J.P. Current Trends in the Management of Lateral Ankle Sprain in the United States. Clin. J. Sport Med. 2017, 27, 145–152. [Google Scholar] [CrossRef]
- Donovan, L.; Hetzel, S.; Laufenberg, C.R.; McGuine, T.A. Prevalence and Impact of Chronic Ankle Instability in Adolescent Athletes. Orthop. J. Sports Med. 2020, 8, 2325967119900962. [Google Scholar] [CrossRef]
- Gribble, P.A.; Bleakley, C.M.; Caulfield, B.M.; Docherty, C.L.; Fourchet, F.; Fong, D.T.-P.; Hertel, J.; Hiller, C.E.; Kaminski, T.W.; McKeon, P.O.; et al. Evidence review for the 2016 International Ankle Consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br. J. Sports Med. 2016, 50, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Corbett, R.O. An Updated Model of Chronic Ankle Instability. J. Athl. Train. 2019, 54, 572–588. [Google Scholar] [CrossRef]
- Gribble, P.A.; Delahunt, E.; Bleakley, C.M.; Caulfield, B.; Docherty, C.L.; Fong, D.T.-P.; Fourchet, F.; Hertel, J.; Hiller, C.E.; Kaminski, T.W.; et al. Selection criteria for patients with chronic ankle instability in controlled research: A position statement of the International Ankle Consortium. J. Athl. Train. 2014, 49, 121–127. [Google Scholar] [CrossRef]
- Tanen, L.; Docherty, C.L.; Van Der Pol, B.; Simon, J.; Schrader, J. Prevalence of chronic ankle instability in high school and division I athletes. Foot Ankle Spéc. 2014, 7, 37–44. [Google Scholar] [CrossRef]
- Mandarakas, M.; Pourkazemi, F.; Sman, A.; Burns, J.; Hiller, C.E. Systematic review of chronic ankle instability in children. J. Foot Ankle Res. 2014, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, Z. Cross-Sectional Studies: Strengths, Weaknesses, and Recommendations. Chest 2020, 158, S65–S71. [Google Scholar] [CrossRef]
- Tayfur, A.; Sendil, A.; Karakaya, J.; Ergun, N. Cross-cultural adaptation, validity, and reliability of Turkish version of Identification of Functional Ankle Instability (IdFAI) scale. Acta Orthop. Traumatol. Turc. 2020, 54, 300–304. [Google Scholar] [CrossRef]
- Vauhnik, R.; Morrissey, M.C.; Rutherford, O.M.; Turk, Z.; Pilih, I.A.; Pohar, M. Knee anterior laxity: A risk factor for traumatic knee injury among sportswomen? Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 823–833. [Google Scholar] [CrossRef]
- Simon, J.; Donahue, M.; Docherty, C.L. Critical review of self-reported functional ankle instability measures: A follow up. Phys. Ther. Sport 2014, 15, 97–100. [Google Scholar] [CrossRef]
- Maricot, A.; Corlùy, H.; De Pauw, K.; Lathouwers, E.; Meeusen, R.; Roelands, B.; Verschueren, J.; Tassignon, B. Deficits in neurocognitive performance in patients with chronic ankle instability during a neurocognitive balance task—A retrospective case-control study. Phys. Ther. Sport 2024, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, J.; Cai, B.; Fan, S.; Jiang, X. Quantitative assessments of static and dynamic balance performance in patients with chronic ankle instability. Medicine 2020, 99, e19775. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Fournier, K.A.; McKeon, P.O. Postural control differs between those with and without chronic ankle instability. Gait Posture 2010, 32, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulos, S.; Roes, K.C.; van der Tweel, I. Sequential designs with small samples: Evaluation and recommendations for normal responses. Stat. Methods Med. Res. 2018, 27, 1115–1127. [Google Scholar] [CrossRef]
