The Use of Serum Scoring Systems in Predicting Liver Fibrosis Caused by Chronic Hepatitis B: A Retrospective Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Data Collection
2.4. Evaluation of Liver Fibrosis
2.5. Noninvasive Serum Scoring
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries; WHO Press: Geneva, Switzerland, 2024. Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 27 March 2025).
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Karacaer, Z.; Avci, Ö.; Karadağ, F.Y. King’s Score may be More Effective in the Determination of Severe Fibrosis in Chronic Hepatitis B Infections. Viral Hepat. Derg. 2017, 23, 20–25. (In Turkish) [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Forns, X.; Ampurdanès, S.; Llovet, J.M.; Aponte, J.; Quintó, L.; Martínez-Bauer, E.; Bruguera, M.; Sánchez-Tapias, J.M.; Rodés, J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002, 36 Pt 1, 986–992. [Google Scholar] [CrossRef]
- Yin, Z.; Zou, J.; Li, Q.; Chen, L. Diagnostic value of FIB-4 for liver fibrosis in patients with hepatitis B: A meta-analysis of diagnostic test. Oncotarget 2017, 8, 22944–22953. [Google Scholar] [CrossRef]
- Kotak, P.S.; Kumar, J.; Kumar, S.; Varma, A.; Acharya, S. Navigating Cirrhosis: A Comprehensive Review of Liver Scoring Systems for Diagnosis and Prognosis. Cureus 2024, 16, e57162. [Google Scholar] [CrossRef]
- El-Kassas, M.; Elakel, W.; Elsharkawy, A.; Asem, N.; Abu-Elfatth, A.; Mostafa, A.; Abdelazeem, A.; El-Serafy, M.; Ibrahem, M.; Ghanem, E.A.; et al. Comparison of different noninvasive scores for assessing hepatic fibrosis in a cohort of chronic hepatitis C patients. Sci. Rep. 2024, 14, 29544. [Google Scholar] [CrossRef]
- Bonacini, M.; Hadi, G.; Govindarajan, S.; Lindsay, K.L. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am. J. Gastroenterol. 1997, 92, 1302–1304. [Google Scholar] [PubMed]
- Okdemir, S.; Cakmak, E. A novel non-invasive score for the prediction of advanced fibrosis in patients with chronic hepatitis B. Ann. Hepatol. 2022, 27, 100544. [Google Scholar] [CrossRef]
- Ekin, N.; Ucmak, F.; Ebik, B.; Tuncel, E.T.; Kacmaz, H.; Arpa, M.; Atay, A.E. GPR, King’s Score and S-Index are superior to other non-invasive fibrosis markers in predicting the liver fibrosis in chronic Hepatitis B patients. Acta Gastro Enterol. Belg. 2022, 85, 62–68. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Wei, S.; Chen, X.; Zhu, H.; Liantang, Y.; Bao, R.; Du, Y. Noninvasive models for the prediction of liver fibrosis in patients with chronic hepatitis B. BMC Gastroenterol. 2024, 24, 183. [Google Scholar] [CrossRef]
