Hyperferritinemia and the Risk of Liver Fibrosis and Liver-Related Events in Patients with Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. Serum Ferritin and Haptoglobin Levels in Relationship with Liver Risk Score and Liver Fibrosis Markers
3.3. Performance of Ferritin and FHR to Predict Advanced Liver Fibrosis and Liver-Related Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cernea, S.; Raz, I. NAFLD in type 2 diabetes mellitus: Still many challenging questions. Diabetes Metab. Res. Rev. 2021, 37, e3386. [Google Scholar] [CrossRef]
- Cernea, S. NAFLD Fibrosis Progression and Type 2 Diabetes: The Hepatic-Metabolic Interplay. Life 2024, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Cullis, J.O.; Fitzsimons, E.J.; Griffiths, W.J.; Tsochatzis, E.; Thomas, D.W.; British Society for Haematology. Investigation and management of a raised serum ferritin. Br. J. Haematol. 2018, 181, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Anastasopoulos, N.A.; Barbouti, A.; Goussia, A.C.; Christodoulou, D.K.; Glantzounis, G.K. Exploring the Role of Metabolic Hyperferritinaemia (MHF) in Steatotic Liver Disease (SLD) and Hepatocellular Carcinoma (HCC). Cancers 2025, 17, 842. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Q.; Yang, Y.; Ma, L. Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. J. Diabetes Investig. 2020, 11, 946–955. [Google Scholar] [CrossRef]
- Harrison, A.V.; Lorenzo, F.R.; McClain, D.A. Iron and the Pathophysiology of Diabetes. Annu. Rev. Physiol. 2023, 85, 339–362. [Google Scholar] [CrossRef]
- El Nakeeb, N.; Saleh, S.A.; Massoud, Y.M.; Hussein, A.; Hamed, R. Serum ferritin as a non-invasive marker in the prediction of hepatic fibrosis among Egyptian patients with non-alcoholic fatty liver disease. JGH Open 2017, 1, 112–119. [Google Scholar] [CrossRef]
- Du, S.X.; Lu, L.L.; Geng, N.; Victor, D.W.; Chen, L.Z.; Wang, C.; Yue, H.Y.; Xin, Y.N.; Xuan, S.Y.; Jin, W.W. Association of serum ferritin with non-alcoholic fatty liver disease: A meta-analysis. Lipids Health Dis. 2017, 16, 228. [Google Scholar] [CrossRef]
- Song, Z.; Miao, X.; Xie, X.; Tang, G.; Deng, J.; Hu, M.; Liu, S.; Leng, S. Associations between serum ferritin baselines and trajectories and the incidence of metabolic dysfunction-associated steatotic liver disease: A prospective cohort study. Lipids Health Dis. 2024, 23, 141. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Belt, P.; Wilson, L.A.; Yeh, M.M.; Neuschwander-Tetri, B.A.; Chalasani, N.; Sanyal, A.J.; Nelson, J.E.; NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 77–85. [Google Scholar] [CrossRef]
