OCT-Based Morphological Classification of Healed Coronary Plaques: Insights from Imaging of Fresh Thrombi at Different Stages of Healing and Implications for Post-Stenting Edge Dissections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Coronary Angiogram Analysis
2.3. OCT Acquisition Technique and Image Analysis
2.3.1. Pilot Subgroup of Patients
2.3.2. Pilot Subgroup Inclusion Criteria
- Presence of visible fresh thrombus.
- Meeting established OCT criteria for red thrombus or white thrombus.
- Detected in at least three consecutive OCT frames.
- Coexistence of a stratified or layered tissue component, consistent with HCP features.
- Located within or adjacent to the thrombus mass.
- No clear thrombus—free interface between the thrombus and the layered component.
- The transition between thrombus and healed tissue had to be continuous, supporting the concept of a “HCP-thrombus continuum”.
- Localization within culprit lesion segments.
- No prior stent at the lesion site.
- High-quality OCT pullback.
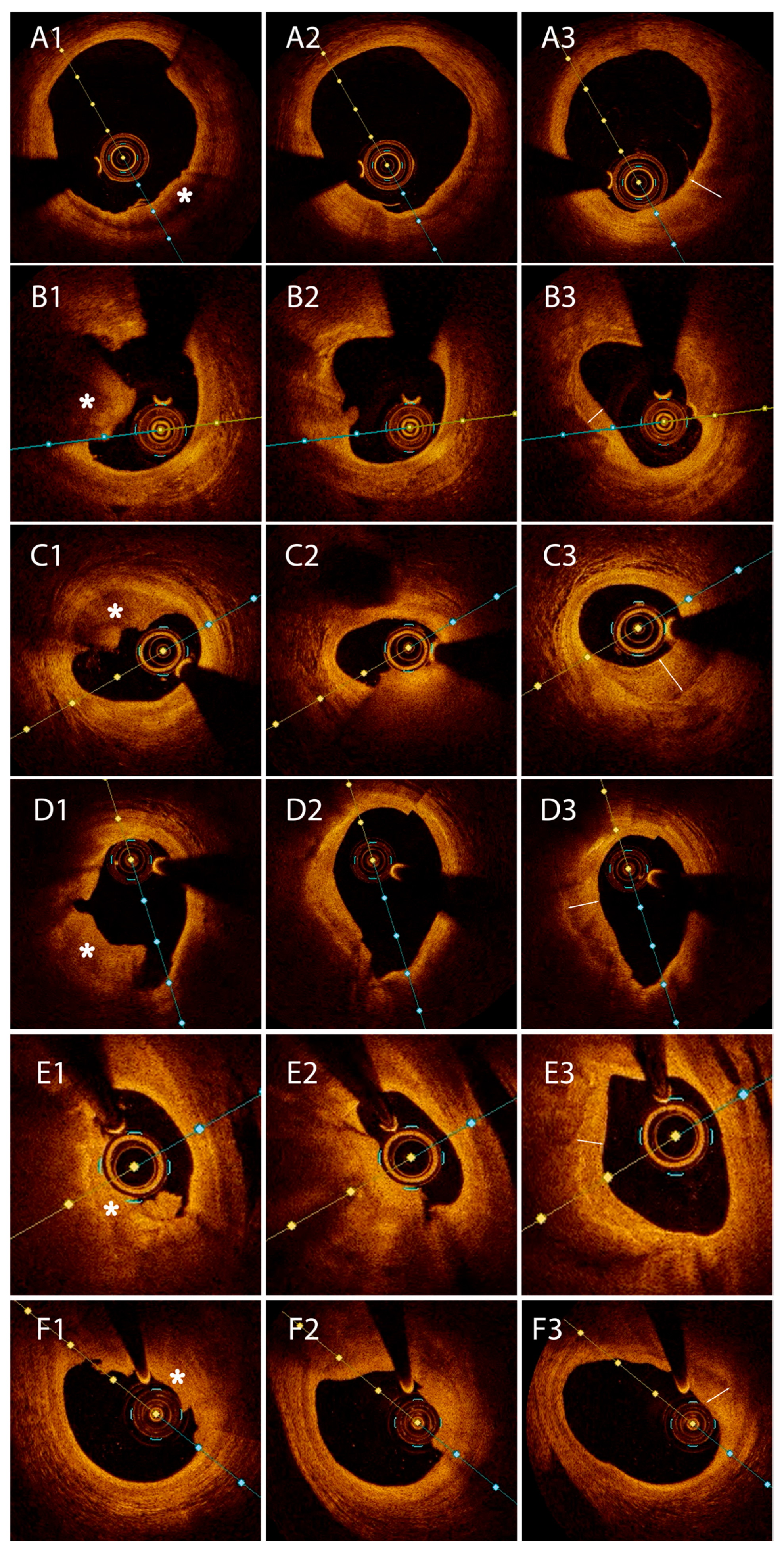
- Type I HCP was defined as an abluminal semilunar tissue with moderate backscattering, in comparison to the underlying fibrous plaque;
- Type II HCP was characterized by healing tissue overlying an LRP with or without a signal-rich ring demarcating the two tissues;
- Type III HCP was assigned when the underlying plaque showed calcification at the interface with the healing layer, with or without a signal-rich ring.
2.4. Statistical Analysis
3. Results
3.1. Pilot Group
3.2. Primary Analysis
3.2.1. Clinical Data
3.2.2. Coronary Angiography and OCT Data
3.3. Secondary Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| AS | Percent area stenosis |
| CAD | Coronary artery disease |
| CN | Calcified nodule |
| ED | Edge dissections |
| HCP | Healed coronary plaques |
| LRP | Lipid-rich plaque |
| LZ | Landing zone |
| MLA | Minimal lumen area |
| NSTE-ACS | Non-ST-segment elevation acute coronary syndromes |
| OCT | Optical coherence tomography |
| PE | Plaque erosion |
| PIT | Pathological intimal thickening |
| PR | Plaque rupture |
| STEMI | ST-segment elevation myocardial infarction |
| TCFA | Thin-cap fibroatheroma |
References
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47 (Suppl. S8), C13–C18. [Google Scholar] [CrossRef]
