Abstract
Background and Objectives: Total neoadjuvant therapy (TNT) for locally advanced rectal cancer (LARC) offers significant advantages in terms of pathologic response and long-term survival; however, it is still unclear which patients will benefit the most from this treatment. This study aims to investigate the role of metabolic parameters on pretreatment positron emission tomography–computed tomography (PET/CT) images in predicting treatment response after TNT. Materials and Methods: The research was conducted using a single-center, retrospective design. Patients treated with total neoadjuvant therapy are included if they have locally advanced rectal cancer (cT3/T4-N0 or cTany-N1/N2). The patient group was categorized into two groups: CR and non-CR. Clinicopathologic features, PET/CT parameters, CA19-9, and CEA values were compared between these two groups. Results: In total, 52 patients were included. The CR group had 21 patients, and the non-CR group had 31 patients. The analysis demonstrated that the CR group had significantly lower metabolic tumor volume (MTV) and total lesion glycolysis (TLG) than the non-CR group (p = 0.022 vs. p = 0.003, p < 0.05). Also, CA19-9 values were lower than the non-CR group, and this difference was statistically remarkable (p = 0.40, p < 0.05). Conclusions: MTV and TLG parameters in PET/CT for pretreatment staging and pretreatment blood CA 19-9 levels are prognostic factors for predicting treatment response, and they may play a crucial role in choosing treatment. Comprehensive research is warranted on this subject with a larger patient population.
1. Introduction
For many years, neoadjuvant long-course chemoradiotherapy (LCRT) or short-course radiotherapy (SCRT), both followed by total mesorectal excision and adjuvant chemotherapy, were standard therapy for locally advanced rectal cancer (LARC) [1,2]. In recent years, the pathological complete response (pCR), disease-free survival (DFS), and overall survival (OS) rates obtained in the Polish II, RAPIDO, STELLAR, and UNICANCER-PRODİGE 23 trials, which investigated the efficacy of total neoadjuvant therapy (TNT), showed that TNT is a new and significant treatment option in LARC treatment [3,4,5,6].
All studies concluded that TNT application had a higher rate of pathological complete response (pCR) than standard neoadjuvant chemoradiotherapy (nCRT). However, these studies had differences in the designs of these trials, and the treatment agents at the endpoints of trials are controversial. Meta-analyses of studies, including clinical trials and other real-life data, showed significant distant recurrence, DFS, and OS contributions [7]. Additionally, TNT provided significant benefits, such as increasing the rate of sphincter-sparing surgery by decreasing tumor size. Meanwhile, the surgical complication rates are similar [8]. After the successful results, TNT has become the standard recommendation, especially with high-risk and lower rectum invasion LARC patients [9]. The increased clinical complete response (cCR) rates have expanded the potential patient population for nonoperative management strategies (NOM). The successful results of studies investigating the watch-and-wait (WW) protocol, particularly the OPRA study, demonstrated the success and feasibility of NOM strategies [10,11]. NOM seems a promising and important option for the future as patients continue their physiologic lives and survival times are at similar levels, although discussions continue for the NOM approach [12].
In recent years, studies have shown that positron emission tomography–computed tomography (PET/CT) parameters are related to treatment response in many cancers. Specifically, total lesion glycolysis (TLG) and metabolic tumor volume (MTV) are related to treatment response and tumor aggressivity [13,14]. Much research has been conducted to foresee TLG and MTV’s predictability of treatment response in LARC patients treated with standard nCRT. These studies have concluded that high TLG and MTV levels are negative cCR predictors [15,16].
It is still controversial who is eligible for the TNT approach after TNT has elevated cCR rates for the potential NOM patient numbers. Before treatment, the CR expected-patient group must be defined well. Based on this, we aim to reveal the relationship between cCR and pCR response with clinicopathological features and values such as mean standardized uptake value (SUVmean), maximum standardized uptake value (SUVmax), TLG, MTV, and mean standardized uptake value (SUVmean) in PET/CTs taken for pretreatment staging in patients treated with TNT with a prediagnosis of LARC.