- Armandi, A.; Sanavia, T.; Younes, R.; Caviglia, G.P.; Rosso, C.; Govaere, O.; Liguori, A.; Francione, P.; Gallego-Duràn, R.; Ampuero, J.; et al. Serum ferritin levels can predict long-term outcomes in patients with metabolic dysfunction-associated steatotic liver disease. Gut 2024, 73, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Corradini, E.; Adams, L.A.; Aigner, E.; Alqahtani, S.; Arrese, M.; Bardou-Jacquet, E.; Bugianesi, E.; Fernandez-Real, J.M.; Girelli, D.; et al. Consensus Statement on the definition and classification of metabolic hyperferritinaemia. Nat. Rev. Endocrinol. 2023, 19, 299–310, Erratum in Nat. Rev. Endocrinol. 2024, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Onișor, D.; Roiban, A.L.; Benedek, T.; Rat, N. Metabolic dysfunction-associated steatotic liver disease-associated fibrosis and cardiac dysfunction in patients with type 2 diabetes. World J. Cardiol. 2024, 16, 580–594. [Google Scholar] [CrossRef]
- Onișor, D.; Roiban, A.L.; Cernea, S. Metabolic Dysfunction-Associated Steatotic Liver Disease in Type 2 Diabetes Patients-The Relationship with Platelets Indicators. Medicina 2024, 60, 2091. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Paik, J.M.; Stepanova, M.; Ong, J.; Alqahtani, S.; Henry, L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J. Hepatol. 2024, 80, 694–701. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Wu, X.; Lu, Y.; She, Q. Advances in diagnostic ultrasound techniques for assessing liver steatosis in nonalcoholic fatty liver disease. iLiver 2023, 2, 214–218. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. Chronic Kidney Disease Epidemiology Collaboration. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, X.R.; Xu, Y. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Otgonsuren, M.; Estep, M.J.; Hossain, N.; Younossi, E.; Frost, S.; Henry, L.; Hunt, S.; Fang, Y.; Goodman, Z.; Younossi, Z.M. Single non-invasive model to diagnose non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). J. Gastroenterol. Hepatol. 2014, 29, 2006–2013. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Tavaglione, F.; Jamialahmadi, O.; De Vincentis, A.; Qadri, S.; Mowlaei, M.E.; Mancina, R.M.; Ciociola, E.; Carotti, S.; Perrone, G.; Bruni, V.; et al. Development and Validation of a Score for Fibrotic Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2023, 21, 1523–1532.e1. [Google Scholar] [CrossRef]