- Mann, J.; Davies, M.J. Mechanisms of progression in native coronary artery disease: Role of healed plaque disruption. Heart 1999, 82, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Kolodgie, F.D.; Farb, A.; Weber, D.K.; Malcom, G.T.; Smialek, J.; Virmani, R. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation 2001, 103, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Joner, M.; Prati, F.; Virmani, R.; Narula, J. Clinical classification of plaque morphology in coronary disease. Nat. Rev. Cardiol. 2014, 11, 379–389. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.-M.; Chowdhary, S.; et al. International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef]
- Onea, H.-L.; Lazar, F.-L.; Olinic, D.-M.; Homorodean, C.; Cortese, B. The role of optical coherence tomography in guiding percutaneous coronary interventions: Is left main the final challenge? Minerva Cardiol Angiol. 2024, 72, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Pang, S.; Chen, X.-Y.; Bourantas, C.V.; Pan, D.-R.; Dong, S.-J.; Wu, W.; Ren, X.-M.; Zhu, H.; Shi, S.-Y.; et al. Comparison of intravascular ultrasound guided versus angiography guided drug eluting stent implantation: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2015, 15, 153. [Google Scholar] [CrossRef]
- Shimokado, A.; Matsuo, Y.; Kubo, T.; Nishiguchi, T.; Taruya, A.; Teraguchi, I.; Shiono, Y.; Orii, M.; Tanimoto, T.; Yamano, T.; et al. In vivo optical coherence tomography imaging and histopathology of healed coronary plaques. Atherosclerosis 2018, 275, 35–42. [Google Scholar] [CrossRef]
- Fracassi, F.; Crea, F.; Sugiyama, T.; Yamamoto, E.; Uemura, S.; Vergallo, R.; Porto, I.; Lee, H.; Fujimoto, J.; Fuster, V.; et al. Healed Culprit Plaques in Patients with Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2019, 73, 2253–2263. [Google Scholar] [CrossRef]
- Wang, C.; Hu, S.; Wu, J.; Yu, H.; Pan, W.; Qin, Y.; He, L.; Li, L.; Hou, J.; Zhang, S.; et al. Characteristics and significance of healed plaques in patients with acute coronary syndrome and stable angina: An in vivo OCT and IVUS study. EuroIntervention 2019, 15, e771–e778. [Google Scholar] [CrossRef]
- Russo, M.; Fracassi, F.; Kurihara, O.; Kim, H.O.; Thondapu, V.; Araki, M.; Shinohara, H.; Sugiyama, T.; Yamamoto, E.; Lee, H.; et al. Healed Plaques in Patients with Stable Angina Pectoris. Arter. Thromb. Vasc. Biol. 2020, 40, 1587–1597. [Google Scholar] [CrossRef]
