Androgen Receptor: Clinical Importance in Breast Cancer Patients Receiving CDK 4/6 Inhibitor Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Pathological Evaluation
- ER: SP1 (Ventana).
- PR: 1E2 (Ventana).
- AR: SP107 (Ventana).
- HER-2: Anti-HER-2/neu; 4B5 (Ventana).
- Ki-67: 30-9 (Ventana).
- 0 or 1+: HER-2 was considered negative.
- 2+: HER-2 was classified as negative or positive by the fluorescence in situ hybridization (FISH) test.
- 3+: HER-2 was evaluated as positive.
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK | Cyclin-Dependent Kinase |
| HR | Hormone Receptor |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
| ET | Endocrine Therapy |
| MBC | Metastatic Breast Cancer |
| PFS | Progression-Free Survival |
| OS | Overall Survival |
| RB1 | Retinoblastoma |
| AR | Androgen Receptor |
| IHC | Immunohistochemistry |
| CAP | College Of American Pathologists |
| ROC | Receiver Operating Characteristic |
| LR | Likelihood Ratio |
References
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.A. Breast cancer statistics 2024. CA A Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zheng, L.; Sun, Z.; Li, J. CDK4/6 inhibitor resistance mechanisms and treatment strategies (Review). Int. J. Mol. Med. 2022, 50, 128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef]
- Cheang, M.C.; Chia, S.K.; Voduc, D.; Gao, D.; Leung, S.; Snider, J.; Waston, M.; Davies, S.; Bernard, P.S.; Parker, J.S.; et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J. Natl. Cancer Inst. 2009, 101, 736–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nair, B.C.; Vadlamudi, R.K. Regulation of hormonal therapy resistance by cell cycle machinery. Gene Ther. Mol. Biol. 2008, 12, 395. [Google Scholar] [PubMed] [PubMed Central]