- Loaeza-del-Castillo, A.; Paz-Pineda, F.; Oviedo-Cárdenas, E.; Sánchez-Avila, F.; Vargas-Vorácková, F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann. Hepatol. 2008, 7, 350–357. [Google Scholar] [CrossRef]
- Serra-Burriel, M.; Juanola, A.; Serra-Burriel, F.; Thiele, M.; Graupera, I.; Pose, E.; Pera, G.; Grgurevic, I.; Caballeria, L.; Piano, S.; et al. Development, validation, and prognostic evaluation of a risk score for long-term liver-related outcomes in the general population: A multicohort study. Lancet 2023, 402, 988–996. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Jiang, X.; Yin, X.; Fekadu, G.; Liu, C.; He, Y.; Chen, H.; Ni, W.; Wang, R.; et al. LiverRisk score: An accurate, cost-effective tool to predict fibrosis, liver-related, and diabetes-related mortality in the general population. Med 2024, 5, 570–582.e4. [Google Scholar] [CrossRef]
- Garcia-Casal, M.N.; Pasricha, S.R.; Martinez, R.X.; Lopez-Perez, L.; Peña-Rosas, J.P. Serum or plasma ferritin concentration as an index of iron deficiency and overload. Cochrane Database Syst. Rev. 2021, 5, CD011817. [Google Scholar] [CrossRef]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef]
- Anderson, E.R.; Shah, Y.M. Iron homeostasis in the liver. Compr. Physiol. 2013, 3, 315–330. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Ruddell, R.G.; Hoang-Le, D.; Barwood, J.M.; Rutherford, P.S.; Piva, T.J.; Watters, D.J.; Santambrogio, P.; Arosio, P.; Ramm, G.A. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009, 49, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.J.; Farnaud, S.J.; Sharp, P.A. Iron and liver fibrosis: Mechanistic and clinical aspects. World J. Gastroenterol. 2019, 25, 521–538. [Google Scholar] [CrossRef]
- Sibille, J.C.; Kondo, H.; Aisen, P. Interactions between isolated hepatocytes and Kupffer cells in iron metabolism: A possible role for ferritin as an iron carrier protein. Hepatology 1988, 8, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rojo, M.A.; Burgess, A.; Glanfield, A.; Hoang-Le, D.; Ramm, G.A. The Role of Receptor-Mediated Endocytosis of H-ferritin in Induction of NFkappaB-dependent Proinflammatory Signalling in Hepatic Stellate Cells: 787. Hepatology 2014, 60, 580A. [Google Scholar]
- Philippe, M.A.; Ruddell, R.G.; Ramm, G.A. Role of iron in hepatic fibrosis: One piece in the puzzle. World J. Gastroenterol. 2007, 13, 4746–4754. [Google Scholar] [CrossRef]