2. Materials and Methods
2.1. Study Population and Data Collection
The research was conducted using a single-center, retrospective design. Between 01.06.2019 and 01.06.2024, patients diagnosed with rectal cancer by histopathologic examination who were in a locally advanced stage (cT3/T4-N0 or cTany-N1/N2) at the time of diagnosis were included in the study. The patients’ data were reviewed using patient files and the hospital’s electronic data system. For diagnostic staging, the American Joint Committee on Cancer (AJCC) tumor/node/metastasis (TNM) classification and staging system 8. Edition used. The LARC staging has been confirmed with digital rectal examination, pelvic magnetic resonance imaging (MRI), and PET/CT. All patients were treated with total neoadjuvant therapy.
Demographic and clinicopathologic characteristics, baseline carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) values, treatment regimens, treatment responses, and lesion and liver SUVmean, SUVmax, and lesion TLG and MTV values on pretreatment PET/CT were recorded. Performance scores were calculated using the Eastern Cooperative Oncology Group Performance Status (ECOG PS).
2.2. Treatment Protocols and 18F-FDG PET/CT Imaging and Analysis
Patients received LCRT or SCRT according to the TNT protocol. Patients who received LCRT were administered a total dose of 50.4–56 Gy to the primary tumor and 45–50.4 Gy to the regional lymph nodes, which include the presacral space, internal iliac nodes, obturator nodes, and ischiorectal fossa. This treatment was delivered in 25–28 fractions with concurrent capecitabine at 825 mg/m2 orally twice daily, five days a week, during the RT period. In contrast, patients receiving SCRT were administered a total of 25 Gy to the primary tumor and the regional lymph nodes in just five fractions. Patients who received LCRT received concurrent chemotherapy, followed by four to six cycles of chemotherapy. Patients who received SCRT received RT only, followed by four or six cycles of chemotherapy. CAPOX and FOLFOX4 were used as systemic chemotherapy regimens.
All 18F-FDG PET/CT scans were conducted using a Discovery ST PET/CT scanner (GE Healthcare, Milwaukee, WI, USA). A cuboid volume of interest (VOI) was defined to encompass the rectal cancer lesion, and the VOI boundaries were automatically delineated along the tumor uptake margins based on a predetermined SUV threshold using PET VCAR software (GE HealthCare, Chicago, IL, USA). The SUVmax was identified as the highest standardized uptake value within the VOI. The MTV was determined as the portion of the tumor exhibiting SUV values equal to or exceeding 40% of the SUVmax within the VOI. The SUVmean was calculated as the average SUV within the VOI. TLG was derived by multiplying the MTV by the SUVmean.
Measurements of tumor length in the vertical axis, SUVmax, SUVmean, MTV, and TLG were recorded before and after 18F-FDG PET/CT imaging. Additionally, SUVmax values of the liver parenchyma were assessed as a standard reference parameter. Two experienced nuclear medicine specialists carried out the final evaluation of CT, PET, and fused PET/CT images.
2.3. Study Design and Statistical Analysis
Treatment response was evaluated using DRE, rectosigmoidoscopy, biopsy, and pelvic MRI. Patients with no residual tumor on DRE, biopsy, and MRI were considered cCR. According to treatment response, patients were divided into two categories: CR and non-CR. Patients who relapsed within the first 6 months after the end of treatment were not considered cCR. Essential demographic characteristics such as age and gender, tumor size, localization (distance from the anal verge), clinical stage at diagnosis (cTxNx), pretreatment CEA, and CA19-9 values were compared between the groups. In addition, SUVmean, SUVmax, TLG, and MTV values on pretreatment PET/CT were compared between the groups.
SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used for all statistics. While evaluating the study data, the conformity of the parameters to normal distribution was evaluated using Kolmogorov–Smirnov and Shapiro–Wilk tests. In the analysis of continuous variables between two groups, the Independent Samples t-test was used if the data were normally distributed, and the Mann–Whitney U Test was used if the data were not normally distributed. The chi-square test was used to analyze categorical variables. Significance was evaluated at p < 0.05 level.