- Gudowska, M.; Gruszewska, E.; Panasiuk, A.; Cylwik, B.; Świderska, M.; Flisiak, R.; Szmitkowski, M.; Chrostek, L. Selected Noninvasive Markers in Diagnosing Liver Diseases. Lab. Med. 2016, 47, 67–72. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Yan, X.; Li, M.; Xia, J.; Liu, Y.; Chen, Y.; Jia, B.; Zhu, L.; Zhu, C.; et al. Albumin-Bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig. Liver Dis. 2019, 51, 1172–1178. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Javed, F.T.; Gull, S.; Kausar, H.; Sarwar, M.T.; Asad, S.; Shahid, I.; Sumrin, A.; Khaliq, S.; et al. A comparison of four fibrosis indexes in chronic HCV: Development of new fibrosis-cirrhosis index (FCI). BMC Gastroenterol. 2011, 11, 44. [Google Scholar] [CrossRef]
- Gudowska, M.; Wrona, A.; Gruszewska, E.; Panasiuk, A.; Cylwik, B.; Swiderska, M.; Filisiak, R.; Szmitkowski, M.; Chrostek, L. Simple non-invasive markers for early diagnosis and determination of the severity of liver diseases. Clin. Exp. Hepatol. 2016, 4, 149–154. [Google Scholar] [CrossRef]
- Qi, X.; An, M.; Wu, T.; Jiang, D.; Peng, M.; Wang, W.; Wang, J.; Zhang, C.; Chess Study Group OBOT. Transient Elastography for Significant Liver Fibrosis and Cirrhosis in Chronic Hepatitis B: A Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2018, 2018, 3406789. [Google Scholar] [CrossRef]
- Xu, X.Y.; Kong, H.; Song, R.X.; Zhai, Y.H.; Wu, X.F.; Ai, W.S.; Liu, H.B.; Mehta, A.S. The effectiveness of noninvasive biomarkers to predict hepatitis B-related significant fibrosis and cirrhosis: A systematic review and meta-analysis of diagnostic test accuracy. PLoS ONE 2014, 9, e100182. [Google Scholar] [CrossRef] [PubMed]
- Bera, C.; Hamdan-Perez, N.; Patel, K. Non-Invasive Assessment of Liver Fibrosis in Hepatitisn B Patients. J. Clin. Med. 2024, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- Medhioub, M.; Ben, S.W.; Khsiba, A.; Ouni, A.; Hamzaoui, L.; Azouz, M.M. Performance of FIB4 and APRI scores for the prediction of fibrosis in patients with chronic hepatitis B virus infection. Tunis. Med. 2020, 98, 998–1004. [Google Scholar] [PubMed]
- Teshale, E.; Lu, M.; Rupp, L.B.; Holmberg, S.D.; Moorman, A.C.; Spradling, P.; Vijayadeva, V.; Boscarino, J.A.; Schmidt, M.A.; Gordon, S.C.; et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: The Chronic Hepatitis Cohort Study (CHeCS). J. Viral Hepat. 2014, 21, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Rungta, S.; Kumari, S.; Verma, K.; Akhtar, G.; Deep, A., Sr.; Swaroop, S. A Comparative Analysis of the APRI, FIB4, and FibroScan Score in Evaluating the Severity of Chronic Liver Disease in Chronic Hepatitis B Patients in India. Cureus 2021, 13, e19342. [Google Scholar] [CrossRef]