- Guo, W.; Weng, T.; Song, Y. Association of serum iron status with MASLD and liver fibrosis. PLoS ONE 2025, 20, e0319057. [Google Scholar] [CrossRef]
- Wang, T.; He, L.; Wang, S.; Ma, D. Association between nonalcoholic steatohepatitis and high serum ferritin levels in type 2 diabetes mellitus. Rev. Assoc. Med. Bras. 2024, 70, e20231405. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Ma, X.Y.; Yi, Y.; Li, L.R.; Xu, Z.Y.; Chang, Y. Association between Serum Ferritin Levels and Metabolic-associated Fatty Liver Disease in Adults: A Cross-sectional Study Based on the NHANES. Curr. Med. Sci. 2024, 44, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Amangurbanova, M.; Huang, D.Q.; Noureddin, N.; Tesfai, K.; Bettencourt, R.; Siddiqi, H.; Lopez, S.J.; Cervantes, V.; Madamba, E.; Loomba, R. A Prospective Study on the Prevalence of MASLD in Patients with Type 2 Diabetes and Hyperferritinaemia. Aliment. Pharmacol. Ther. 2025, 61, 456–464. [Google Scholar] [CrossRef]
- Suresh, D.; Li, A.; Miller, M.J.; Wijarnpreecha, K.; Chen, V.L. Associations between metabolic hyperferritinaemia, fibrosis-promoting alleles and clinical outcomes in steatotic liver disease. Liver Int. 2024, 44, 389–398. [Google Scholar] [CrossRef]
- Song, B.G.; Goh, M.J.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Sinn, D.H. Serum Ferritin Levels and Liver-Related Events in Individuals With Steatotic Liver Disease: A Longitudinal Cohort Study. Aliment. Pharmacol. Ther. 2025, 61, 491–500. [Google Scholar] [CrossRef]
- Ramasamy, J. Diagnostic Utility of Serum Ferritin in Identifying Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease (NAFLD): A Cross-Sectional Study Using National Health and Nutrition Examination Survey (NHANES-2017-2020) Data. Indian. J. Clin. Biochem. 2025, 40, 80–88. [Google Scholar] [CrossRef]
- Trasolini, R.; Cox, B.; Galts, C.; Yoshida, E.M.; Marquez, V. Elevated serum ferritin in non-alcoholic fatty liver disease is not predictive of fibrosis. Can. Liver J. 2022, 5, 152–159. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Seleem, W.M.; El-Kalla, F.; AbdAlkhalik Basha, M.; Abd-Elsalam, S. Efficacy of a non-invasive model in predicting the cardiovascular morbidity and histological severity in non-alcoholic fatty liver disease. Diabetes Metab. Syndr. 2019, 13, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- Qadri, S.; Yki-Järvinen, H. Surveillance of the liver in type 2 diabetes: Important but unfeasible? Diabetologia 2024, 67, 961–973. [Google Scholar] [CrossRef]