3. Results
3.1. Characteristics of Patients
A total of 307 patients diagnosed with rectal cancer were scanned. The study included 52 patients who underwent TNT for LARC and had PET/CT for pretreatment staging. There were 21 patients in the CR group and 31 in the non-CR group. The median age of the patients ranged between 40 and 89 years. An amount of 35 (67.3%) patients were men, and 17 (32.6%) were women; 48 of 52 patients had an ECOG PS of 0 or 1. In 29 patients, the largest diameter of the tumor was 5 cm or less, while the largest diameter was larger than 5 cm in 23 patients. In 31 patients, the tumor was located in the distal, 14 in the middle, and 7 in the upper rectum. All 40 patients whose microsatellite status was reached were microsatellite stable (MSS). At the time of diagnosis, eight patients had cT2, 41 patients had cT3, three patients had cT4 disease, four patients had cN0, 37 had cN1, and 11 had cN2 disease. In total, five patients received short-course radiotherapy, while 47 received long-course radiotherapy. In addition, 49 patients were treated with the radiotherapy concurrent capecitabine followed by CAPOX, while three patients were treated with the radiotherapy concurrent 5-Fluorouracil infusion followed by the FOLFOX regimen. All patients received 4–6 cycles of CAPOX therapy. Patient characteristics and other findings are summarized in Table 1.

Table 1.
Baseline demographic and clinicopathological characteristics of the patients and treatment modalities implemented.
3.2. Pretreatment PET/CT Measurements and Treatment Response
The mean lesion SUVmean was 10.14 ± 5.55, SUVmax was 17.59 ± 9.51, lesion-to-liver SUVmean was 5.04 ± 2.48, and SUVmax was 6.46 ± 3.05 on pretreatment PET/CTs. The median MTV value was 19.72 ± 10.13 cm3, while the median TLG value was 190.76 ± 137.23. The findings and distribution according to the groups are summarized in Table 2.

Table 2.
Pretreatment PET/CT measurements, encompassing SUVmean, SUVmax, MTV, and TLG.
A total of 13 patients evaluated as cCR after TNT was completed were followed up with NOM. Figure 1 shows MRI images of a patient with complete response, and Figure 2 shows PET/CT images. It shows that 39 patients underwent surgery, and 8 of them had pCR. A total of 9 patients had ypT0 (one of them was ypT0N1), 1 had ypT1, 6 had ypT2, 21 had ypT3, and 1 had ypT4. In addition, 27 patients had no pathologically involved lymph nodes (ypN0), 9 had ypN1, and 2 had ypN2. These findings and tumor regression grading distribution are summarized in Table 3.
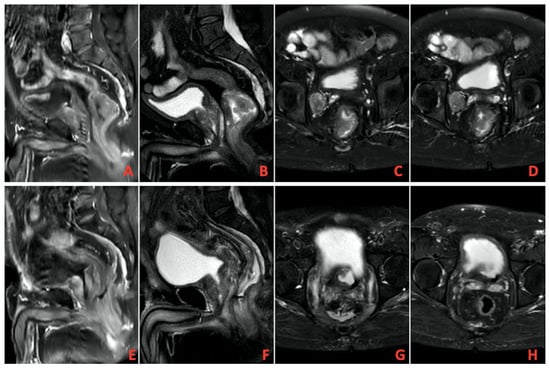
Figure 1.
(A–D) Pretreatment imaging, (E–H) Posttreatment imaging on magnetic resonance imaging (MRI).
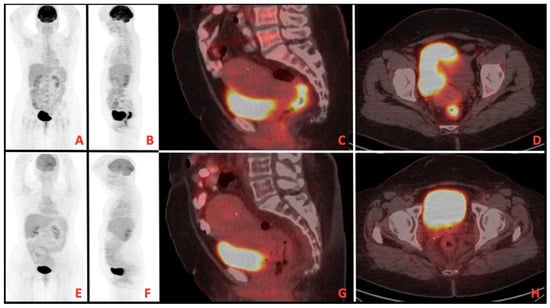
Figure 2.
(A–D) Pretreatment imaging, (E–H) posttreatment imaging on positron emission tomography/computed tomography (PET/CT).