- Wang, H.W.; Peng, C.Y.; Lai, H.C.; Su, W.P.; Lin, C.H.; Chuang, P.H.; Chen, S.H.; Chen, C.H.; Hsu, W.F.; Huang, G.T. New noninvasive index for predicting liver fibrosis in Asian patients with chronic viral hepatitis. Sci. Rep. 2017, 7, 3259. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, C.F.; Zhao, Y.P.; Liu, H.L.; Zheng, R.D.; Xian, J.C.; Xu, H.T.; Mao, Y.M.; Zeng, M.D.; Lu, L.G. Simpler score of routine laboratory tests predicts liver fibrosis in patients with chronic hepatitis B. J. Gastroenterol. Hepatol. 2010, 25, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Hasegawa, K.; Ishii, A.; Takata, R.; Enomoto, H.; Yoh, K.; Kishino, K.; Shimono, Y.; Iwata, Y.; Nakano, C.; et al. A proposed predictive model for advanced fibrosis in patients with chronic hepatitis B and its validation. Medicine 2016, 95, e4679. [Google Scholar] [CrossRef]
- Coskun, B.D.; Altinkaya, E.; Sevinc, E.; Ozen, M.; Karaman, H.; Karaman, A.; Poyrazoglu, O. The diagnostic value of a globulin/platelet model for evaluating liver fibrosis in chronic hepatitis B patients. Rev. Esp. de Enfermedades Dig. 2015, 107, 740–744. [Google Scholar] [CrossRef]
- Vu, H.; Huy, D.; Minh Nhut, B.N.; Quan, P.A.; Quyen, N.Q.; Thu Thuy, T.T.; Khanh Tuong, T.T.; Minh Duc, N. The value of APGA score, fibrosis index for diagnosing liver fibrosis in patients with chronic hepatitis B. La Clin. Ter. 2024, 175, 137–145. [Google Scholar] [CrossRef]
- Zhu, W.W.; Guo, J.J.; Guo, L.; Jia, H.L.; Zhu, M.; Zhang, J.B.; Loffredo, C.A.; Forgues, M.; Huang, H.; Xing, X.J.; et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 3944–3954. [Google Scholar] [CrossRef]
- Zhang, T.T.; Ye, S.S.; Liang, J.; Bai, L. Prognostic value of non-invasive fibrosis indices post-curative resection in hepatitis-B-associated hepatocellular carcinoma patients. Exp. Biol. Med. 2020, 245, 703–710. [Google Scholar] [CrossRef]
- Imai, H.; Kamei, H.; Onishi, Y.; Ishizu, Y.; Ishigami, M.; Goto, H.; Ogura, Y. Diagnostic usefulness of APRI and FIB-4 for the prediction of liver fibrosis after liver transplantation in patients infected with hepatitis C virus. Transplant. Proc. 2018, 50, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Wang, J.; Song, X.; Kang, L.; Lin, J.; Hu, Q.; Sun, W.; Gao, Y. Development and validation of a nomogram for predicting advanced liver fibrosis in patients with chronic hepatitis B. Front. Mol. Biosci. 2024, 11, 1452841. [Google Scholar] [CrossRef]