- Adams, L.A. Biomarkers of liver fibrosis. J. Gastroenterol. Hepatol. 2011, 26, 802–809. [Google Scholar] [CrossRef]
- Cheng, C.H.; Hao, W.R.; Cheng, T.H. Multifaceted role of haptoglobin: Implications for disease development. World J. Hematol. 2024, 11, 98807. [Google Scholar] [CrossRef]
- Alboraie, M.A.; Afifi, M.E.; Elghamry, F.G.; Shalaby, H.A.; Elshennawy, G.E.; Abdelaziz, A.A.; Shaheen, M.U.; Abo El-Seoud, A.R. Egy-score predicts severe hepatic fibrosis and cirrhosis in Egyptians with chronic liver diseases: A pilot study. Hepat. Mon. 2013, 13, e10810. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Seo, Y.S.; Um, S.H.; Won, N.H.; Yoo, H.; Jung, E.S.; Kwon, Y.D.; Park, S.; Keum, B.; Kim, Y.S.; et al. Usefulness of non-invasive markers for predicting significant fibrosis in patients with chronic liver disease. J. Korean Med. Sci. 2010, 25, 67–74. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Sun, W.; Ye, Y.; Wang, X. Proteomic identification network analysis of haptoglobin as a key regulator associated with liver fibrosis. Appl. Biochem. Biotechnol. 2013, 169, 832–846. [Google Scholar] [CrossRef] [PubMed]
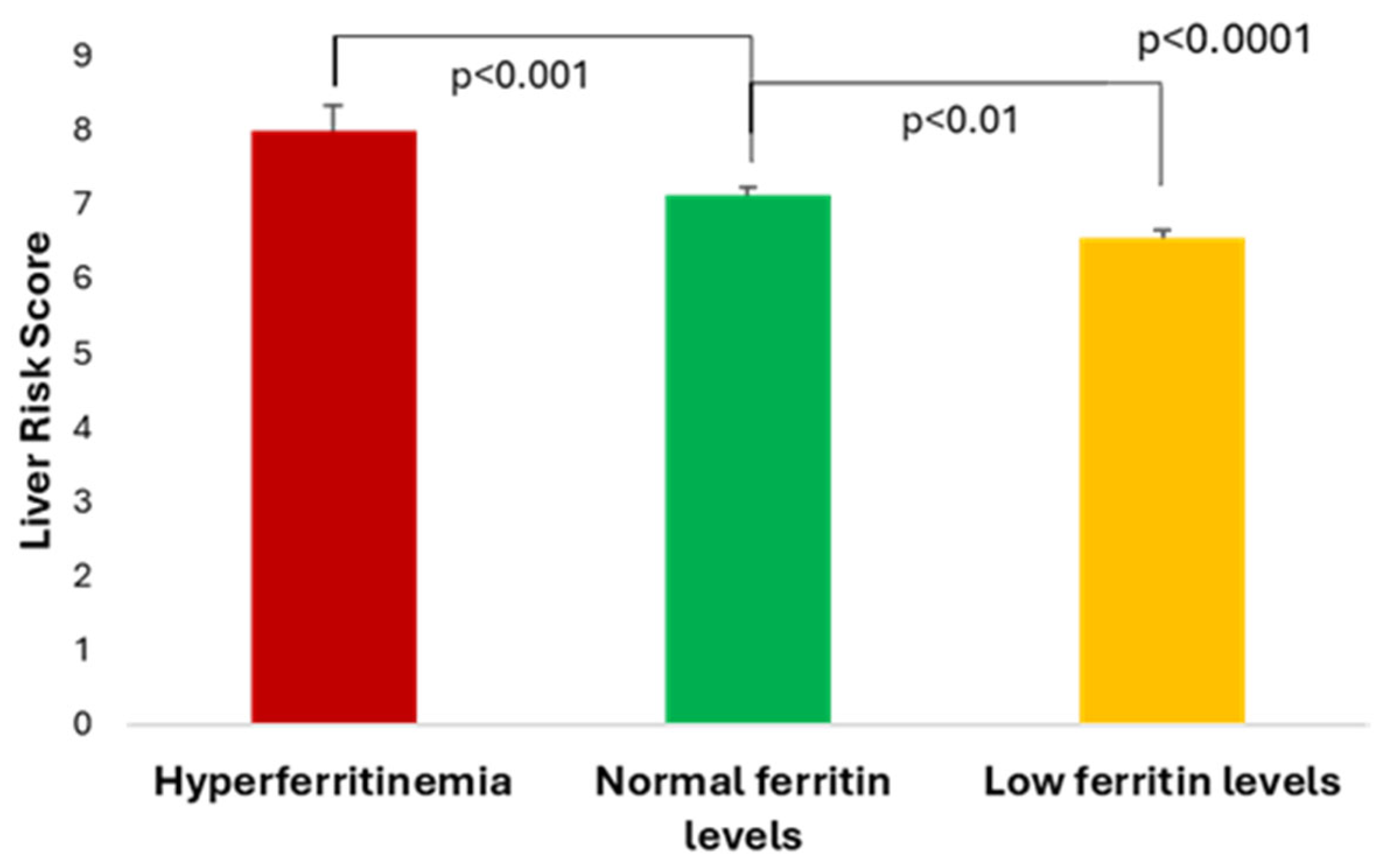
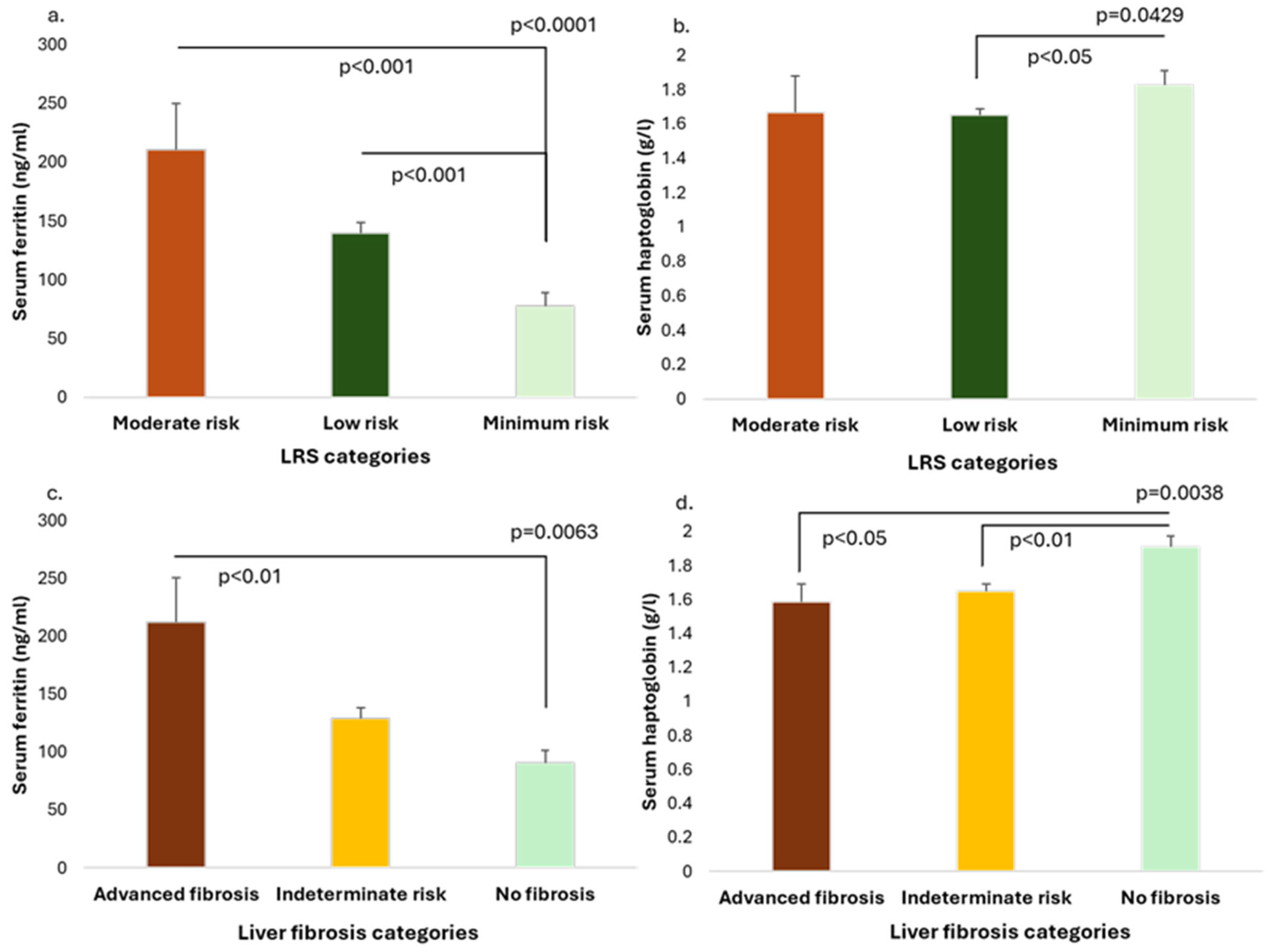
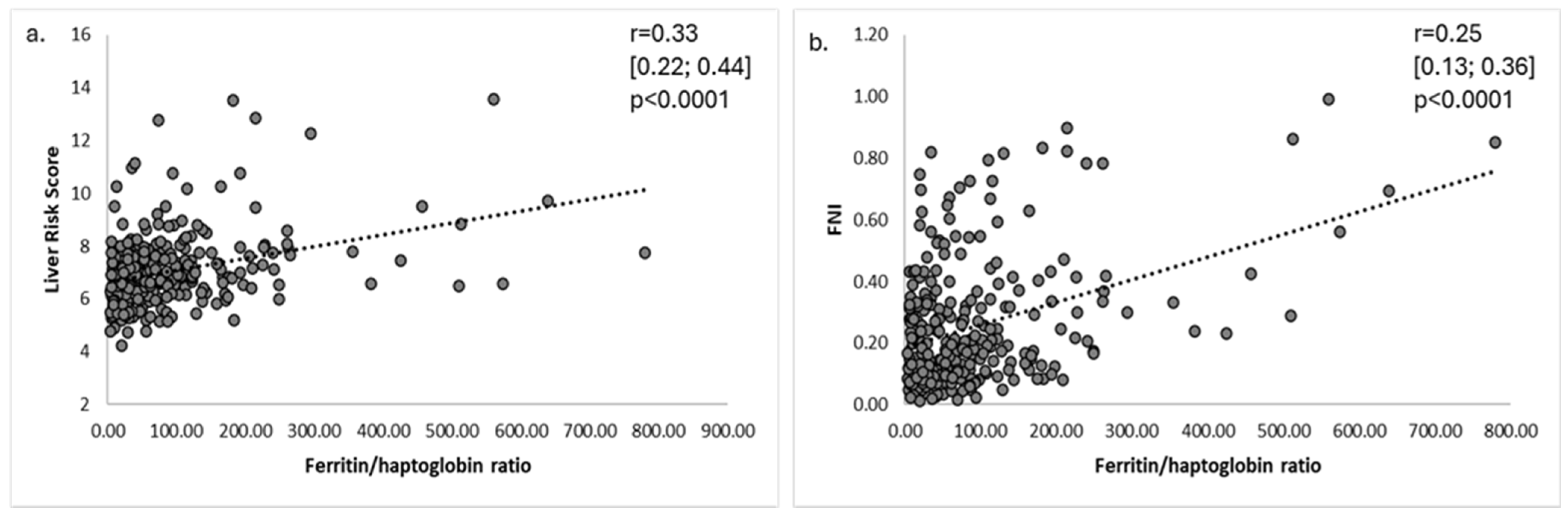
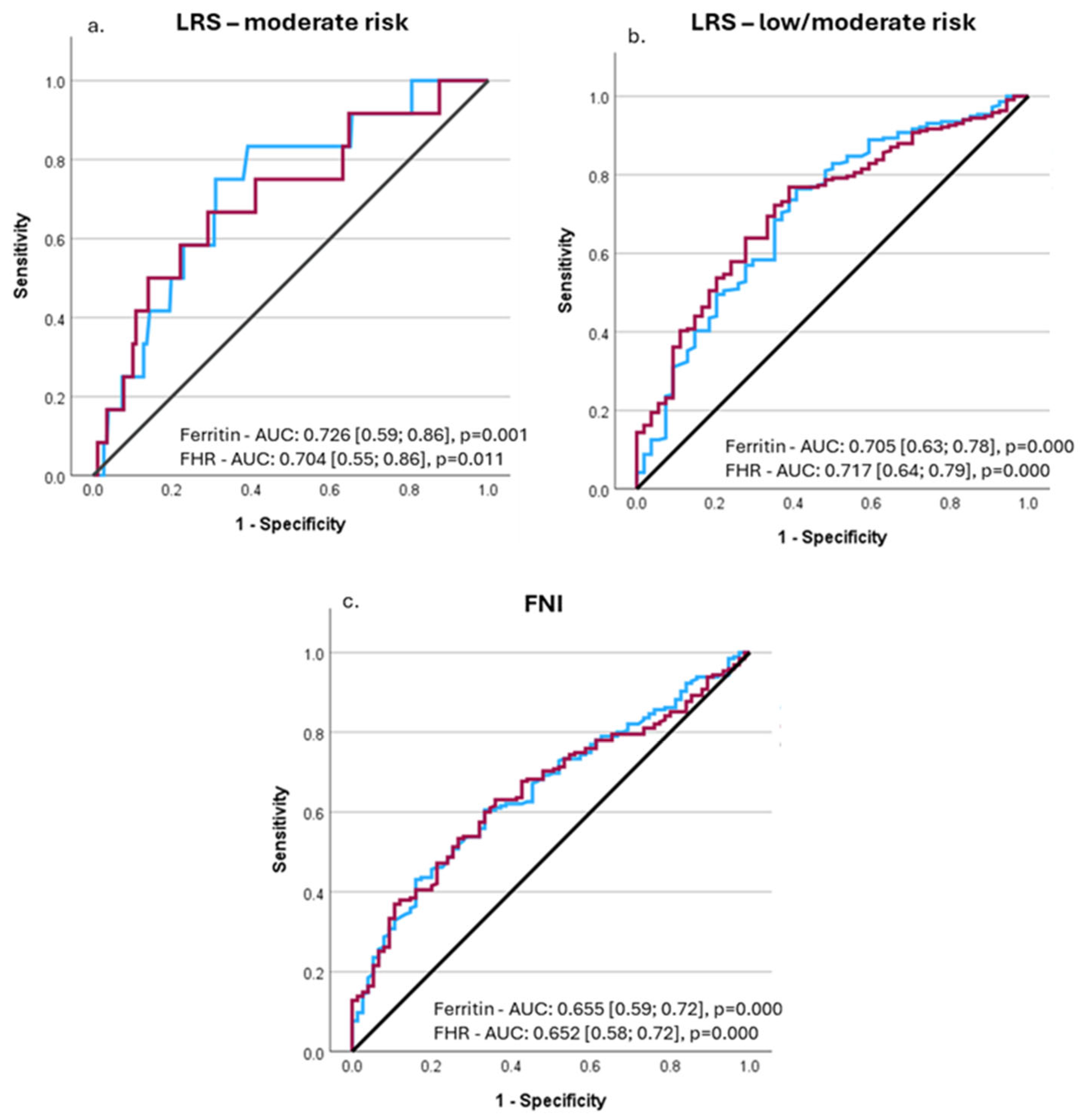
| Hyperferritinemia (>ULN) n = 34 | Normal Ferritin Levels (50-ULN) n = 163 | Low Ferritin Levels (<50 ng/mL) n = 74 | p | |
|---|---|---|---|---|
| Serum ferritin (ng/mL) | 335.0 [189.0] | 115.0 [80.3] | 26.84 ± 11.67 | <0.0001 |
| Clinical and anthropometric parameters | ||||
| Age (years) | 63.56 ± 8.93 | 66.0 [10.0] | 65.77 ± 8.40 | 0.4034 |
| Diabetes duration (years) | 8.03 ± 4.59 ** | 9.0 [6.0] * | 11.0 [5.0] *,** | 0.0029 |
| Sex (F/M) (number (%)) | 12 (35.3)/22 (64.7) | 79 (48.5)/84 (51.5) | 58 (78.4)/16 (21.6) | <0.0001 |
| Systolic BP (mmHg) | 134.94 ± 14.03 | 135.46 ± 15.88 | 133.75 [17.5] | 0.6402 |
| Diastolic BP (mmHg) | 84.10 ± 9.10 | 82.00 [12.50] | 80.0 [12.13] | 0.0725 |
| BMI (kg/m2) | 33.48 ± 5.17 | 33.76 [7.06] | 34.39 ± 6.13 | 0.7839 |
| AMMi | 0.79 [0.31] ** | 0.75 [0.29] ** | 0.67 [0.17] **,** | 0.0002 |
| Fat mass (kg) | 33.45 ± 10.67 | 32.09 [12.98] | 31.41 [14.22] | 0.8769 |
| WHtR | 0.68 ± 0.07 | 0.68 ± 0.07 | 0.69 ± 0.09 | 0.3274 |
| Waist circumference (cm) | 113.74 ± 10.24 | 111.91 ± 11.43 | 110.22 ± 12.72 | 0.3214 |
| Hip circumference (cm) | 109.21 ± 10.37 | 107.0 [14.00] | 109.51 ± 11.28 | 0.9082 |