- Gültürk, I.; Colak, R.; Kapar, C.; Guliyev, M.; Yıldırım, C.; Yilmaz, M. Could macrocytosis predict survival in advanced breast cancer patients that were treated with CDK 4–6 inhibitors? Breast 2024, 78, 103820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamilton, E.; Infante, J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016, 45, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.; Loibl, S.; Turner, N.C. The CDK4/6 inhibitor revolution-a game-changing era for breast cancer treatment. Nat. Rev. Clin. Oncol. 2024, 21, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Magge, T.; Rajendran, S.; Brufsky, A.M.; Foldi, J. CDK4/6 inhibitors: The Devil is in the Detail. Curr. Oncol. Rep. 2024, 26, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Kolyvas, E.A.; Caldas, C.; Kelly, K.; Ahmad, S.S. Androgen receptor function and targeted therapeutics across breast cancer subtypes. Breast Cancer Res. BCR 2022, 24, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anestis, A.; Zoi, I.; Papavassiliou, A.G.; Karamouzis, M.V. Androgen Receptor in Breast Cancer-Clinical and Preclinical Research Insights. Molecules 2020, 25, 358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, A.F.; Karami, S.; Peidl, A.S.; Waiters, K.D.; Babajide, M.F.; Bawa-Khalfe, T. Androgen Receptor in Hormone Receptor-Positive Breast Cancer. Int. J. Mol. Sci. 2023, 25, 476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Kim, S.I.; Park, B.-W.; Lee, K.S. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.T.; Nguyen, C.V.; Mai, N.T.; Vu, H.T.N.; Pham, K.H.; Tran, G.L. Impact of Androgen Receptor Expression and the AR:ER Ratio on the Survival Outcomes in the Diverse Subgroups of Vietnamese Breast Cancer: A Single Institutional Retrospective Cohort Analysis. Technol. Cancer Res. Treat. 2022, 21, 15330338221080941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sgroi, D.C.; Treuner, K.; Zhang, Y.; Piper, T.; Salunga, R.; Ahmed, I.; Doos, L.; Thornber, S.; Taylor, K.J.; Brachtel, E.; et al. Correlative studies of the Breast Cancer Index (HOXB13/IL17BR) and ER, PR, AR, AR/ER ratio and Ki67 for prediction of extended endocrine therapy benefit: A Trans-aTTom study. Breast Cancer Res. BCR 2022, 24, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fitzgibbons, P.L.; Dillon, D.A.; Alsabeh, R.; Berman, M.A.; Hayes, D.F.; Hicks, D.G.; Hughes, K.S.; Nofech-Mozes, S. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch. Pathol. Lab. Med. 2014, 138, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MavGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J. Strategies for subtypes--dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bronte, G.; Rocca, A.; Ravaioli, S.; Puccetti, M.; Tumedei, M.M.; Scarpi, E.; Andreis, D.; Maltoni, R.; Sarti, S.; Cecconetto, L.; et al. Androgen receptor in advanced breast cancer: Is it useful to predict the efficacy of anti-estrogen therapy? BMC Cancer 2018, 18, 348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karaboyun, K.; Iriagac, Y.; Cavdar, E.; Avci, O.; Seber, E.S. A New Mortality Prediction Model in Advanced Stage Cancer Patients Requiring Hospitalisation while Receiving Active Systemic Therapy. Age 2023, 62, 10–87. [Google Scholar]
- Okour, M.; Jacobson, P.A.; Israni, A.; Brundage, R.C. Comparative Evaluation of Median Versus Youden Index Dichotomization Methods: Exposure–Response Analysis of Mycophenolic Acid and Acyl-Glucuronide Metabolite. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven-Law, G.; Milioli, H.H.; Roden, D.; Jindal, S.; Hui, M.; Finlay-Schultz, J.; Ebrahimie, E.; et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat. Med. 2021, 27, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Elebro, K.; Borgquist, S.; Simonsson, M.; Markkula, A.; Jirström, K.; Ingvar, C.; Rose, C.; Jernström, H. Combined Androgen and Estrogen Receptor Status in Breast Cancer: Treatment Prediction and Prognosis in a Population-Based Prospective Cohort. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3640–3650. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D'Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. BCR 2014, 16, R7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Amicis, F.; Thirugnansampanthan, J.; Cui, Y.; Selever, J.; Beyer, A.; Parra, I.; Weigel, N.L.; Herynk, M.H.; Tsimelzon, A.; Lewis, M.T.; et al. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res. Treat. 2010, 121, 1–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rangel, N.; Rondon-Lagos, M.; Annaratone, L.; Osella-Abate, S.; Metovic, J.; Mano, M.P.; Bertero, L.; Cassoni, P.; Sapino, A.; Castellano, I. The role of the AR/ER ratio in ER-positive breast cancer patients. Endocr.-Relat. Cancer 2018, 25, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, S.; Maltoni, R.; Pasculli, B.; Parrella, P.; Giudetti, A.M.; Vergara, D.; Tumedei, M.M.; Pirini, F.; Bravaccini, S. Androgen receptor in breast cancer: The “5W” questions. Front. Endocrinol. 2022, 13, 977331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rechoum, Y.; Rovito, D.; Iacopetta, D.; Barone, I.; Andò, S.; Weigel, N.L.; O’malley, B.W.; Brown, P.H.; Fuqua, S.A.W. AR collaborates with ERα in aromatase inhibitor-resistant breast cancer. Breast Cancer Res. Treat. 2014, 147, 473–485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.C.; Chen, C.; Bolton, E.C. Androgen Receptor-Mediated Growth Suppression of HPr-1AR and PC3-Lenti-AR Prostate Epithelial Cells. PLoS ONE 2015, 10, e0138286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| n | (%) | Median PFS (Month) | p * | ||
|---|---|---|---|---|---|
| Age | <55 | 19 | 46.3% | 25.4 | 0.036 |
| ≥55 | 22 | 53.7% | 34.4 | ||
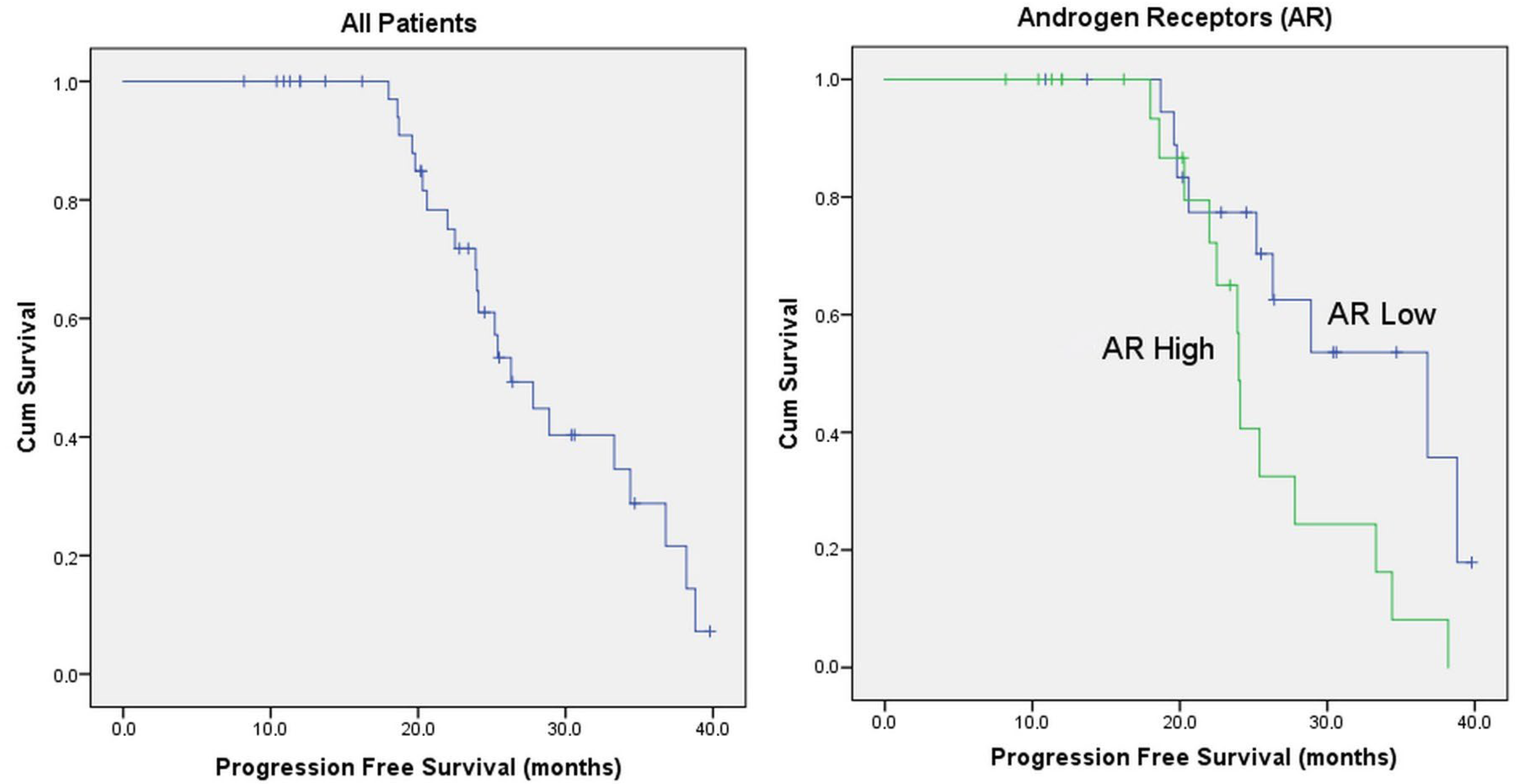
| AR | Low (<%50) | 20 | 48.8% | 36.8 | 0.025 |
| High (≥%50) | 21 | 51.2% | 24.0 | ||
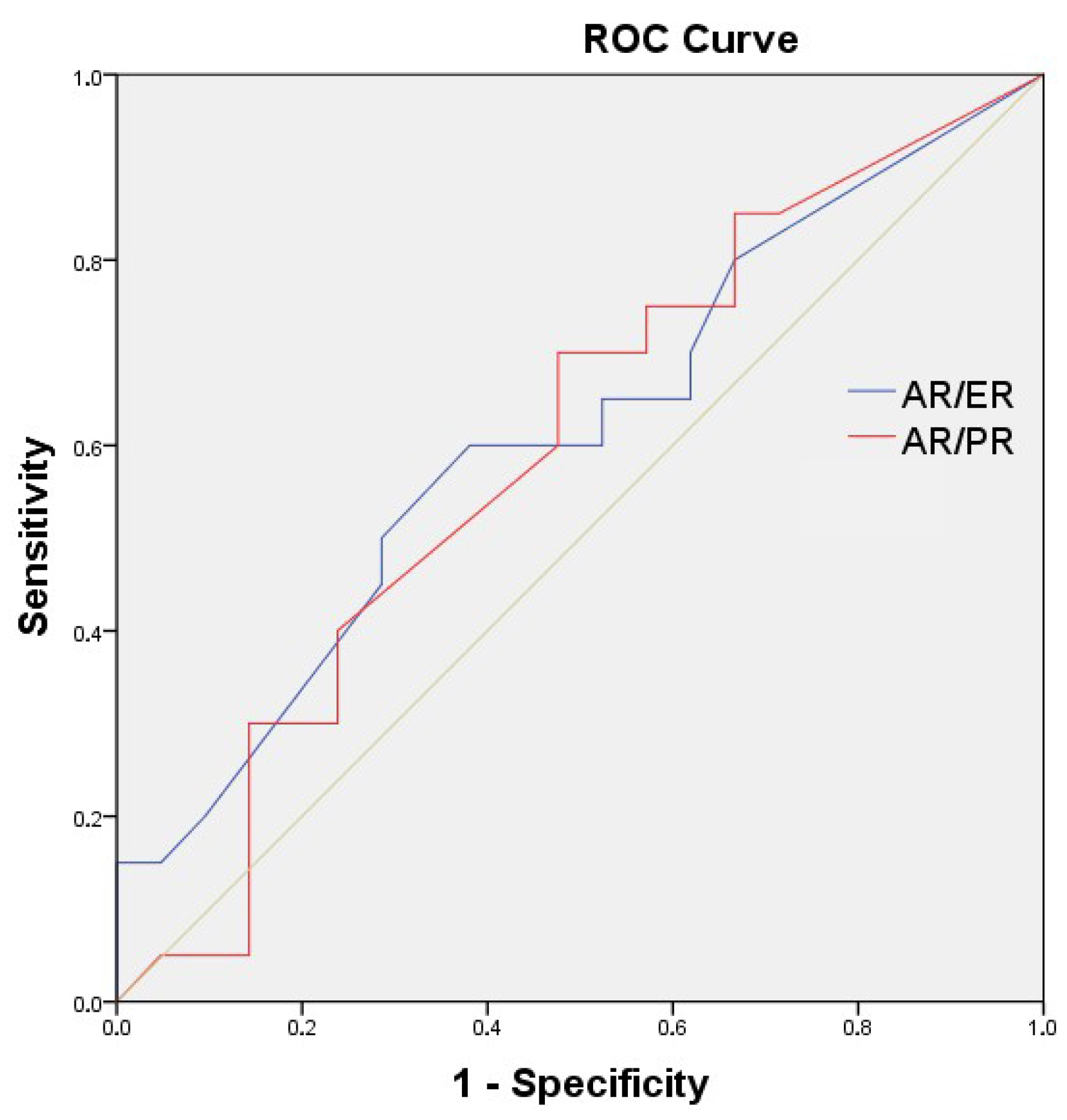
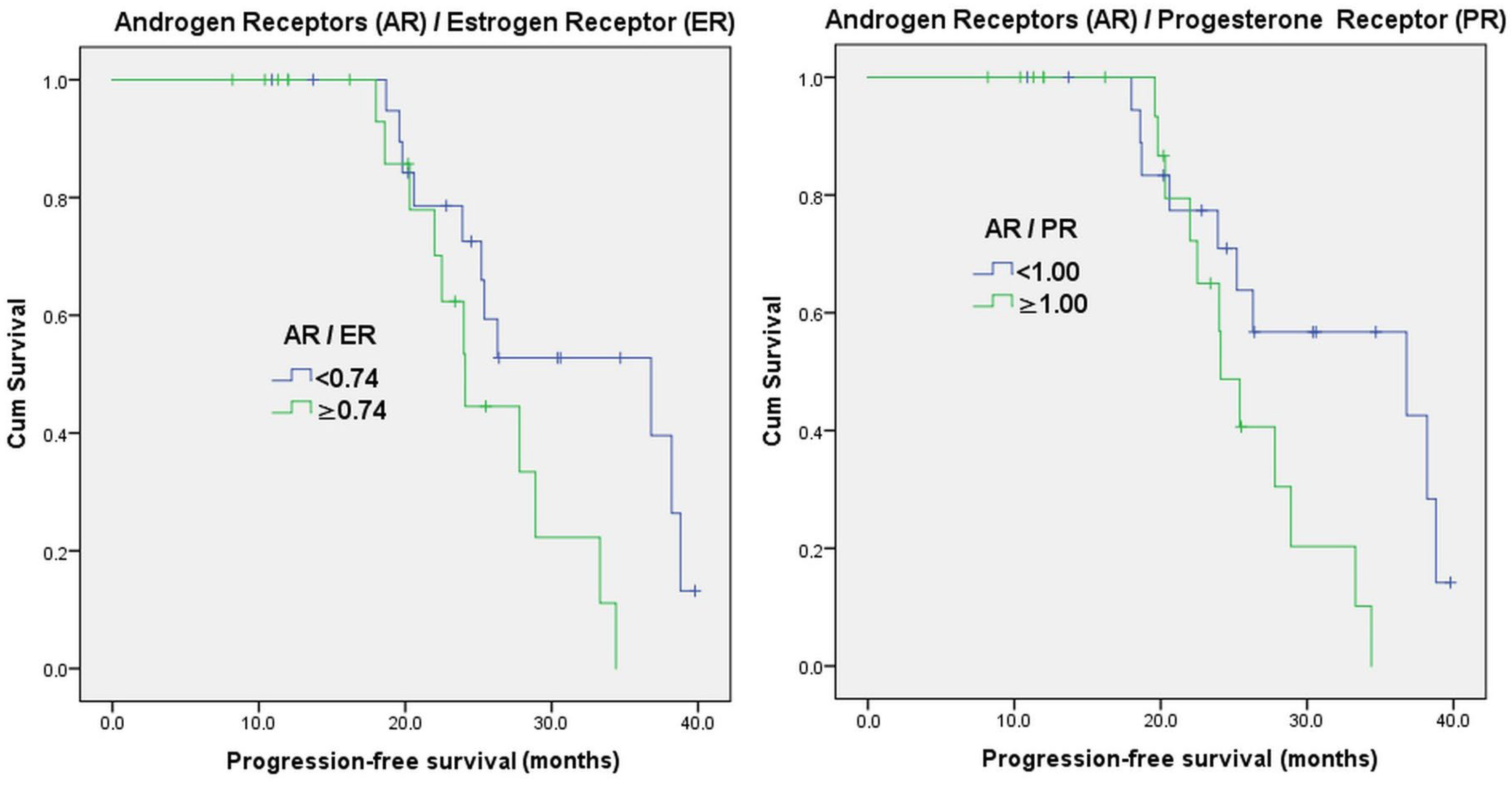
| AR/ER ratio | <0.74 | 21 | 51.2% | 36.8 | 0.042 |
| ≥0.74 | 20 | 48.8% | 24.1 | ||