- Mandarakas, M.; Hiller, C.E.; Rose, K.J.; Burns, J. Measuring Ankle Instability in Pediatric Charcot-Marie-Tooth Disease. J. Child Neurol. 2013, 28, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.L.; Davenport, T.E.; Fraser, J.J.; Sawdon-Bea, J.; Carcia, C.R.; Carroll, L.A.; Kivlan, B.R.; Carreira, D. Ankle Stability and Movement Coordination Impairments: Lateral Ankle Ligament Sprains Revision 2021. J. Orthop. Sports Phys. Ther. 2021, 51, CPG1–CPG80. [Google Scholar] [CrossRef]
- Glatthorn, J.F.; Gouge, S.; Nussbaumer, S.; Stauffacher, S.; Impellizzeri, F.M.; Maffiuletti, N.A. Validity and reliability of Optojump photoelectric cells for estimating vertical jump height. J. Strength Cond. Res. 2011, 25, 556–560. [Google Scholar] [CrossRef]
- Patti, A.; Gervasi, M.; Giustino, V.; Figlioli, F.; Canzone, A.; Drid, P.; Thomas, E.; Messina, G.; Vicari, D.S.S.; Palma, A.; et al. The Influence of Ankle Mobility and Foot Stability on Jumping Ability and Landing Mechanics: A Cross-Sectional Study. J. Funct. Morphol. Kinesiol. 2024, 9, 160. [Google Scholar] [CrossRef]
- Cook, G.; Burton, L.; Hoogenboom, B.J.; Voight, M. Functional movement screening: The use of fundamental movements as an assessment of function-part 1. Int. J. Sports Phys. Ther. 2014, 9, 396. [Google Scholar]
- Cook, G.; Burton, L.; Hoogenboom, B.J.; Voight, M. Functional movement screening: The use of fundamental movements as an assessment of function-part 2. Int. J. Sports Phys. Ther. 2014, 9, 549. [Google Scholar] [PubMed]
- O’bRien, W.; Khodaverdi, Z.; Bolger, L.; Tarantino, G.; Philpott, C.; Neville, R.D. The assessment of functional movement in children and adolescents: A systematic review and meta-analysis. Sports Med. 2022, 52, 37–53. [Google Scholar] [CrossRef]
- Zarei, M.; Soltanirad, S.; Kazemi, A.; Hoogenboom, B.J.; Hosseinzadeh, M. Composite functional movement screen score predicts injuries in youth volleyball players: A prospective cohort study. Sci. Rep. 2022, 12, 20207. [Google Scholar] [CrossRef] [PubMed]
- Vehrs, P.R.; Uvacsek, M.; Johnson, A.W. Assessment of dysfunctional movements and asymmetries in children and adolescents using the Functional Movement Screen—A narrative review. Int. J. Environ. Res. Public Health 2021, 18, 12501. [Google Scholar] [CrossRef]
- Plisky, P.J.; Gorman, P.P.; Butler, R.J.; Kiesel, K.B.; Underwood, F.B.; Elkins, B. The reliability of an instrumented device for measuring components of the star excursion balance test. N. Am. J. Sports Phys. Ther. 2009, 4, 92–99. [Google Scholar]
- Bulow, A.; Anderson, J.E.; Leiter, J.R.; MacDonald, P.B.; Peeler, J. The Modified Star Excursion Balance and Y-Balance Test Results Differ When Assessing Physically Active Healthy Adolescent Females. Int. J. Sports Phys. Ther. 2019, 14, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Plisky, P.; Schwartkopf-Phifer, K.; Huebner, B.; Garner, M.B.; Bullock, G. Systematic Review and Meta-Analysis of the Y-Balance Test Lower Quarter: Reliability, Discriminant Validity, and Predictive Validity. Int. J. Sports Phys. Ther. 2021, 16, 1190–1209. [Google Scholar] [CrossRef] [PubMed]
