Development and Validation of a NEWS2-Enhanced Multivariable Prediction Model for Clinical Deterioration and In-Hospital Mortality in Hospitalized Adults
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Electronic Health Record
2.3. Ethical Aspects
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Description of HCR Patients and Death Patients
3.2. Comparison of NEWS2, Barthel, and ReTos with HCR and Death
3.3. Predictive Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NEWS2 | The National Early Warning Score 2 |
| EWS | Early warning systems |
| ICU | Intensive care unit |
| CVC | Central venous catheter |
| HCR | High clinical risk |
| AUC | Area under the curve |
| EHR | Electronic health record |
| UOC | Hospital operating units |
| ADLs | Electronic health record |
| IQR | Interquartile range |
References
- Smith, M.B.; Chiovaro, J.C.; O’Neil, M.; Kansagara, D.; Quiñones, A.R.; Freeman, M.; Slatore, C.G. Early warning system scores for clinical deterioration in hospitalized patients: A systematic review. Ann. Am. Thorac. Soc. 2014, 11, 1454–1465. [Google Scholar] [CrossRef]
- Guan, G.; Lee, C.M.Y.; Begg, S.; Crombie, A.; Mnatzaganian, G. The use of early warning system scores in pre-hospital and emergency department settings to predict clinical deterioration: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0265559. [Google Scholar] [CrossRef]
- Tirkkonen, J.; Olkkola, K.T.; Huhtala, H.; Tenhunen, J.; Hoppu, S. Medical emergency team activation: Performance of conventional dichotomised criteria versus national early warning score. Acta Anaesthesiol. Scand. 2014, 58, 411–419. [Google Scholar] [CrossRef]
- Jones, D.; Mitchell, I.; Hillman, K.; Story, D. Defining clinical deterioration. Resuscitation 2013, 84, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Jung, H. Indicators of clinical deterioration in adult general ward patients from nurses’ perspectives: A mixed-methods systematic review. BMC Nurs. 2024, 23, 861. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.; Ojemeni, M.M.; Kalamani, R.; Tong, J.; Crecelius, M.L. Relationship between nurse burnout, patient and organizational outcomes: Systematic review. Int. J. Nurs. Stud. 2021, 119, 103933. [Google Scholar] [CrossRef]
- Twigg, D.E.; Whitehead, L.; Doleman, G.; El-Zaemey, S. The impact of nurse staffing methodologies on nurse and patient outcomes: A systematic review. J. Adv. Nurs. 2021, 77, 4599–4611. [Google Scholar] [CrossRef]
- Allen, S.B. The nurse-patient assignment: Purposes and decision factors. JONA J. Nurs. Adm. 2015, 45, 628–635. [Google Scholar] [CrossRef]
- Van Galen, L.S.; Struik, P.W.; Driesen, B.E.; Merten, H.; Ludikhuize, J.; Van Der Spoel, J.I.; Nanayakkara, P.W. Delayed recognition of deterioration of patients in general wards is mostly caused by human related monitoring failures: A root cause analysis of unplanned ICU admissions. PLoS ONE 2016, 11, e0161393. [Google Scholar] [CrossRef] [PubMed]
- Barwise, A.; Thongprayoon, C.; Gajic, O.; Jensen, J.; Herasevich, V.; Pickering, B.W. Delayed rapid response team activation is associated with increased hospital mortality, morbidity, and length of stay in a tertiary care institution. Crit. Care Med. 2016, 44, 54–63. [Google Scholar] [CrossRef]
- Areia, C.; Biggs, C.; Santos, M.; Thurley, N.; Gerry, S.; Tarassenko, L.; Vollam, S. The impact of wearable continuous vital sign monitoring on deterioration detection and clinical outcomes in hospitalised patients: A systematic review and meta-analysis. Crit. Care 2021, 25, 351. [Google Scholar] [CrossRef]