| AR/PR ratio | <1.00 | 20 | 48.8% | 36.8 | 0.036 |
| ≥1.00 | 21 | 51.2% | 24.1 | ||
| Menopause status | Pre-peri | 12 | 29.3% | 22.0 | 0.039 |
| Post | 29 | 70.7% | 33.3 | ||
| Breast cancer surgery | Yes | 20 | 48.8% | 26.3 | 0.641 |
| No | 21 | 51.2% | 24.1 | ||
| Chemotherapy | No | 33 | 80.5% | 25.2 | 0.059 |
| Yes | 8 | 19.5% | 28.9 | ||
| Diagnostic stage | Stage (1-2-3) | 11 | 26.8% | 24.1 | 0.245 |
| Stage 4 | 30 | 73.2% | 27.8 | ||
| Adjuvant hormonotherapy | No | 30 | 73.2% | 27.8 | 0.245 |
| Yes | 11 | 26.8% | 24.1 | ||
| Lymph node metastasis | No | 13 | 31.7% | 28.9 | 0.701 |
| Yes | 28 | 68.3% | 25.4 | ||
| Visceral metastasis | No | 26 | 63.4% | 28.9 | 0.018 |
| Yes | 15 | 36.6% | 20.6 | ||
| Bone metastasis | No | 8 | 19.5% | 34.4 | 0.682 |
| Yes | 33 | 80.5% | 25.4 | ||
| Radiotherapy | No | 20 | 48.8% | 34.4 | 0.094 |
| Yes | 21 | 51.2% | 23.9 | ||
| Grade | 1-2 | 26 | 63.4% | 27.8 | 0.267 |
| 3 | 15 | 36.6% | 22.0 | ||
| Histology | Ductal | 34 | 82.9% | 25.2 | 0.048 |
| Others | 7 | 17.1% | 38.2 | ||
| Treatment | Ribociclib | 21 | 51.2% | 24.1 | 0.091 |
| Palbociclib | 20 | 48.8% | 34.4 |
| AR | ER | PR | Ki-67 | |||||
|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | |
| Age | −0.093 | 0.564 | 0.102 | 0.528 | −0.028 | 0.861 | −0.136 | 0.398 |
| AR | −0.116 | 0.472 | 0.395 | 0.011 | −0.108 | 0.500 | ||
| ER | 0.189 | 0.235 | 0.138 | 0.388 | ||||
| PR | −0.067 | 0.678 | ||||||
| Variable | Category | HR (95% CI) | p |
|---|---|---|---|
| Age | Continuous | 0.955 (0.914–0.999) | 0.044 |
| ER | Continuous | 1.002 (0.979–1.025) | 0.864 |
| PR | Continuous | 1.003 (0.990–1.015) | 0.690 |
| AR | Continuous | 1.014 (1.002–1.026) | 0.023 |
| AR/ER ratio | 0.74</≥0.74 | 2.522 (1.004–6.336) | 0.049 |
| AR/PR ratio | 1.00</≥1.00 | 2.659 (1.029–6.869) | 0.043 |
| Ki-67 | Continuous | 1.008 (0.986–1.030) | 0.485 |
| Grade | 1-2/3 | 1.712 (0.656–4.470) | 0.272 |