- Brumitt, J.; Patterson, C.; Dudley, R.; Sorenson, E.; Hill, G.; Peterson, C. Comparison of lower quarter Y-Balance Test scores for female collegiate volleyball players based on competition level, position, and starter status. Int. J. Sports Phys. Ther. 2019, 14, 415–423. [Google Scholar] [CrossRef]
- Smith, C.A.; Chimera, N.J.; Warren, M. Association of y balance test reach asymmetry and injury in division I athletes. Med. Sci. Sports Exerc. 2015, 47, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Slinde, F.; Suber, C.; Suber, L.; Edwén, C.E.; Svantesson, U. Test-retest reliability of three different countermovement jumping tests. J. Strength Cond. Res. 2008, 22, 640–644. [Google Scholar] [CrossRef]
- Sattler, T.; Sekulic, D.; Hadzic, V.; Uljevic, O.; Dervisevic, E. Vertical jumping tests in volleyball: Reliability, validity, and playing-position specifics. J. Strength Cond. Res. 2012, 26, 1532–1538. [Google Scholar] [CrossRef]
- D’ISanto, T.; Di Tore, P.A.; Altavilla, G. Correlation of the anthropometric characteristics and the ability to jump in volleyball. J. Hum. Sport Exerc. 2018, 13, S393–S400. [Google Scholar] [CrossRef]
- Cichanowski, H.R.; Schmitt, J.S.; Johnson, R.J.; Niemuth, P.E. Hip strength in collegiate female athletes with patellofemoral pain. Med. Sci. Sports Exerc. 2007, 39, 1227–1232. [Google Scholar] [CrossRef]
- Hébert, L.J.; Maltais, D.B.; Lepage, C.; Saulnier, J.; Crête, M. Hand-held dynamometry isometric torque reference values for children and adolescents. Pediatr. Phys. Ther. 2015, 27, 414–423. [Google Scholar] [CrossRef]
- Krause, D.A.; Neuger, M.D.; Lambert, K.A.; Johnson, A.E.; DeVinny, H.A.; Hollman, J.H. Effects of examiner strength on reliability of hip-strength testing using a handheld dynamometer. J. Sport Rehabil. 2014, 23, 56–64. [Google Scholar] [CrossRef]
- Hannon, J.P.; Wang-Price, S.; Garrison, J.C.; Goto, S.; Bothwell, J.M.; Bush, C.A. Normalized Hip and Knee Strength in Two Age Groups of Adolescent Female Soccer Players. J. Strength Cond. Res. 2022, 36, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, E.; Tomczak, M. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. TRENDS Sport Sci. 2014, 21, 19–25. [Google Scholar]
- Lee, D.K. Alternatives to P value: Confidence interval and effect size. Korean J. Anesthesiol. 2016, 69, 555–562. [Google Scholar] [CrossRef]
- Rosenthal, J.A. Qualitative Descriptors of Strength of Association and Effect Size. J. Soc. Serv. Res. 1996, 21, 37–59. [Google Scholar] [CrossRef]
- Suphasubtrakul, T.; Lekskulchai, R.; Jalayondeja, C. Balance, strength and physical activity after ankle sprain: Comparison between children with chronic ankle instability and copers. Phys. Ther. Sport 2024, 65, 49–53. [Google Scholar] [CrossRef]
- Ko, J.; Rosen, A.B.; Brown, C.N. Functional performance deficits in adolescent athletes with a history of lateral ankle sprain(s). Phys. Ther. Sport 2018, 33, 125–132. [Google Scholar] [CrossRef]