- Kaya, A.; Barutcu, S.; Gulsen, M.T. Evaluation of fibrosis with noninvasive biochemical tests in chronic viral hepatitis B. Hepatol. Forum 2023, 4, 25–29. [Google Scholar] [CrossRef]
- Şahin, T.; Süleymanlar, İ. Evaluation of Liver Fibrosis by Ten Noninvasive Methods in Evaluation of Liver Fibrosis by Ten Noninvasive Methods in Patients with Chronic Hepatitis B: A Comparative Study. Akdeniz Med. J. 2018, 4, 18–24. (In Turkish) [Google Scholar] [CrossRef]
- Eminler, A.T.; Ayyildiz, T.; Irak, K.; Kiyici, M.; Gurel, S.; Dolar, E.; Gulten, M.; Nak, S.G. AST/ALT ratio is not useful in predicting the degree of fibrosis in chronic viral hepatitis patients. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1361–1366. [Google Scholar] [CrossRef]
- Kalkan, Ç.; Yılmaz, Y.; Erdoğan, B.D.; Savaş, B.; Yurdcu, E.; Çalışkan, A.; Keskin, O.; Gencdal, G.; Zeybel, M.; Törüner, M.; et al. Non-invasive fibrosis markers for assessment of liver fibrosis in chronic hepatitis delta. J. Viral Hepat. 2023, 30, 406–416. [Google Scholar] [CrossRef]
- Ö. zçelik, A.; Çelik, M.; Şahin, A.; Ceylan, M.R.; Güler Dincer, N. Comparison of Invasive and Non-Invasive Liver Fibrosis Indicators in Chronic Hepatitis B Patients. J. Harran Univ. Med. Fac. 2024, 21, 533–539. (In Turkish) [Google Scholar]
- Pramono, L.K.; Tjandrawati, A.; Turbawaty, D.K.; Rostini, T.; Bestari, M.B.; Haryono; Budiman, D.; Nugraha, P.; Uhlmann, D. Macrophage-2-Binding Protein Glycosylation Isomer (M2BPGi) and AGAP Score as Markers of Noninvasive Test for Liver Fibrosis versus FibroScan in Chronic Hepatitis B Patients: A Retrospective Observational Study. Int. J. Hepatol. 2024, 2024, 6635625. [Google Scholar] [CrossRef]
- Hamidi, A.A.; Oncul, A.; Ozguven, B.Y.; Sevgi, D.Y.; Gunduz, A.; Uzun, N.; Dokmetas, I. Diagnostic accuracy of different noninvasive scores for detecting advanced fibrosis in chronic hepatitis B. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Lu, W.; Zhou, X.; Huang, D.; Wang, Y.; Li, X.; Yan, L.; Lin, W.; Song, S.; Zhang, Z.; et al. A Novel Non-invasive Model Based on GPR for the Prediction of Liver Fibrosis in Patients With Chronic Hepatitis B. Front. Med. 2021, 8, 727706. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, P.J. Classification of chronic viral hepatitis: A need for reassessment. J. Hepatol. 1991, 13, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Dilcan, K.H.; Gozdas, H.T. Noninvasive evaluation of significant liver fibrosis in chronic hepatitis B patients. Acta Gastro Enterol. Belg. 2024, 87, 388–392. [Google Scholar] [CrossRef]