Table 3.
Treatment outcomes.
3.3. Comparison of CR and Non-CR Groups
The median age of the patients was 60.47 ± 11.95 years in the CR group and 62.93 ± 11.78 years in the non-CR group. The proportion of male patients was 71.4% in the CR group and 64.5% in the non-CR group. The mean diameter of the largest tumor was 5.69 ± 2.03 cm in the CR group and 6.14 ± 1.74 cm in the non-CR group. According to Distance from Anal Verge measurements, the median distance was 5.16 ± 3.32 cm in the CR group and 6.23 ± 4.03 cm in the non-CR group. When the CR and non-CR groups were analyzed according to the clinical stage distribution (cT and cN) before treatment, no significant difference was found between the two groups (p = 0.105 and p = 0.228, respectively).
Regarding PET/CT parameters, lesion-to-liver SUVmean values were 4.49 ± 2.19 in the CR group and 5.42 ± 2.62 in the non-CR group, and this difference was not statistically significant (p = 0.414). Lesion-to-liver SUVmax values were 5.59 ± 2.61 in the CR group and 7.05 ± 3.03 in the non-CR group, and the difference between the groups was not statistically significant (p = 0.355). MTV was 15.92 ± 6.52 cm3 in the CR group and 22.29 ± 11.37 cm3 in the non-CR group. This difference was statistically significant (p = 0.022). TLG values were 129.91 ± 60.77 SUV × cm3 in the CR group, and 231.98 ± 158.77 SUV × cm3 in the non-CR group, and the difference was significant (p = 0.003).
The median CEA levels were 4.2 (0–25.4) ng/mL in the CR group and 6.1 (0–87.5) ng/mL in the non-CR group, and this difference between the groups was insignificant (p = 0.077). CA19-9 levels were 65 (0–525) U/mL in the CR group and 117 (0–1358) U/mL in the non-CR group. This difference was statistically significant (p = 0.040). All these findings are summarized in Table 4.

Table 4.
Comparison of patient characteristics and PET/CT parameters regarding CR and Non-CR groups.
4. Discussion
In TNT-treated LARC patients, there was a significant correlation between MTV and TLG values measured on pretreatment PET/CT and patients’ response to treatment. MTV and TLG values were statistically significantly lower in the pCR group compared to the non-PCR group. Considering the increasing use of TNT and NOM strategies, these pretreatment assessments may help clinicians predict the patient’s response to treatment and determine the treatment modalities chosen.
The relationship between standard nCRT, MTV, and TLG has been examined in previous years. Many studies have found that high pretreatment MTV and TLG values are associated with lower pathologic response rates and negative survival outcomes after nCRT [17,18]. Also, decreased MTV and TLG values have been reported to indicate a favorable response to treatment [19,20]. In our study, a statistically significant difference was detected between the MTV and TLG rates between the CR group and the non-CR group, and this result emphasizes the value of MTV and TLG while predicting treatment response. Some studies have shown a relationship between SUVmax values and complete response in patients receiving standard CRT [21,22]. However, our results showed no statistical difference between SUVmean and SUVmax between CR and non-CR groups. Studies involving more patients are needed to eliminate the contradictions in this regard.
Previous studies found a significant relationship between CA19-9 and CEA values measured before treatment in LARC patients in terms of treatment response, disease recurrence, and overall survival [23,24]. In our study, CA19-9 and CEA values were numerically higher in the non-PCR group compared to the CR response group. However, when comparing the CR and non-CR groups, this difference was statistically significant only between CA19-9 values. This result indicates that CA19-9 values measured before treatment may be predictive in predicting treatment response. Survival results are currently immature as median DFS and OS were not reached.
In previous studies, the CR rates obtained with TNT varied between 22.4% and 29.9% [25,26]. In our study, this rate was found to be 40.3%. The main reason was that 90.3% of our patients had received LCRT, and 63.8% had received 56 Gy. Plus, while 38.1% of our patients were pCR, 61.9% were cCR. Although the patients who were accepted as cCR were patients who did not relapse within 6 months, we think that the higher rate of cCR compared to pCR may have increased the total number of CRs.