- Churpek, M.M.; Yuen, T.C.; Winslow, C.; Meltzer, D.O.; Kattan, M.W.; Edelson, D.P. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit. Care Med. 2016, 44, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Escobar, G.J.; Liu, V.X.; Schuler, A.; Lawson, B.; Greene, J.D.; Kipnis, P. Automated identification of adults at risk for in-hospital clinical deterioration. N. Engl. J. Med. 2020, 383, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Edelson, D.P. Risk stratification of hospitalized patients on the wards. Chest 2013, 143, 1758–1765. [Google Scholar] [CrossRef]
- Morgan, R.J.M.; Williams, F.; Wright, M.M. An early warning scoring system for detecting developing critical illness. Clin. Intensive Care 1997, 8, 100. [Google Scholar]
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admissions. QJM 2001, 94, 521–526. [Google Scholar] [CrossRef]
- Alam, N.; Hobbelink, E.L.; van Tienhoven, A.J.; van de Ven, P.M.; Jansma, E.P.; Nanayakkara, P.W. The impact of the use of the Early Warning Score (EWS) on patient outcomes: A systematic review. Resuscitation 2014, 85, 587–594. [Google Scholar] [CrossRef]
- National Early Warning Score (NEWS). Standardising the assessment of acute illness severity in the NHS. In Report of a Working Party; Royal College of Physicians: London, UK, 2012. [Google Scholar]
- Prytherch, D.R.; Smith, G.B.; Schmidt, P.E.; Featherstone, P.I. ViEWS—Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation 2010, 81, 932–937. [Google Scholar] [CrossRef]
- Hodgson, L.E.; Congleton, J.; Venn, R.; Forni, L.G.; Roderick, P.J. NEWS 2–too little evidence to implement? Clin. Med. 2018, 18, 371–373. [Google Scholar] [CrossRef]
- Smith, G.B.; Redfern, O.C.; Pimentel, M.A.; Gerry, S.; Collins, G.S.; Malycha, J.; Prytherch, D.; Schmidt, P.E.; Watkinson, P.J. The national early warning score 2 (NEWS2). Clin. Med. 2019, 19, 260. [Google Scholar] [CrossRef]
- Haegdorens, F.; Monsieurs, K.G.; De Meester, K.; Van Bogaert, P. The optimal threshold for prompt clinical review: An external validation study of the national early warning score. J. Clin. Nurs. 2020, 29, 4594–4603. [Google Scholar] [CrossRef]
- Haegdorens, F.; Lefebvre, J.; Wils, C.; Franck, E.; Van Bogaert, P. Combining the Nurse Intuition Patient Deterioration Scale with the National Early Warning Score provides more Net Benefit in predicting serious adverse events: A prospective cohort study in medical, surgical, and geriatric wards. Intensive Crit. Care Nurs. 2024, 83, 103628. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.; Kellett, J. The United Kingdom’s National Early Warning Score: Should everyone use it? A narrative review. Intern. Emerg. Med. 2023, 18, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, L.E.; Dimitrov, B.D.; Congleton, J.; Venn, R.; Forni, L.G.; Roderick, P.J. A validation of the National Early Warning Score to predict outcome in patients with COPD exacerbation. Thorax 2017, 72, 23–30. [Google Scholar] [CrossRef]
- Silcock, D.J.; Corfield, A.R.; Gowens, P.A.; Rooney, K.D. Validation of the National Early Warning Score in the prehospital setting. Resuscitation 2015, 89, 31–35. [Google Scholar] [CrossRef]
- Smith, G.B.; Prytherch, D.R.; Meredith, P.; Schmidt, P.E.; Featherstone, P.I. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation 2013, 84, 465–470. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, H.; Chen, L.; Wu, S.; Wang, S. National early warning score in predicting severe adverse outcomes of emergency medicine patients: A retrospective cohort study. J. Multidiscip. Healthc. 2021, 14, 2067–2078. [Google Scholar] [CrossRef]