- Erdogan, S.; Dogan, H.O.; Sezer, S.; Uysal, S.; Ozhamam, E.; Kayacetin, S.; Koca, Y. The diagnostic value of non-invasive tests for the evaluation of liver fibrosis in chronic hepatitis B patients. Scand. J. Clin. Lab. Investig. 2013, 73, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Q.; Wu, Z.; Zhao, H.; Wang, G.Q. China HepB-Related Fibrosis Assessment Research Group Evaluation and comparison of thirty noninvasive models for diagnosing liver fibrosis in chinese hepatitis B patients. J. Viral Hepat. 2019, 26, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Ozyalvacli, G.; Kucukbayrak, A.; Kurt, M.; Gurel, K.; Gunes, O.; Ustun, C.; Akdeniz, H. Non-invasive fibrosis tests are correlated with necroinflammatory activity of liver in patients with chronic hepatitis B. Clin. Ter. 2014, 165, e199–e204. [Google Scholar]
- Kang, N.L.; Zhang, J.M.; Lin, M.X.; Chen, X.D.; Huang, Z.X.; Zhu, Y.Y.; Liu, Y.R.; Zeng, D.W. Serum ceruloplasmin can predict liver fibrosis in hepatitis B virus-infected patients. World J. Gastroenterol. 2020, 26, 3952–3962. [Google Scholar] [CrossRef]
- Tag-Adeen, M.; Omar, M.Z.; Abd-Elsalam, F.M.; Hasaneen, A.; Mohamed, M.A.; Elfeky, H.M.; Said, E.M.; Abdul-Aziz, B.; Osman, A.H.; Ahmed, E.S.; et al. Assessment of liver fibrosis in Egyptian chronic hepatitis B patients: A comparative study including 5 noninvasive indexes. Medicine 2018, 97, e9781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Y.; Yu, P.; Liu, Y.; Mei, M.; Bian, Z.; Shao, W.; Lv, J.; Li, X.; Lu, W.; et al. Retrospective evaluation of non-invasive assessment based on routine laboratory markers for assessing advanced liver fibrosis in chronic hepatitis B patients. Int. J. Gen. Med. 2022, 15, 5159–5171. [Google Scholar] [CrossRef]
- Köse, Ş.; Şentürk, H.; Tatar, G.; Bayram, A.; Öztürk, R. Pentraxin-3: A novel marker for indicating liver fibrosis in chronic hepatitis B patients. Turk. J. Gastroenterol. 2021, 32, 731–738. [Google Scholar] [CrossRef]
- Tsuji, Y.; Namisaki, T.; Kaji, K.; Takaya, H.; Nakanishi, K.; Sato, S.; Saikawa, S.; Sawada, Y.; Kitagawa, K.; Shimozato, N.; et al. Comparison of serum fibrosis biomarkers for diagnosing significant liver fibrosis in patients with chronic hepatitis B. Exp. Ther. Med. 2020, 20, 985–995. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, J.; Huang, C.; Wang, X.; Xu, Z. A noninvasive diagnostic model for significant liver fibrosis in patients with chronic hepatitis B based on CHI3L1 and routine clinical indicators. Ann. Palliat. Med. 2021, 10, 5509–5519. [Google Scholar] [CrossRef]