While standard nCRT was used in the Polish II, RAPIDO, and STELLAR studies, LCRT was used in the UNICANCER-PRODIGO 23 study. In the preoperative period, FOLFIRINOX (folinic acid, 5-fluorouracil, irinotecan, oxaliplatin) was used in the UNICANCER-PRODIGO 23 study, FOLFOX4 (folinic acid, 5-fluorouracil, oxaliplatin), or CAPOX (capecitabine and oxaliplatin) in the RAPIDO study, FOLFOX4 in Polish II, and CAPOX in the STELLAR study [3,4,5,6]. Unlike other studies, the NOM approach was also used in patients with complete response in the STELLAR study [5]. Looking at 3-year DFS, an increase was observed in the TNT arm compared to standard CRT in the RAPIDO and UNICANCER-PRODİGE 23 trials. However, OS was similar in both groups in the RAPIDO study. In the STELLAR study, while DFS was similar between the groups, an increase in OS was observed in the TNT arm. Although the treatment modalities and survival analyses in these major studies differed, increased CR rates were obtained with the TNT approach compared to the standard CRT approach in all modalities applied [3,4,5,6]. However, there are still no precise data on which patient groups achieved higher CR rates in all these studies. In selecting the treatment modality to be applied in elderly, fragile patients with uncertain complete responses in whom surgical decision-making is complex, patient groups that will respond well to TNT should be better defined.
Regardless of whether standard neoadjuvant nCRT or TNT is used, the feasibility of NOM in patients who achieve complete or near-complete response has been a topic of ongoing debate for a long time [27,28,29]. Primarily, the OPRA study provides crucial information in this regard. The OPRA study applied the WW strategy to 74% of patients with a complete or nearly complete response, and tumor regrowth developed in 36%. When the operated patients in this group were compared with those initially treated with TME for non-CR, the 5-year DFS was 64% in both groups, and there was no difference between them [11]. One of the three patients who underwent WW recurred. From this point of view, we believe that the clinicopathologic features of the group with recurrence should be defined in more detail. Studies comparing TLG, MTV, and CA 19-9 levels with recurrence rate may help clinicians.
The limitations of our study were that it included a small group of patients, the follow-up period was short, and PET/CT was not performed for treatment response in every patient before and after TNT. Subgroup analyses could not be detailed due to the small number of patients. In addition, since TNT is a new treatment, data on recurrence rate, median DFS, and OS were inaccessible. Nevertheless, our study differs from other studies in that it focuses on a new approach, such as TNT, and, as far as we can see, is the first study on this subject.
5. Conclusions
Following the outstanding results of TNT, the number of patients achieving CR and, accordingly, the importance of NOM strategies are increasing. With the increase in the number of patients with CR and WW, the frequency of follow-up and the choice of diagnostic tools used in follow-up gain importance. In order to determine all these more clearly, patient groups and tumor characteristics need to be more accurately defined. Accordingly, measuring parameters such as MTV and TLG on PET/CT for preoperative staging are important prognostic factors in predicting treatment response. Low preoperative MTV and TLG values may be considered a predictive factor for complete response in patients who cannot be evaluated for response with rectosigmoidoscopy and pelvic MRI when deciding on NOM. Although the findings of our study reveal the relationship between treatment response and PET/CT parameters in the context of TNT an area that has not been previously explored, comprehensive studies with larger patient populations are warranted in this field.
Author Contributions
Conceptualization, Z.B. and A.Ç.; methodology, A.D., O.K., H.A., M.A. and İ.V.B.; software, Y.A. and B.P.; validation, S.I., M.S., S.Ö., H.A., M.A. and İ.V.B.; formal analysis, B.P. and N.M.; investigation, Z.B., Z.C.B.G., T.B.A., E.K. and N.S.; resources, P.E., M.A.T., A.D., N.S., S.I. and S.Ö.; data curation, T.B.A., Y.A. and P.E.; writing—original draft preparation, A.K.G.; writing—review and editing, A.K.G.; visualization, Z.B., Z.C.B.G. and N.M.; supervision, A.K.G., O.K., S.Ö., M.A. and İ.V.B.; project administration, A.K.G. and İ.V.B.; funding acquisition, E.K. and A.Ç. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Marmara University Faculty of Medicine Ethics Committee on 15 November 2024 with protocol number 15.11.2024.1299.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this study’s findings are not publicly available due to privacy reasons but are available from the corresponding author.