| Laboratory parameters | ||||
| Fasting BG (mg/dL) | 137.95 [37.05] | 138.63 [30.59] | 135.35 [32.27] | 0.1591 |
| HbA1c (%) | 7.05 [0.80] * | 6.80 [0.9] * | 6.8 [0.85] | 0.0433 |
| Total cholesterol (mg/dL) | 153.02 [49.19] | 157.16 [45.97] | 150.42 (45.65] | 0.2878 |
| HDL cholesterol (mg/dL) | 44.57 ± 10.11 | 43.85 [11.45] | 47.22 ± 10.96 | 0.1593 |
| LDL cholesterol (mg/dL) | 88.41 ± 34.43 | 85.73 [39.37] | 77.32 [40.36] | 0.1750 |
| Triglycerides (mg/dL) | 132.38 [96.31] | 163.98 [98.34] * | 132.95 [77.36] * | 0.0289 |
| Uric acid (mg/dL) | 5.78 ± 1.49 | 5.89 [1.86] | 5.60 ± 1.37 | 0.1332 |
| C-peptide (ng/mL) | 3.98 ± 1.57 ** | 3.32 [2.07] ** | 2.79 ± 1.28 **,** | <0.0001 |
| HOMA-IR | 3.45 ± 1.51 *** | 2.82 [1.82] *** | 2.35 ± 1.09 ***,*** | <0.0001 |
| Albumin (g/dL) | 4.68 ± 0.25 | 4.66 ± 0.24 | 4.61 ± 0.21 | 0.4773 |
| ALAT (U/L) | 24.20 [27.18] *** | 18.81 [16.19] ***,*** | 13.69 [10.98] *** | <0.0001 |
| ASAT (U/L) | 27.54 [23.05] *,** | 20.79 [9.95] * | 19.08 [7.33] ** | 0.0024 |
| GGT (U/L) | 49.09 [44.28] *,*** | 29.90 [25.02] *,*** | 21.98 [20.92] ***,*** | <0.0001 |
| Direct bilirubin (mg/dL) | 0.23 ± 0.08 ** | 0.21 [0.10] *** | 0.16 [0.09] **,*** | 0.0001 |
| Haptoglobin (g/L) | 1.63 ± 0.59 | 1.64 ± 0.57 | 1.81 ± 0.65 | 0.1152 |
| SHBG (nmol/L) | 33.67 ± 14.44 | 32.00 [18.70] * | 37.45 [20.88] * | 0.0153 |
| Creatinine (mg/dL) | 0.88 ± 0.18 | 0.85 [0.27] * | 0.75 [0.25] * | 0.0078 |
| eGFR (ml/min/1.73m2) | 93.86 [66.20] | 89.96 [21.30] | 91.26 [27.58] | 0.5293 |
| Red blood cell count (106/μL) | 4.99 ± 0.44 * | 4.81 ± 0.52 | 4.70 ± 0.51 * | 0.0209 |
| Hemoglobin (g/dL) | 15.01 ± 1.41 *** | 14.44 ± 1.47 *** | 13.30 [1.80] ***,*** | <0.0001 |
| Hematocrit (%) | 45.24 ± 3.98 *** | 43.62 ± 4.28 *** | 41.35 ± 4.53 ***,*** | <0.0001 |
| MCV (fL) | 91.5 [7.98] ** | 90.9 [5.10] *** | 88.65 [4.80] **,*** | 0.0001 |
| MCH (pg) | 30.15 ± 2.55 *** | 30.11 ± 1.72 *** | 28.9 [2.13] ***,*** | <0.0001 |
| MCHC (g/dL) | 33.18 ± 0.65 ** | 33.1 [0.90] *** | 32.54 ± 0.92 **,*** | <0.0001 |
| Leucocyte count (103/μL) | 7.88 ± 1.76 | 7.53 ± 1.83 | 7.86 ± 2.0 | 0.3434 |
| Neutrophil count (103/μL) | 4.59 ± 1.39 | 4.28 [1.71] | 4.78 ± 1.60 | 0.4493 |
| Lymphocyte count (103/μL) | 2.51 ± 0.66 | 2.24 ± 0.69 | 2.32 ± 0.67 | 0.1130 |
| Monocyte count (103/μL) | 0.50 ± 0.15 | 0.45 [0.17] | 0.48 ± 0.13 | 0.5361 |
| Eosinophil count (103/μL) | 0.21 [0.15] | 0.18 [0.16] | 0.18 [0.18] | 0.6626 |