- Gerry, S.; Bonnici, T.; Birks, J.; Kirtley, S.; Virdee, P.S.; Watkinson, P.J.; Collins, G.S. Early warning scores for detecting deterioration in adult hospital patients: Systematic review and critical appraisal of methodology. BMJ 2020, 369, m1501. [Google Scholar] [CrossRef] [PubMed]
- Haegdorens, F.; Wils, C.; Franck, E. Predicting patient deterioration by nurse intuition: The development and validation of the nurse intuition patient deterioration scale. Int. J. Nurs. Stud. 2023, 142, 104467. [Google Scholar] [CrossRef]
- Bedoya, A.D.; Clement, M.E.; Phelan, M.; Steorts, R.C.; O’Brien, C.; Goldstein, B.A. Minimal impact of implemented early warning score and best practice alert for patient deterioration. Crit. Care Med. 2019, 47, 49–55. [Google Scholar] [CrossRef]
- Ko, R.E.; Kim, Z.; Jeon, B.; Ji, M.; Chung, C.R.; Suh, G.Y.; Cho, B.H. Deep Learning-Based Early Warning Score for Predicting Clinical Deterioration in General Ward Cancer Patients. Cancers 2023, 15, 5145. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; So, K.; Prabhakar, S.K.; Kim, C.; Lee, J.J.; Sohn, J.H.; Won, D.O. Early warning score and feasible complementary approach using artificial intelligence-based bio-signal monitoring system: A review. Biomed. Eng. Lett. 2025, 15, 717–734. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Bellandi, T.; Brandi, A.; Rasero, L.; Rizzini, D.; Rodella, S.; Simonetti, M.; Tartaglia, R. La Prevenzione Delle Cadute in Ospedale; Edizioni ETS: Pisa, Italy, 2011. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef]
- McGinley, A.; Pearse, R.M. A national early warning score for acutely ill patients. BMJ 2012, 345, e5310. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, P.; Greaves, J.; Cook, G.; Fraser, A.; Bainbridge, L. A study to introduce National Early Warning Scores (NEWS) in care homes: Influence on decision-making and referral processes. Nurs. Open 2022, 9, 519–526. [Google Scholar] [CrossRef]
- Subbe, C.P.; Bannard-Smith, J.; Bunch, J.; Champunot, R.; DeVita, M.A.; Durham, L.; Welch, J. Quality metrics for the evaluation of Rapid Response Systems: Proceedings from the third international consensus conference on Rapid Response Systems. Resuscitation 2019, 141, 1–12. [Google Scholar] [CrossRef]
- Riccalton, V.; Threlfall, L.; Ananthakrishnan, A.; Cong, C.; Milne-Ives, M.; Le Roux, P.; Meinert, E. Modifications to the National Early Warning Score 2: A Scoping Review. BMC Med. 2025, 23, 154. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Moreno-Torres, V.; Royuela, A.; Múñez, E.; Ortega, A.; Gutierrez, Á.; Mills, P.; Ramos-Martínez, A. Better prognostic ability of NEWS2, SOFA and SAPS-II in septic patients. Med. Clin. 2022, 159, 224–229. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yeo, J.H.; Kang, M.J.; Lee, J.H.; Cho, K.W.; Hwang, S.; Kim, Y.W. Performance assessment of the SOFA, APACHE II scoring system, and SAPS II in intensive care unit organophosphate poisoned patients. J. Korean Med. Sci. 2013, 28, 1822. [Google Scholar] [CrossRef]
- Colussi, G.; Perrotta, G.; Pillinini, P.; Dibenedetto, A.G.; Da Porto, A.; Catena, C.; Sechi, L.A. Prognostic scores and early management of septic patients in the emergency department of a secondary hospital: Results of a retrospective study. BMC Emerg. Med. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Regione Toscana. Delibera n.416 del 19-04-2021. Available online: https://www301.regione.toscana.it/bancadati/atti/Contenuto.xml?id=5287555&nomeFile=Delibera_n.416_del_19-04-2021 (accessed on 30 June 2025).