Conflicts of Interest
The authors state no conflicts of interest.
References
- Li, Y.; Wang, J.; Ma, X.; Tan, L.; Yan, Y.; Xue, C.; Hui, B.; Liu, R.; Ma, H.; Ren, J. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int. J. Biol. Sci. 2016, 12, 1022–1031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv22–iv40, Erratum in Ann. Oncol. 2018, 29 (Suppl. S4), iv263. https://doi.org/10.1093/annonc/mdy161. [Google Scholar] [CrossRef] [PubMed]
- Ciseł, B.; Pietrzak, L.; Michalski, W.; Wyrwicz, L.; Rutkowski, A.; Kosakowska, E.; Cencelewicz, A.; Spałek, M.; Polkowski, W.; Jankiewicz, M.; et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: Long-term results of the randomized Polish II study. Ann. Oncol. 2019, 30, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42, Erratum in Lancet Oncol. 2021, 22, e42. https://doi.org/10.1016/S1470-2045(20)30781-6. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.-M.; Jiang, J.; Li, N.; Liu, W.-Y.; Chen, S.-L.; Li, S.; Lu, N.-N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.C.; Soucisse, M.; Michael, M.; Tie, J.; Ngan, S.Y.; Leong, T.; McCormick, J.; Warrier, S.K.; Heriot, A.G. Total Neoadjuvant Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Metaanalysis of Oncological and Operative Outcomes. Ann. Surg. Oncol. 2021, 28, 7476–7486. [Google Scholar] [CrossRef] [PubMed]
- Riesco-Martinez, M.C.; Fernandez-Martos, C.; Gravalos-Castro, C.; Espinosa-Olarte, P.; La Salvia, A.; Robles-Diaz, L.; Modrego-Sanchez, A.; Garcia-Carbonero, R. Impact of Total Neoadjuvant Therapy vs. Standard Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Cancers 2020, 12, 3655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scott, A.J.; Kennedy, E.B.; Berlin, J.; Brown, G.; Chalabi, M.; Cho, M.T.; Cusnir, M.; Dorth, J.; George, M.; Kachnic, L.A.; et al. Management of Locally Advanced Rectal Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 3355–3375. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, T.; Xiao, L.; Yang, S.; Liu, Q.; Gao, Y.; Chen, G.; Xiao, W. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1555–e1566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verheij, F.S.; Omer, D.M.; Williams, H.; Lin, S.T.; Qin, L.X.; Buckley, J.T. Long-Term Results of Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J. Clin. Oncol. 2024, 42, 500–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, H.; Lee, C.; Garcia-Aguilar, J. Nonoperative management of rectal cancer. Front. Oncol. 2024, 14, 1477510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pak, K.; Cheon, G.J.; Nam, H.-Y.; Kim, S.-J.; Kang, K.W.; Chung, J.-K.; Kim, E.E.; Lee, D.S. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: A systematic review and meta-analysis. J. Nucl. Med. 2014, 55, 884–890. [Google Scholar] [CrossRef]
- Im, H.J.; Pak, K.; Cheon, G.J.; Kang, K.W.; Kim, S.J.; Kim, I.J.; Chung, J.K.; Kim, E.E.; Lee, D.S. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: A meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ebinç, S.; Güzel, Y.; Oruç, Z.; Kömek, H.; Kalkan, Z.; Can, C.; Taşdemir, B.; Urakçi, Z.; Kaplan, M.A.; Küçüköner, M.; et al. 18 F-FDG PET/CT parameters for prediction of response to neoadjuvant therapy and prognosis in rectal cancer. Nucl. Med. Commun. 2023, 44, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Park, H.L.; Yoon, N.; Kim, J.H.; Oh, J.K.; Buyn, J.H.; Choi, E.K.; Hong, J.H. Prognostic Impact of Total Lesion Glycolysis (TLG) from Preoperative 18F-FDG PET/CT in Stage II/III Colorectal Adenocarcinoma: Extending the Value of PET/CT for Resectable Disease. Cancers 2022, 14, 582. [Google Scholar] [CrossRef]