| Basophil count (103/μL) | 0.04 [0.03] | 0.04 [0.02] | 0.04 [0.02] | 0.5284 |
| Platelet count (103/μL) ^ | 226.97 ± 48.86 * | 228.28 ± 59.40 ** | 258.02 ± 75.95 *,** | 0.0028 |
| Inflammatory indexes | ||||
| NLR | 1.93 ± 0.67 | 1.96 [1.04] | 1.92 [1.17] | 0.4001 |
| SIII | 436.28 ± 174.28 | 431.63 [277.30] | 519.46 [326.18] | 0.0720 |
| SIRI | 0.97 ± 0.46 | 0.89 [0.58] | 0.91 [0.73] | 0.7737 |
| MLR | 0.21 ± 0.07 | 0.20 [0.10] | 0.20 [0.11] | 0.5533 |
| Liver steatosis and fibrosis indexes | ||||
| FLI | 91.60 [21.25] | 91.00 [18.6] | 81.60 [31.00] | 0.0399 |
| ION | 28.55 ± 14.68 *** | 21.17 [18.75] ***,*** | 15.42 ± 10.97 *** | <0.0001 |
| FIB-4 ^ | 1.52 [1.52] | 1.35 [0.83] | 1.31 [0.78] | 0.3450 |
| FNI | 0.33 [0.42] **,*** | 0.19 [0.23] ** | 0.15 [0.22] *** | 0.0006 |
| APRI ^ | 0.31 [0.37] *** | 0.26 [0.19] * | 0.21 [0.14] ***,* | 0.0023 |
| Parameter | r [95% CI] | p |
|---|---|---|
| Clinical and anthropometric parameters | ||
| Age (years) | 0.18 [−0.30; −0.06] | 0.0028 |
| Diabetes duration (years) | −0.29 [−0.40; −0.17] | <0.0001 |
| Diastolic BP (mmHg) | 0.18 [0.06; 0.30] | 0.0029 |
| Sex (F = 1/M = 2) | 0.41 [0.30; 0.50] | <0.0001 |
| Waist circumference (cm) | 0.16 [0.04; 0.28] | 0.0077 |
| WHR | 0.18 [0.06; 0.29] | 0.0034 |
| AMMi | 0.34 [0.23; 0.44] | <0.0001 |
| Laboratory parameters | ||
| C-peptide (ng/mL) | 0.28 [0.16; 0.39] | <0.0001 |
| HOMA-IR | 0.28 [0.16; 0.39] | <0.0001 |
| Albumin (g/dL) | 0.19 [0.07; 0.30] | 0.0021 |
| ASAT (U/L) | 0.26 [0.15; 0.37] | <0.0001 |
| ALAT (U/L) | 0.34 [0.23; 0.44] | <0.0001 |
| GGT (U/L) | 0.40 [0.29; 0.49] | <0.0001 |
| Direct bilirubin (mg/dL) | 0.27 [0.15; 0.38] | <0.0001 |
| SHBG (nmol/L) | −0.17 [−0.28; −0.05] | 0.0059 |
| Creatinine (mg/dL) | 0.22 [0.10; 0.34] | 0.0002 |
| Red blood cell count (106/μL) | 0.23 [0.11; 0.35] | 0.0001 |
| Hemoglobin (g/dL) | 0.40 [0.30; 0.50] | <0.0001 |
| Hematocrit (%) | 0.36 [0.24; 0.46] | <0.0001 |
| MCV (fL) | 0.22 [0.10; 0.34] | 0.0002 |
| MCH (pg) | 0.31 [0.20; 0.42] | <0.0001 |
| MCHC (g/dL) | 0.34 [0.23; 0.44] | <0.0001 |
| Platelet count (103/μL) | −0.18 [−0.30; −0.06] | 0.0028 |
| SIII | −0.13 [−0.25; −0.01] | 0.0293 |
| Liver steatosis and fibrosis markers | ||
| FLI | 0.19 [0.07; 0.31] | 0.0015 |
| ION | 0.31 [0.20; 0.42] | <0.0001 |
| FNI | 0.26 [0.14; 0.37] | <0.0001 |
| APRI | 0.23 [0.11; 0.34] | 0.0001 |
| Liver Risk Score | 0.32 [0.20; 0.42] | <0.0001 |
| Parameter | Standardized β coefficient (SE) | 95% CI | t Ratio | p |