- Maeda, N.; Ikuta, Y.; Tsutsumi, S.; Arima, S.; Ishihara, H.; Ushio, K.; Mikami, Y.; Komiya, M.; Nishikawa, Y.; Nakasa, T.; et al. Relationship of Chronic Ankle Instability with Foot Alignment and Dynamic Postural Stability in Adolescent Competitive Athletes. Orthop. J. Sports Med. 2023, 11, 23259671231202220. [Google Scholar] [CrossRef]
- Chimera, N.J.; Smith, C.A.; Warren, M. Injury History, sex, and performance on the functional movement screen and Y balance test. J. Athl. Train. 2015, 50, 475–485. [Google Scholar] [CrossRef] [PubMed]
- McCann, R.S.; Crossett, I.D.; Terada, M.; Kosik, K.B.; Bolding, B.A.; Gribble, P.A. Hip strength and star excursion balance test deficits of patients with chronic ankle instability. J. Sci. Med. Sport 2017, 20, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.B.; Johnson, N.F.; Terada, M.; Thomas, A.C.; Mattacola, C.G.; Gribble, P.A. Decreased dynamic balance and dorsiflexion range of motion in young and middle-aged adults with chronic ankle instability. J. Sci. Med. Sport 2019, 22, 976–980. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Tillman, M.D.; Chmielewski, T.L.; Cauraugh, J.H.; Naugle, K.E.; Borsa, P.A. Self-Assessed Disability and Functional Performance in Individuals with and Without Ankle Instability: A Case Control Study. J. Orthop. Sports Phys. Ther. 2009, 39, 458–467. [Google Scholar] [CrossRef]
- Ma, S.; Xu, Y.; Xu, S. Effects of Physical Training Programs on Healthy Athletes’ Vertical Jump Height: A Systematic Review with Meta-Analysis. J. Sports Sci. Med. 2025, 24, 236–257. [Google Scholar] [CrossRef]
- Rosen, A.B.; Needle, A.R.; Ko, J. Ability of Functional Performance Tests to Identify Individuals With Chronic Ankle Instability: A Systematic Review With Meta-Analysis. Clin. J. Sport Med. 2019, 29, 509–522. [Google Scholar] [CrossRef]
- Bain, K.A.; Clawson, P.A.; Slone, S.A.; Gribble, P.A.; Hoch, J.M.; Hoch, M.C.; Kosik, K.B. Isometric Hip strength and patient-reported outcomes of individuals with and without chronic ankle instability. J. Sport Rehabil. 2021, 31, 53–59. [Google Scholar] [CrossRef]
- McCann, R.S.; Bolding, B.A.; Terada, M.; Kosik, K.B.; Crossett, I.D.; Gribble, P.A. Isometric Hip Strength and Dynamic Stability of Individuals With Chronic Ankle Instability. J. Athl. Train. 2018, 53, 672–678. [Google Scholar] [CrossRef]
- Khalaj, N.; Vicenzino, B.; Smith, M.D. Hip and knee muscle torque and its relationship with dynamic balance in chronic ankle instability, copers and controls. J. Sci. Med. Sport 2021, 24, 647–652. [Google Scholar] [CrossRef]
- Jaber, H.; Lohman, E.; Daher, N.; Bains, G.; Nagaraj, A.; Mayekar, P.; Shanbhag, M.; Alameri, M.; Jan, Y.-K. Neuromuscular control of ankle and hip during performance of the star excursion balance test in subjects with and without chronic ankle instability. PLoS ONE 2018, 13, e0201479. [Google Scholar] [CrossRef] [PubMed]
- Velarde-Sotres, Á.; Bores-Cerezal, A.; Alemany-Iturriaga, J.; Calleja-González, J. Tensiomyography, functional movement screen and counter movement jump for the assessment of injury risk in sport: A systematic review of original studies of diagnostic tests. Front. Sports Act. Living 2025, 7, 1565900. [Google Scholar] [CrossRef] [PubMed]