- Sakin, A.; Sahin, S.; Karyagar, S.S.; Karyagar, S.; Atci, M.; Akboru, M.H.; Cihan, S. The Predictive Value of Baseline Volumetric PET/CT Parameters on Treatment Response and Prognosis in Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. J. Gastrointest. Cancer 2022, 53, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, J.G.; Lee, S.W.; Chae, Y.S.; Kang, B.W.; Lee, Y.J.; Park, J.S.; Choi, G.S. Clinical implications of initial FDG-PET/CT in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Cancer Chemother. Pharmacol. 2013, 71, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Pyo, D.H.; Choi, J.Y.; Lee, W.Y.; Yun, S.H.; Kim, H.C.; Huh, J.W.; Park, Y.A.; Shin, J.K.; Cho, Y.B. A Nomogram for Predicting Pathological Complete Response to Neoadjuvant Chemoradiotherapy Using Semiquantitative Parameters Derived From Sequential PET/CT in Locally Advanced Rectal Cancer. Front. Oncol. 2021, 11, 742728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rymer, B.; Curtis, N.J.; Siddiqui, M.R.; Chand, M. FDG PET/CT Can Assess the Response of Locally Advanced Rectal Cancer to Neoadjuvant Chemoradiotherapy: Evidence From Meta-analysis and Systematic Review. Clin. Nucl. Med. 2016, 41, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, E.; Lambreton, F.; Yu, J.Q.; Li, T.; Denlinger, C.S.; Meyer, J.E.; Sigurdson, E.R.; Farma, J.M. Impact of PET/CT for Restaging Patients with Locally Advanced Rectal Cancer After Neoadjuvant Chemoradiation. J. Surg. Res. 2019, 243, 242–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maffione, A.M.; Marzola, M.C.; Capirci, C.; Colletti, P.M.; Rubello, D. Value of (18)F-FDG PET for Predicting Response to Neoadjuvant Therapy in Rectal Cancer: Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2015, 204, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Gu, B.; Shi, L.; Wang, X.; Ye, W.; Zhou, W.; Sun, X. Prognostic value of CEA and CA19-9 in patients with local advanced rectal cancer receiving neoadjuvant chemoradiotherapy, radical surgery and postoperative chemotherapy. Transl. Cancer Res. 2021, 10, 88–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.N.; Ouyang, P.Y.; Xiao, W.W.; Yu, X.; You, K.Y.; Zeng, Z.F.; Xu, R.H.; Gao, Y.H. Elevated CA19-9 as the Most Significant Prognostic Factor in Locally Advanced Rectal Cancer Following Neoadjuvant Chemoradiotherapy. Medicine 2015, 94, e1793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrelli, F.; Trevisan, F.; Cabiddu, M.; Sgroi, G.; Bruschieri, L.; Rausa, E.; Ghidini, M.; Turati, L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann. Surg. 2020, 271, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.T.; Hunt, S.R. Watch and Wait: Is Surgery Always Necessary for Rectal Cancer? Curr. Treat Options Oncol. 2016, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Malcomson, L.; Emsley, R.; Gollins, S.; Maw, A.; Myint, A.S.; Rooney, P.S.; Susnerwala, S.; Blower, A.; Saunders, M.P.; et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): A propensity-score matched cohort analysis. Lancet Oncol. 2016, 17, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.E.; Georgescu, M.T.; Bobircă, F.T.; Georgescu, T.F.; Doran, H.; Pătraşcu, T. Synchronous Locally Advanced Rectal Cancer with Clinical Complete Remission and Important Downstaging after Neoadjuvant Radiochemotherapy—Personalised Therapeutic Approach. Chirurgia 2017, 112, 726–733. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).