| Menopausal status | Pre-peri/post | 0.739 (0.550–0.995) | 0.046 |
| Breast cancer surgery | No/yes | 1.223 (0.525–2.849) | 0.642 |
| Chemotherapy | No/yes | 0.796 (0.321–1.971) | 0.621 |
| Diagnostic stage | 1/2/3/4 | 0.822 (0.566–1.194) | 0.303 |
| Adjuvant hormonotherapy | No/yes | 1.709 (0.685–4.263) | 0.251 |
| Lymph node metastasis | No/yes | 1.195 (0.482–2.964) | 0.701 |
| Visceral metastasis | No/yes | 3.769 (1.160–12.248) | 0.027 |
| Bone metastasis | No/yes | 1.259 (0.418–3.789) | 0.682 |
| Radiotherapy | No/yes | 2.088 (0.868–5.023) | 0.100 |
| Histology | Ductal/Others | 0.303 (0.087–1.053) | 0.060 |
| Treatment | Ribo/palbo | 0.460 (0.183–1.157) | 0.099 |
| Multivariate Model 1 * | |||
| AR | Continuous | 1.015 (1.003–1.027) | 0.018 |
| Visceral metastasis | No/yes | 4.065 (1.245–13.280) | 0.020 |
| Multivariate Model 2 ** | |||
| AR/PR ratio | 1.00</≥1.00 | 2.852 (1.104–7.368) | 0.030 |
| Visceral metastasis | No/yes | 4.204 (1.283–13.771) | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saray, S.; Yılmaz, T.; Kanmaz, H.; İriağaç, Y. Androgen Receptor: Clinical Importance in Breast Cancer Patients Receiving CDK 4/6 Inhibitor Treatment. Medicina 2025, 61, 1464. https://doi.org/10.3390/medicina61081464
Saray S, Yılmaz T, Kanmaz H, İriağaç Y. Androgen Receptor: Clinical Importance in Breast Cancer Patients Receiving CDK 4/6 Inhibitor Treatment. Medicina. 2025; 61(8):1464. https://doi.org/10.3390/medicina61081464
Chicago/Turabian StyleSaray, Seray, Tufan Yılmaz, Hüseyin Kanmaz, and Yakup İriağaç. 2025. "Androgen Receptor: Clinical Importance in Breast Cancer Patients Receiving CDK 4/6 Inhibitor Treatment" Medicina 61, no. 8: 1464. https://doi.org/10.3390/medicina61081464
APA StyleSaray, S., Yılmaz, T., Kanmaz, H., & İriağaç, Y. (2025). Androgen Receptor: Clinical Importance in Breast Cancer Patients Receiving CDK 4/6 Inhibitor Treatment. Medicina, 61(8), 1464. https://doi.org/10.3390/medicina61081464