|---|---|---|---|---|
| a. Serum ferritin (R2: 0.344, p < 0.001) | ||||
| Diabetes duration | −0.140 (1.4426) | −6.441; −0.825 | −2.548 | 0.011 |
| ASAT | 0.204 (0.915) | 0.363; 3.968 | 2.366 | 0.019 |
| GGT | 0.129 (0.178) | 0.036; 0.737 | 2.169 | 0.031 |
| Hemoglobin | 0.165 (5.163) | 3.386; 23.722 | 2.625 | 0.009 |
| AMMi | 0.139 (48.304) | 6.674; 196.925 | 2.107 | 0.036 |
| b. Liver Risk Score (R2: 0.400, p < 0.001) | ||||
| Ferritin | 0.203 (0.001) | 0.001; 0.003 | 3.783 | <0.001 |
| Direct bilirubin | 0.207 (0.757) | 1.448; 4.428 | 3.883 | <0.001 |
| Haptoglobin | −0.145 (0.120) | −0.581; −0.109 | −2.875 | 0.004 |
| HbA1c | 0.437 (0.089) | 0.574; 0.923 | 8.431 | <0.001 |
| BMI | 0.195 (0.014) | 0.024; 0.080 | 3.673 | 0.001 |
| AMMi | 0.112 (0.461) | 0.008; 1.823 | 1.986 | 0.048 |
| c. FNI (R2: 0.329, p < 0.001) | ||||
| Ferritin | 0.198 (0.000) | 0.000; 0.001 | 3.367 | <0.001 |
| Haptoglobin | −0.131 (0.018) | −0.080; −0.010 | 2.507 | 0.013 |
| HOMA-IR | 0.122 (0.009) | 0.000; 0.035 | 1.996 | 0.047 |
| GGT | 0.305 (0.000) | 0.001; 0.002 | 5.454 | <0.001 |
| d. ION (R2: 0.272; p < 0.001) | ||||
| Age | −0.229 (0.113) | −0.628; −0.183 | −3.583 | <0.001 |
| Ferritin | 0.189 (0.007) | 0.008; 0.036 | 3.126 | 0.002 |
| BMI | 0.328 (0.160) | 0.599; 1.227 | 5.719 | <0.001 |
| eGFR | −0.306 (0.054) | −0.372; −0.157 | −4.855 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernea, S.; Roiban, A.L.; Onișor, D. Hyperferritinemia and the Risk of Liver Fibrosis and Liver-Related Events in Patients with Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease. Medicina 2025, 61, 1518. https://doi.org/10.3390/medicina61091518
Cernea S, Roiban AL, Onișor D. Hyperferritinemia and the Risk of Liver Fibrosis and Liver-Related Events in Patients with Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease. Medicina. 2025; 61(9):1518. https://doi.org/10.3390/medicina61091518
Chicago/Turabian StyleCernea, Simona, Andrada Larisa Roiban, and Danusia Onișor. 2025. "Hyperferritinemia and the Risk of Liver Fibrosis and Liver-Related Events in Patients with Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease" Medicina 61, no. 9: 1518. https://doi.org/10.3390/medicina61091518
APA StyleCernea, S., Roiban, A. L., & Onișor, D. (2025). Hyperferritinemia and the Risk of Liver Fibrosis and Liver-Related Events in Patients with Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease. Medicina, 61(9), 1518. https://doi.org/10.3390/medicina61091518