- Jensen, J.K.; Skår, R.; Tveit, B. Introducing the National Early Warning Score—A qualitative study of hospital nurses’ perceptions and reactions. Nurs. Open 2019, 6, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.A.; Redfern, O.C.; Gerry, S.; Collins, G.S.; Malycha, J.; Prytherch, D.; Watkinson, P.J. A comparison of the ability of the National Early Warning Score and the National Early Warning Score 2 to identify patients at risk of in-hospital mortality: A multi-centre database study. Resuscitation 2019, 134, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rothman, M.J.; Rothman, S.I.; Beals, J., IV. Development and validation of a continuous measure of patient condition using the electronic medical record. J. Biomed. Inform. 2013, 46, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Davis, A.; Fischhoff, B.; Yecies, E.; Grace, J.; Klobuka, A.; Mohan, D.; Hanmer, J. Comparing the predictive ability of a commercial artificial intelligence early warning system with physician judgement for clinical deterioration in hospitalised general internal medicine patients: A prospective observational study. BMJ Open 2019, 9, e032187. [Google Scholar] [CrossRef]
- Shin, Y.; Cho, K.J.; Chang, M.; Youk, H.; Kim, Y.J.; Park, J.Y.; Yoo, D. The development and validation of a novel deep-learning algorithm to predict in-hospital cardiac arrest in ED-ICU (emergency department-based intensive care units): A single center retrospective cohort study. Signa Vitae 2024, 20, 83. [Google Scholar]
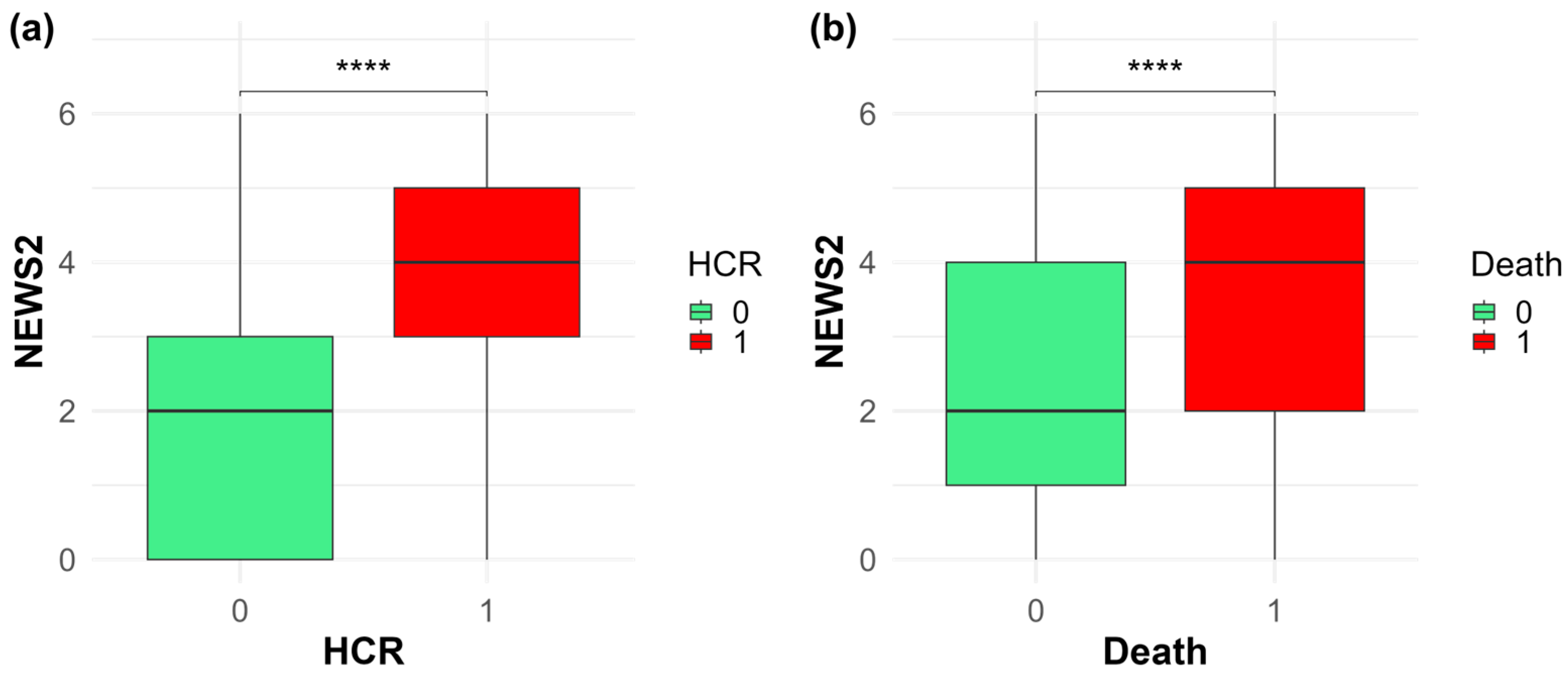
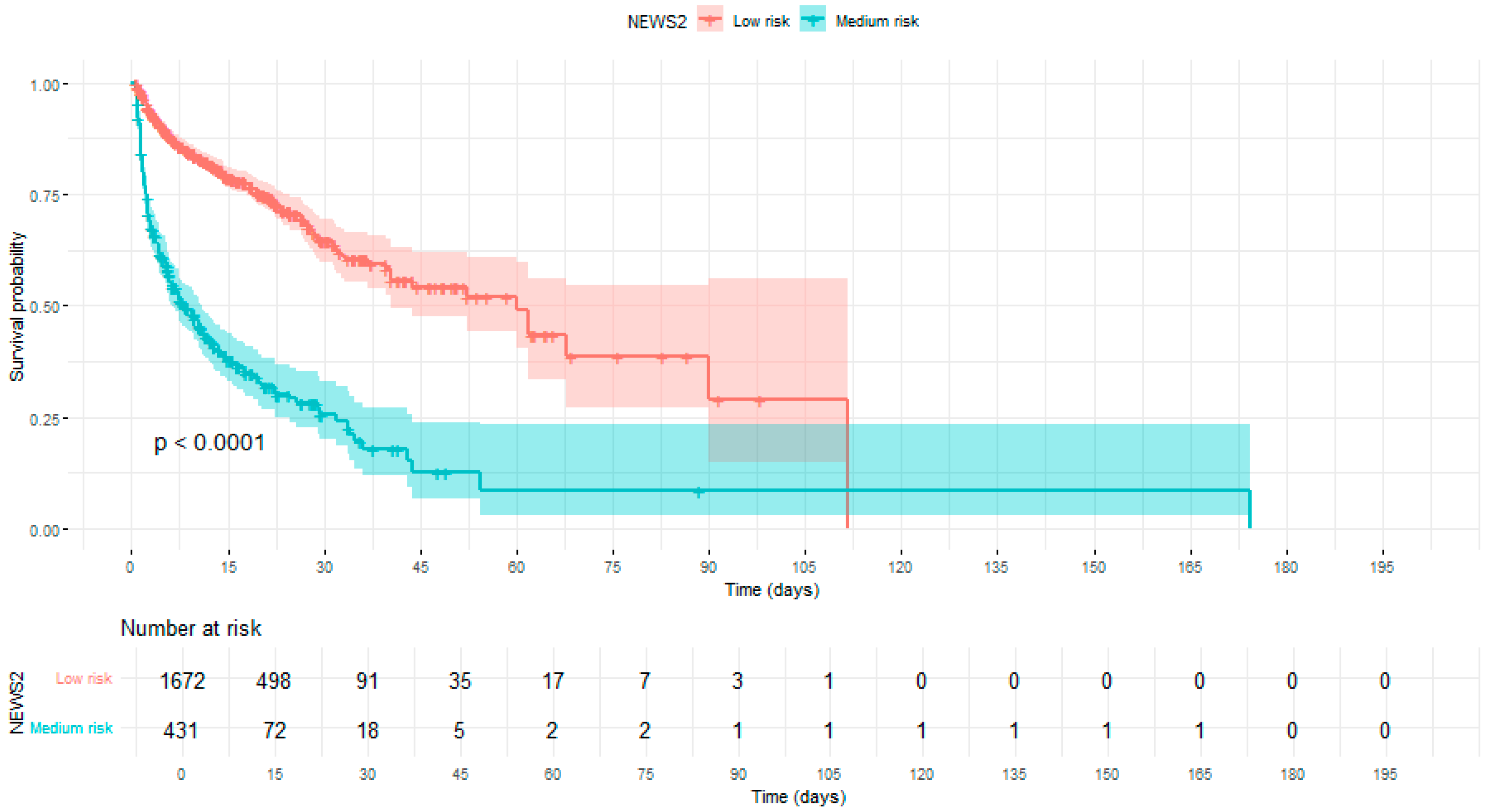
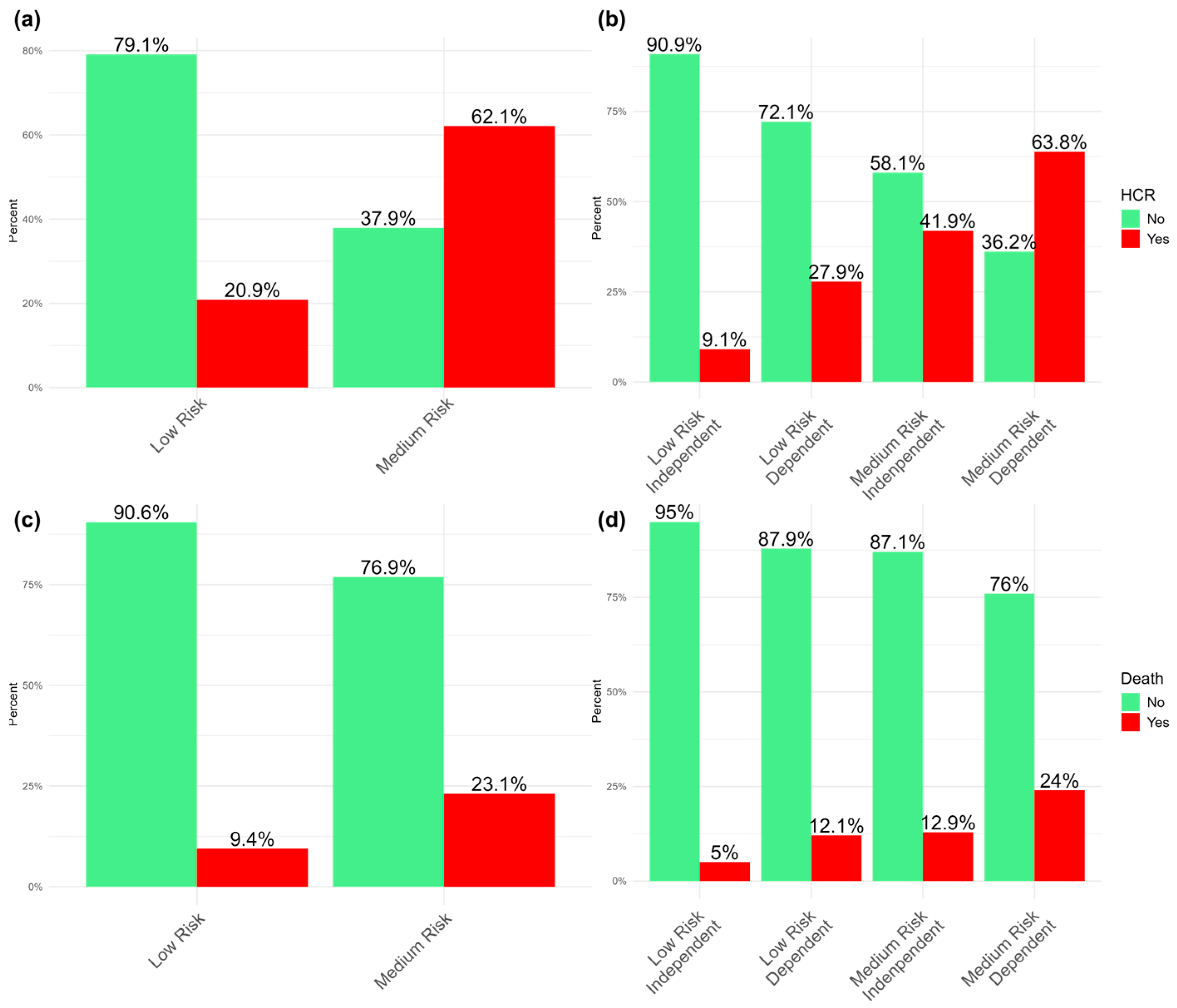
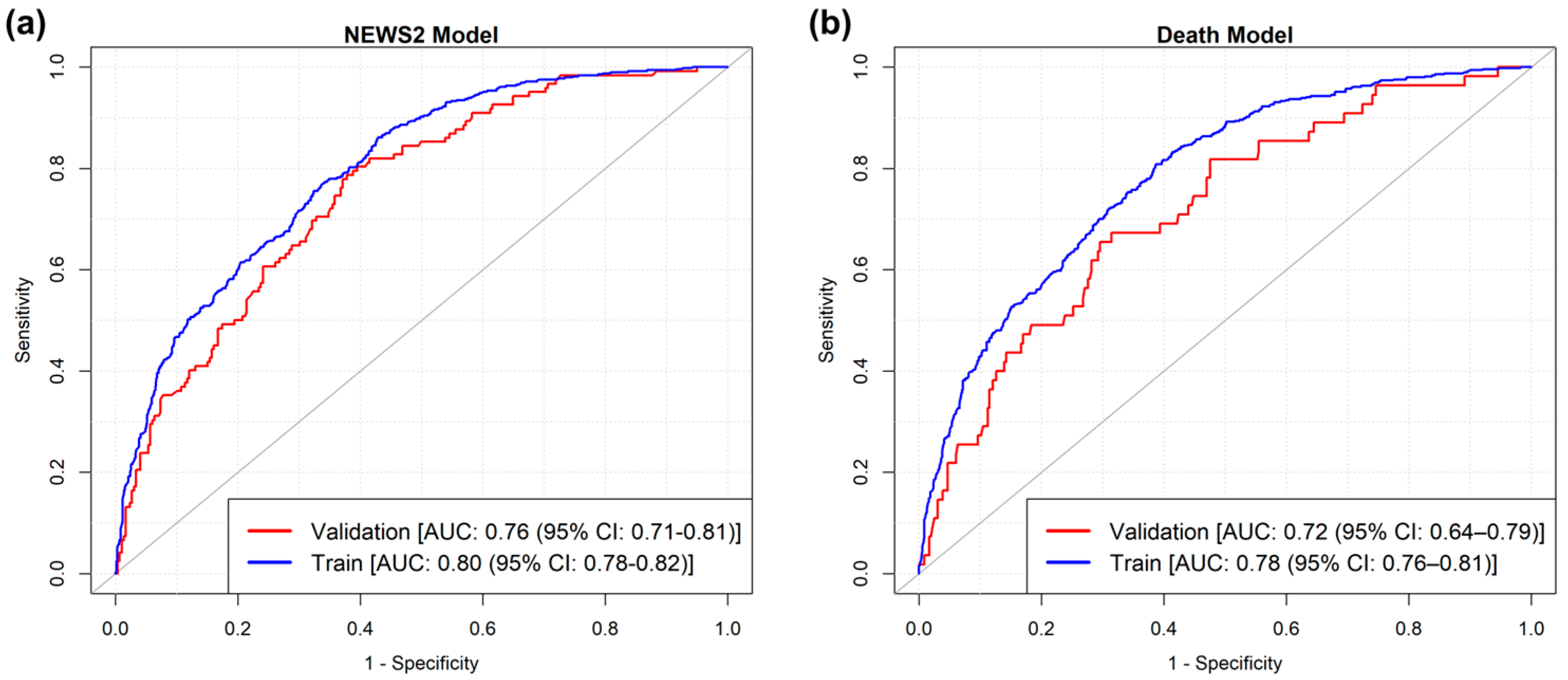
| Variabile | NEWS ≤ 7 | NEWS ≥ 7 | p | Survived | Died | p |
|---|---|---|---|---|---|---|
| n | 1496 | 612 | 1848 | 260 | ||