- Quatman-Yates, C.C.; Quatman, C.E.; Meszaros, A.J.; Paterno, M.V.; Hewett, T.E. A systematic review of sensorimotor function during adolescence: A developmental stage of increased motor awkwardness? Br. J. Sports Med. 2012, 46, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Letafatkar, A.; Hadadnezhad, M.; Shojaedin, S.; Mohamadi, E. Relationship between functional movement screening score and history of injury. Int. J. Sports Phys. Ther. 2014, 9, 21–27. [Google Scholar]
- Jaimes, C.; Khwaja, A.; Chauvin, N.A. Ankle and Foot Injuries in the Young Athlete. Semin. Musculoskelet. Radiol. 2018, 22, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Dallinga, J.M.; van der Does, H.T.; Benjaminse, A.; Lemmink, K.A. Dynamic postural stability differences between male and female players with and without ankle sprain. Phys. Ther. Sport 2016, 17, 69–75. [Google Scholar] [CrossRef] [PubMed]
| CAI Group (n = 23) | Control Group (n = 23) | p-Value | |||
|---|---|---|---|---|---|
| X (SD) | M (25–75%) | X (SD) | M (25–75%) | ||
| Age (year) | 14.91 (0.9) | 15 (14–15) | 14.96 (0.9) | 15 (14–16) | pa = 0.796 |
| Body weight (kg) | 63.54 (8.1) | 63 (59–71) | 58.64 (7.4) | 59.1 (54–63) | pa = 0.062 |
| Height (cm) | 174.03 (7.5) | 174 (170–177.7) | 170.85 (8.5) | 172 (164–177) | pa = 0.191 |
| Body mass index (kg/m2) | 20.99 (2.5) | 20.86 (19.4–22.5) | 20.03 (1.5) | 19.79 (19.4–21.1) | pa = 0.177 |
| Starting age of volleyball specific training (year) | 10.57 (1.5) | 11 (10–12) | 10.09 (1.7) | 10 (9–11) | pa = 0.215 |
| IdFAI score of the dominant lower limb (0–37) | 15.5 (5.2) | 14 (11–18) | 4.9 (3.3) | 5 (1.5–7.5) | pa < 0.001 * |
| n (%) | n (%) | ||||
| Lower-limb dominance | pb = 1.0 | ||||
| Right | 15 (65.2) | 15 (65.2) | |||
| Left | 8 (34.8) | 8 (34.8) | |||
| CAI Group (n = 23) | Control Group (n = 23) | p-Value | Effect Size [95% CI] | ||||
|---|---|---|---|---|---|---|---|
| X (SD) | M (25–75%) | X (SD) | M (25–75%) | Ζ | r | ||
| Composite FMS score (0–27) | 15 (2) | 15 (13–17) | 16.65 (1.6) | 17 (16–18) | pa = 0.007 * | −2.69 | −0.40 [(−0.62)–(−0.13)] |
| Movement sub-score of the FMS (0–9) | 6.57 (1.2) | 7 (6–7) | 7.78 (0.9) | 8 (7–8) | pa = 0.001 * | −3.32 | −0.49 [(−0.69)–(−0.23)] |
| n (%) | n (%) | Χ2 | Φ | ||||
| Composite FMS score | pb = 0.022 * | 5.25 | 0.34 [(0.09)–(0.57)] | ||||
| ≤14 | 10 (43.5) | 3 (13.1) | |||||
| >14 | 13 (56.5) | 20 (86.9) | |||||
| CAI Group (n = 23) | Control Group (n = 23) | p-Value | Effect Size [95% CI] | ||||
|---|---|---|---|---|---|---|---|
| X (SD) | M (25–75%) | X (SD) | M (25–75%) | Ζ | r | ||
| YBT-Anterior (%) | 67.6 (12.9) | 74.1 (55.5–78.8) | 76.9 (14.1) | 84.6 (61.1–89.1) | pa = 0.004 * | −2.87 | −0.42 [(−0.63)–(−0.15)] |
| YBT-Posteromedial (%) | 74.8 (18) | 78.9 (60.8–92) | 83.6 (12.9) | 84.9 (74.3–90.5) | pa = 0.147 | −1.45 | −0.21 [(−0.47)–(0.07)] |
| YBT-Posterolateral (%) | 79.8 (17.7) | 79.6 (63.9–96) | 87 (13.7) | 90.2 (69.3–97.6) | pa = 0.170 | −1.37 | −0.20 [(−0.46)–(0.08)] |
| YBT-Composite (%) | 74.1 (15.7) | 79.3 (58.2–89.7) | 82.5 (12.8) | 87.5 (68.4–92.5) | pa = 0.057 | −1.90 | −0.28 [(−0.54)–(0.02)] |
| YBT-Anterior asymmetry (cm) | 4.9 (4) | 3.5 (1.2–9.6) | 2.2 (1.2) | 2.3 (1.3–3) | pa = 0.073 | −1.79 | −0.26 [(−0.52)–(0.03)] |