- Dong, H.; Xu, C.; Zhou, W.; Liao, Y.; Cao, J.; Li, Z.; Hu, B. The combination of 5 serum markers compared to FibroScan to predict significant liver fibrosis in patients with chronic hepatitis B virus. Clin. Chim. Acta 2018, 483, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Purnak, T.; Beyazit, Y.; Oztas, E.; Yesil, Y.; Efe, C.; Torun, S.; Celik, T.; Tenlik, I.; Kurt, M.; Ozaslan, E. Serum angiotensin-converting enzyme level as a marker of fibrosis in patients with chronic hepatitis B. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 244–249. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Yan, L.; Zhao, H.; Wang, G.; China HepB-Related Fibrosis Assessment Research Group. Serum platelet-derived growth factor BB levels: A potential biomarker for the assessment of liver fibrosis in patients with chronic hepatitis B. Int. J. Infect. Dis. 2016, 49, 94–99. [Google Scholar] [CrossRef]
- Johnson, G.; Moore, S.W. Why has butyrylcholinesterase been retained? Structural and functional diversification in a duplicated gene. Neurochem. Int. 2012, 61, 783–797. [Google Scholar] [CrossRef]
- Andersson, M.L.; Møller, A.M.; Wildgaard, K. Butyrylcholinesterase deficiency and its clinical importance in anaesthesia: A systematic review. Anaesthesia 2019, 74, 518–528. [Google Scholar] [CrossRef]
- Pohanka, M. Butyrylcholinesterase as a biochemical marker. Bratisl. Lek. Listy 2013, 114, 726–734. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
| Patients | Mean or n (%) |
|---|---|
| Age | 43 ± 13 (21–77) |
| 45.8 ± 13.5 |
| 42.1 ± 12.4 |
| Sex | |
| 111 (44.5) |
| 138 (55.5) |
| Fibrosis | 249 |
| 200 (80.3) |
| 44 (17.7) |
| 5 (2) |
| Histological activity İndex (HAI) | 249 |
| 39 (15.6) |
| 141 (56.7) |
| 60 (24) |
| 9 (3.6) |
| Platelet count (109/L) | 239.61 ± 59.34 |
| 262.22 ± 64.35 |
| 218.33 ± 45.07 |
| AST (U/L) | 26.64 ± 13.39 |
| 23.23 ± 6.16 |
| 29.79 ± 16.89 |
| ALT (U/L) | 30.17 ± 38.75 |
| 20.02 ± 9.02 |
| 39.55 ± 51.39 |
| Total bilurubin (mg/dL) | 0.63 ± 0.33 |
| 0.53 ± 0.21 |
| 0.73 ± 0.40 |
| Albumin (g/L) | 41.76 ± 2.7 |
| 41.42 ± 2.60 |
| 42.07 ± 2.91 |
| INR | 1.04 ±0.08 |
| 1.04 ± 0.09 |
| 1.05 ± 0.08 |
| ALP (U/L) | 77.28 ± 24.90 |
| 72.97 ± 22.32 |
| 81.56 ± 26.73 |
| GGT (U/L) | 23.60 ± 22.69 |
| 18.27 ± 13.54 |
| 28.76 ± 28.04 |
| Cholesterol (mg/dL) (in 53 Patients) | 176.34 ± 34.70 |
| 186.04 ± 35.67 |
| 167.68 ± 31.99 |
| AFP μ/L | 3.85 ± 7.96 |
| 4.21 ± 11.1 |
| 3.52 ± 2.72 |
| HBV DNA IU/mL | 64,142.741 ± 301,427.605 |
| 40,721.124 ± 174,142.665 |
| 83,288.983 ± 374,377.896 |
| HBeAg negative | 230 (92.4) |
| 110 (48) |
| 120 (52) |
| HBeAg positive | 19 (7.6) |
| 10 (54) |
| 9 (46) |
| Scoring Method (Number of Calculated Case) | Mean ± SD | Median (Min–Max)/% |
|---|---|---|