| Male | 769 (51.4) | 324 (52.9) | 0.553 | 966 (52.3) | 127 (48.8) | 0.333 |
| Cvc | 59 (3.9) | 62 (10.1) | <0.001 | 101 (5.5) | 20 (7.7) | 0.193 |
| Revolving Door | 113 (7.6) | 62 (10.1) | 0.063 | 144 (7.8) | 31 (11.9) | 0.032 |
| Age | 79 [67, 86] | 84 [75, 89] | <0.001 | 80 [69, 87] | 85.00 [76, 88] | <0.001 |
| Barthel | 50 [10, 92.5] | 5 [0, 45] | <0.001 | 35.00 [5, 80] | 5.00 [0, 45] | <0.001 |
| NEWS2 | 2 [0, 3] | 4 [3, 5] | <0.001 | 2 [1, 4] | 4 [2, 5] | <0.001 |
| ReTos | 6 [3, 8] | 7.00 [5, 10] | <0.001 | 6 [4, 9] | 7 [4, 9] | 0.168 |
| Died | 59 (3.9) | 201 (32.8) | <0.001 | |||
| High Risk | 411 (22.2) | 201 (77.3) | <0.001 |
| HCR | ||||
|---|---|---|---|---|
| OR Univariate | OR Multivariate | p Value | Variable Importance | |
| CVC | 3.04 (2.00–4.67) | 3.81 (2.34–6.27) | <0.001 | 5.33 |
| Age | 1.038 (1.03–1.05) | 1.02 (1.01–1.03) | <0.001 | 4.00 |