| n (%) | n (%) | Χ2 | Φ | ||||
| YBT-Anterior asymmetry | pb = 0.001 * | 11.28 | 0.50 [(0.23)–(0.69)] | ||||
| ≥4 cm | 11 (47.8) | 1 (4.4) | |||||
| <4 cm | 12 (52.2) | 22 (95.6) | |||||
| CAI Group (n = 23) | Control Group (n = 23) | p-Value | Effect Size [95% CI] | ||||
|---|---|---|---|---|---|---|---|
| X (SD) | M (25–75%) | X (SD) | M (25–75%) | Ζ | r | ||
| SJwH (cm) | 22.6 (3.4) | 23.1 (20.3–25) | 22.7 (3.2) | 22.8 (20.7–25) | pa = 0.843 | −0.20 | −0.03 [(−0.32)–(0.26)] |
| CMJwH (cm) | 24.9 (3.1) | 25 (23.1–27.5) | 24.3 (3.2) | 24.4 (22.1–26.6) | pa = 0.455 | −0.75 | −0.11 [(−0.38)–(0.17)] |
| CMJwA (cm) | 29.4 (3.9) | 29.1 (26.5–31.9) | 28.9 (3.5) | 28.5 (26.1–31.3) | pa = 0.503 | −0.67 | −0.10 [(−0.37)–(0.18)] |
| CAI Group (n = 23) | Control Group (n = 23) | p-Value | Effect Size [95% CI] | ||||
|---|---|---|---|---|---|---|---|
| X (SD) | M (25–75%) | X (SD) | M (25–75%) | Ζ | r | ||
| Hip flexors (Nm/kg) | 1.40 (0.31) | 1.38 (1.19–1.64) | 1.63 (0.21) | 1.62 (1.52–1.77) | pa = 0.007 * | −2.69 | −0.40 [(−0.62)–(−0.13)] |
| Hip extensors (Nm/kg) | 2.38 (0.35) | 2.44 (2.12–2.49) | 2.59 (0.35) | 2.62 (2.32–2.76) | pa = 0.062 | −1.87 | −0.28 [(−0.54)–(0.02)] |
| Hip abductors (Nm/kg) | 2.08 (0.67) | 2.13 (1.54–2.51) | 2.26 (0.40) | 2.24 (1.92–2.59) | pa = 0.253 | −1.14 | −0.17 [(−0.44)–(0.11)] |
| Hip adductors (Nm/kg) | 1.12 (0.28) | 1.11 (0.88–1.36) | 1.26 (0.17) | 1.25 (1.16–1.34) | pa = 0.044 * | −2.11 | −0.31 [(−0.56)–(−0.01)] |
| Hip internal rotators (Nm/kg) | 0.74 (0.13) | 0.73 (0.66–0.83) | 0.75 (0.14) | 0.75 (0.66–0.88) | pa = 0.965 | −0.04 | −0.01 [(−0.29)–(0.28)] |
| Hip external rotators (Nm/kg) | 0.59 (0.07) | 0.60 (0.55–0.64) | 0.60 (0.09) | 0.61 (0.55–0.66) | pa = 0.886 | −0.14 | −0.02 [(−0.30)–(0.27)] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akoğlu, A.S.; Adın, R.M.; Ada, A.M.; Bayrakcı Tunay, V.; Erden, Z. Comparison of Functional Movement, Balance, Vertical Jumping, Hip Strength and Injury Risk in Adolescent Female Volleyball Players with and Without Chronic Ankle Instability. Medicina 2025, 61, 1547. https://doi.org/10.3390/medicina61091547
Akoğlu AS, Adın RM, Ada AM, Bayrakcı Tunay V, Erden Z. Comparison of Functional Movement, Balance, Vertical Jumping, Hip Strength and Injury Risk in Adolescent Female Volleyball Players with and Without Chronic Ankle Instability. Medicina. 2025; 61(9):1547. https://doi.org/10.3390/medicina61091547
Chicago/Turabian StyleAkoğlu, Abdullah Sinan, Rıdvan M. Adın, Ahmet Mustafa Ada, Volga Bayrakcı Tunay, and Zafer Erden. 2025. "Comparison of Functional Movement, Balance, Vertical Jumping, Hip Strength and Injury Risk in Adolescent Female Volleyball Players with and Without Chronic Ankle Instability" Medicina 61, no. 9: 1547. https://doi.org/10.3390/medicina61091547
APA StyleAkoğlu, A. S., Adın, R. M., Ada, A. M., Bayrakcı Tunay, V., & Erden, Z. (2025). Comparison of Functional Movement, Balance, Vertical Jumping, Hip Strength and Injury Risk in Adolescent Female Volleyball Players with and Without Chronic Ankle Instability. Medicina, 61(9), 1547. https://doi.org/10.3390/medicina61091547