| APRI (204) | 0.265 ± 0.15 | 0.225 (0.092–1.111) |
| LOK (198) | 0.351 ± 0.192 | 0.33 (0.05–1.1) |
| FORNS (54) | 4.028 ± 1.726 | 4.09 (1–8.98) |
| FIB-4 (204) | 1.053 ± 0.564 | 0.912 (0.287–3.5) |
| FI (140) | −36.074 ± 2.768 | −36.32 (−42.97–−25.72) |
| FIBROALPHA (249) | 1.287 ± 0.143 | 1.302 (0.872–2.125) |
| KING (202) | 5.408 ± 3.865 | 4.525 (0–26.067) |
| BONACINI (198) | 4.232 ± 1.216 | 4 (1–7) |
| AGAP (192) | 0.805 ± 1.762 | 0.343 (0.043–15.942) |
| GPR (192) | 0.2 ± 0.2 | 0.2 (0.1–1.8) |
| AAR (205) | 1.127 ± 0.424 | 1.09 (0.21–3.3) |
| GUCI (200) | 0.274 ± 0.164 | 0.242 (0–1.278) |
| ALBI (139) | −2.881 ± 0.325 | −2.913 (−3.729–−0.95) |
| FCI (128) | 0.094 ± 0.099 | 0.069 (0–0.855) |
| FIBRO-Q (200) | 2.393 ± 1.525 | 2.024 (0–8.254) |
| SINDEX (139) | 0.066 ± 0.084 | 0.045 (0–0.603) |
| FIBROSIS | ||
| ISHAK 0–2 | 200 | 80.3 |
| ISHAK 3–4 | 44 | 17.7 |
| ISHAK 5–6 | 5 | 2 |
| FIBROSIS | ||
| ISHAK < 3 | 200 | 80.3 |
| ISHAK ≥ 3 | 49 | 19.7 |
| ISHAK < 3 | ISHAK ≥ 3 | Total | Test Statistics | p Value | |
|---|---|---|---|---|---|
| APRI | 0.22 (0.092–0.662) | 0.299 (0.101–1.111) | 0.225 (0.092–1.111) | 1810.000 | <0.001 x |
| LOK | 0.29 (0.05–1.1) | 0.45 (0.1–0.89) | 0.33 (0.05–1.1) | 2228.000 | 0.002 x |
| FORNS | 3.68 ± 1.497 | 4.787 ± 1.983 | 4.028 ± 1.726 | −2.274 | 0.027 y |
| FIB4 | 0.833 (0.287–2.577) | 1.357 (0.337–3.5) | 0.912 (0.287–3.5) | 1773.000 | <0.001 x |
| FI | −36.306 ± 2.651 | −35.321 ± 3.041 | −36.074 ± 2.768 | −1.801 | 0.074 y |
| FIBROALPHA | 1.29 (0.872–1.846) | 1.35 (1.02–2.125) | 1.302 (0.872–2.125) | 3498.000 | 0.002 x |
| KING | 4.137 (0–15.639) | 7.159 (2.112–26.067) | 4.525 (0–26.067) | 1488.000 | <0.001 x |
| BONACINI | 4 (1–6) | 5 (1–7) | 4 (1–7) | 2186.000 | 0.001 x |
| AGAP | 0.307 (0.043–8.377) | 0.594 (0.072–15.942) | 0.343 (0.043–15.942) | 1384.000 | <0.001 x |
| GPR | 0.2 (0.1–1.5) | 0.2 (0.1–1.8) | 0.2 (0.1–1.8) | 1761.000 | <0.001 x |
| AAR | 1.07 (0.21–3.3) | 1.135 (0.39–1.83) | 1.09 (0.21–3.3) | 3380.500 | 0.903 x |
| GUCI | 0.217 (0–0.695) | 0.306 (0.117–1.278) | 0.242 (0–1.278) | 1642.000 | <0.001 x |
| ALBI | −2.922 (−3.729–−0.95) | −2.824 (−3.385–−2.213) | −2.913 (−3.729–−0.95) | 1377.000 | 0.066 x |
| FCI | 0.062 (0–0.366) | 0.092 (0–0.855) | 0.069 (0–0.855) | 1062.500 | 0.009 x |
| FIBROQ | 1.883 (0–7.512) | 3.056 (0.415–8.254) | 2.024 (0–8.254) | 2225.000 | 0.002 x |
| SINDEX | 0.04 (0–0.302) | 0.056 (0–0.603) | 0.045 (0–0.603) | 1046.000 | <0.001 x |
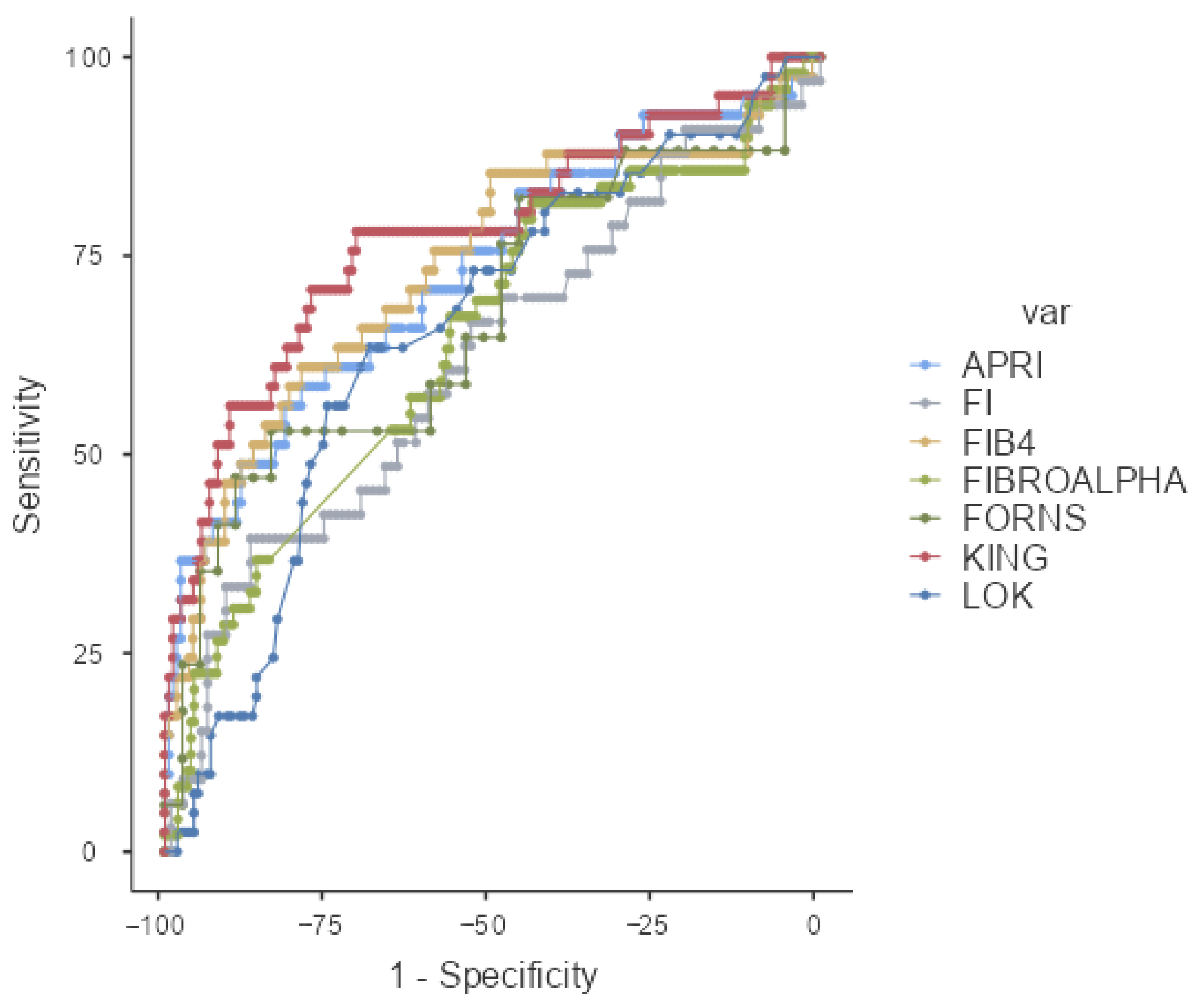
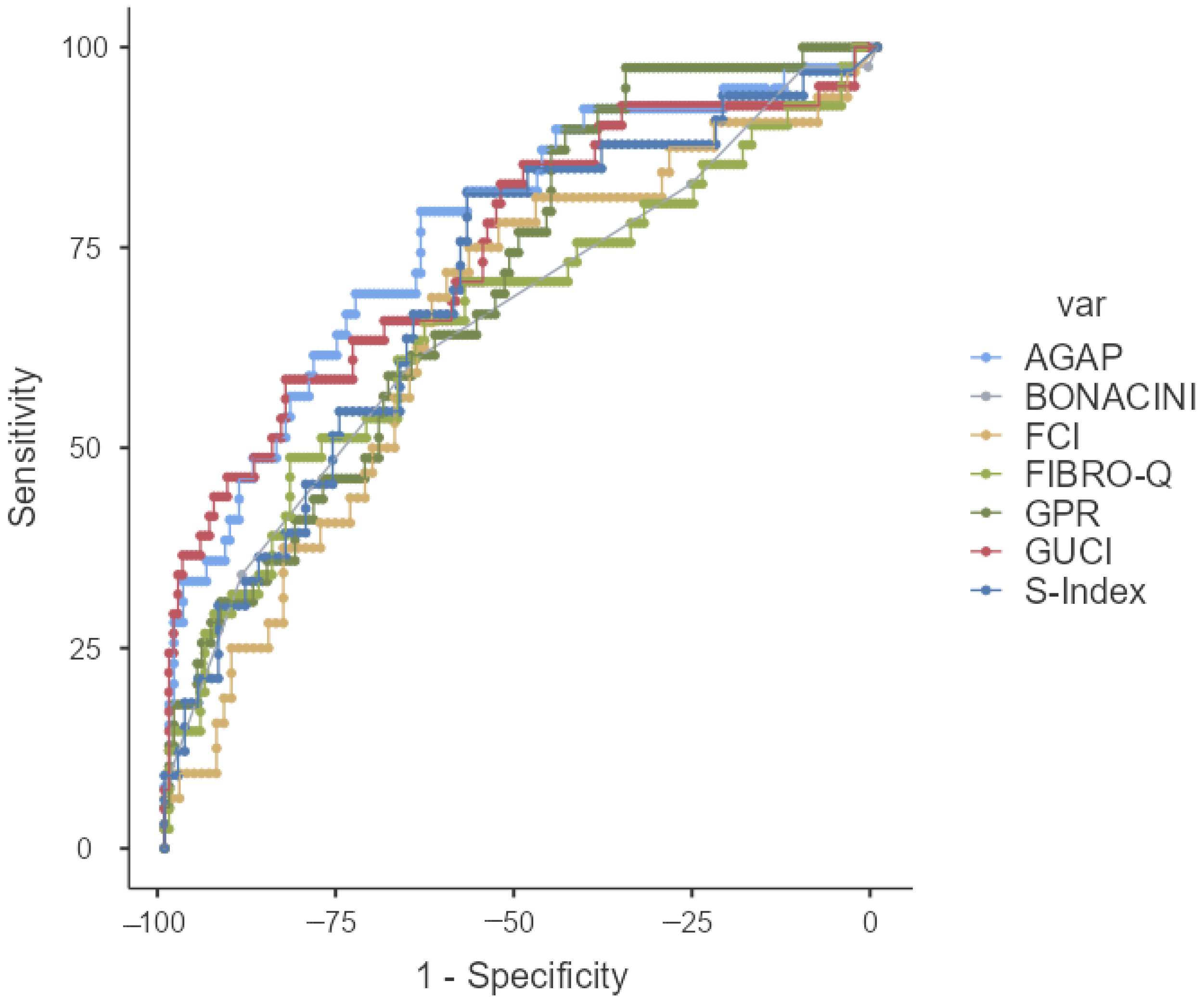
| AUC (%95 CI) | p | Cut-Off | Sensitivity% | Specificity% | PPV% | NPV% | |