| Barthel | 0.976 (0.97–0.98) | 0.988 (0.98–0.99) | <0.001 | 5.49 |
| NEWS2 | 1.72 (1.61–1.84) | 1.57 (1.46–1.69) | <0.001 | 12.50 |
| Death | ||||
| OR Univariate | OR Multivariate | p Value | Variable Importance | |
| Age | 1.03 (1.02–1.05) | 1.018 (1.00–1.03) | 0.008 | 2.64 |
| Barthel | 0.984 (0.98–0.99) | 0.99 (0.99–1) | 0.002 | 3.11 |
| NEWS2 | 1.336 (1.24–1.44) | 1.228 (1.13–1.34) | <0.001 | 4.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Conte, S.; Fruscoloni, G.; Cartocci, A.; Vitiello, M.; De Marco, M.F.; Cevenini, G.; Barbini, P. Development and Validation of a NEWS2-Enhanced Multivariable Prediction Model for Clinical Deterioration and In-Hospital Mortality in Hospitalized Adults. Medicina 2025, 61, 1543. https://doi.org/10.3390/medicina61091543
Lo Conte S, Fruscoloni G, Cartocci A, Vitiello M, De Marco MF, Cevenini G, Barbini P. Development and Validation of a NEWS2-Enhanced Multivariable Prediction Model for Clinical Deterioration and In-Hospital Mortality in Hospitalized Adults. Medicina. 2025; 61(9):1543. https://doi.org/10.3390/medicina61091543
Chicago/Turabian StyleLo Conte, Sofia, Guido Fruscoloni, Alessandra Cartocci, Martin Vitiello, Maria Francesca De Marco, Gabriele Cevenini, and Paolo Barbini. 2025. "Development and Validation of a NEWS2-Enhanced Multivariable Prediction Model for Clinical Deterioration and In-Hospital Mortality in Hospitalized Adults" Medicina 61, no. 9: 1543. https://doi.org/10.3390/medicina61091543
APA StyleLo Conte, S., Fruscoloni, G., Cartocci, A., Vitiello, M., De Marco, M. F., Cevenini, G., & Barbini, P. (2025). Development and Validation of a NEWS2-Enhanced Multivariable Prediction Model for Clinical Deterioration and In-Hospital Mortality in Hospitalized Adults. Medicina, 61(9), 1543. https://doi.org/10.3390/medicina61091543