|---|---|---|---|---|---|---|---|
| APRI | 0.729 (0.634–0.824) | <0.001 | ≥0.29 | 56.10 | 81.60 | 43.40 | 88.08 |
| LOK | 0.654 (0.560–0.747) | 0.002 | ≥0.39 | 63.41 | 68.79 | 34.67 | 87.80 |
| FORNS | 0.673 (0.506–0.841) | 0.042 | ≥4.91 | 52.94 | 83.78 | 60.00 | 79.49 |
| FIB4 | 0.735 (0.639–0.831) | <0.001 | ≥1.23 | 60.98 | 79.14 | 42.37 | 88.97 |
| FI | 0.619 (0.503–0.734) | 0.040 | ≥−33.58 | 39.39 | 86.92 | 48.15 | 82.30 |
| FIBROALPHA | 0.643 (0.553–0.734) | 0.002 | ≥1.26 | 81.63 | 44.00 | 26.32 | 90.72 |
| KING | 0.775 (0.684–0.865) | <0.001 | ≥5.21 | 78.05 | 70.81 | 40.51 | 92.68 |
| BONACINI | 0.660 (0.561–0.760) | 0.002 | ≥5.0 | 60.98 | 64.97 | 31.25 | 86.44 |
| AGAP | 0.768 (0.681–0.885) | <0.001 | ≥3.37 | 79.49 | 64.05 | 36.05 | 92.45 |
| GPR | 0.705 (0.619–0.791) | <0.001 | ≥0.14 | 89.74 | 43.79 | 28.93 | 94.37 |
| AAR | 0.506 (0.409–0.603) | 0.901 | - | - | - | - | - |
| GUCI | 0.748 (0.656–0.840) | <0.001 | ≥0.29 | 58.54 | 83.02 | 47.06 | 88.59 |
| ABLI | 0.606 (0.494–0.719) | 0.066 | - | - | - | - | - |
| FCI | 0.654 (0.543–0.765) | 0.009 | ≥0.07 | 71.88 | 60.42 | 37.70 | 86.57 |
| FIBROQ | 0.659 (0.556–0.761) | 0.002 | ≥3.32 | 48.78 | 82.39 | 41.67 | 86.18 |
| SINDEX | 0.701 (0.598–0.804) | 0.001 | ≥0.04 | 81.82 | 57.55 | 37.50 | 91.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özgüler, M.; Durak, S.; Solmaz, Ö.A.; Eser Karlıdağ, G.; Gündağ, Ö.; Kırık, Y.; Tanır, B.; Selim Kara, S. The Use of Serum Scoring Systems in Predicting Liver Fibrosis Caused by Chronic Hepatitis B: A Retrospective Case-Control Study. Medicina 2025, 61, 1490. https://doi.org/10.3390/medicina61081490
Özgüler M, Durak S, Solmaz ÖA, Eser Karlıdağ G, Gündağ Ö, Kırık Y, Tanır B, Selim Kara S. The Use of Serum Scoring Systems in Predicting Liver Fibrosis Caused by Chronic Hepatitis B: A Retrospective Case-Control Study. Medicina. 2025; 61(8):1490. https://doi.org/10.3390/medicina61081490
Chicago/Turabian StyleÖzgüler, Müge, Samet Durak, Özgen Arslan Solmaz, Gülden Eser Karlıdağ, Ömür Gündağ, Yasemin Kırık, Büşra Tanır, and Sümeyye Selim Kara. 2025. "The Use of Serum Scoring Systems in Predicting Liver Fibrosis Caused by Chronic Hepatitis B: A Retrospective Case-Control Study" Medicina 61, no. 8: 1490. https://doi.org/10.3390/medicina61081490
APA StyleÖzgüler, M., Durak, S., Solmaz, Ö. A., Eser Karlıdağ, G., Gündağ, Ö., Kırık, Y., Tanır, B., & Selim Kara, S. (2025). The Use of Serum Scoring Systems in Predicting Liver Fibrosis Caused by Chronic Hepatitis B: A Retrospective Case-Control Study. Medicina, 61(8), 1490. https://doi.org/10.3390/medicina61081490