- Yin, Y.; Fang, C.; Jiang, S.; Wang, J.; Wang, Y.; Guo, J.; Lei, F.; Sun, S.; Pei, X.; Jia, R.; et al. Culprit and Non-Culprit Plaque Characteristics with vs. without a Healed Phenotype in Patients with Acute Myocardial Infarction Caused by Plaque Erosion—A 3-Vessel OCT Study. Circ. J. 2022, 86, 846–854. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Onea, H.-L.; Spinu, M.; Homorodean, C.; Olinic, M.; Lazar, F.-L.; Ober, M.C.; Stoian, D.; Itu, L.M.; Olinic, D.M. Distinctive Morphological Patterns of Complicated Coronary Plaques in Acute Coronary Syndromes: Insights from an Optical Coherence Tomography Study. Diagnostics 2022, 12, 2837. [Google Scholar] [CrossRef]
- Onea, H.-L.; Spinu, M.; Homorodean, C.; Ober, M.C.; Olinic, M.; Lazar, F.-L.; Achim, A.; Tataru, D.A.; Olinic, D.M. Superficial Calcified Plates Associated to Plaque Erosions in Acute Coronary Syndromes. Life 2023, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Subban, V.; Raffel, O.C. Optical coherence tomography: Fundamentals and clinical utility. Cardiovasc. Diagn. Ther. 2020, 10, 1389–1414. [Google Scholar] [CrossRef] [PubMed]
- Milzi, A.; Burgmaier, M.; Burgmaier, K.; Hellmich, M.; Marx, N.; Reith, S. Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: An intracoronary optical coherence tomography study. Cardiovasc. Diabetol. 2017, 16, 152. [Google Scholar] [CrossRef]
- Ong, D.S.; Lee, J.S.; Soeda, T.; Higuma, T.; Minami, Y.; Wang, Z.; Lee, H.; Yokoyama, H.; Yokota, T.; Okumura, K.; et al. Coronary Calcification and Plaque Vulnerability: An Optical Coherence Tomographic Study. Circ. Cardiovasc. Imaging 2016, 9, e003929. [Google Scholar] [CrossRef]
- Onea, H.-L.; Olinic, M.; Lazar, F.-L.; Homorodean, C.; Ober, M.C.; Spinu, M.; Achim, A.; Tataru, D.A.; Olinic, D.M. A Review Paper on Optical Coherence Tomography Evaluation of Coronary Calcification Pattern: Is It Relevant Today? J. Cardiovasc. Dev. Dis. 2024, 11, 231. [Google Scholar] [CrossRef]
- Sugiyama, T.; Yamamoto, E.; Fracassi, F.; Lee, H.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; et al. Calcified Plaques in Patients with Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2019, 12, 531–540. [Google Scholar] [CrossRef]
- Rittersma, S.Z.; van der Wal, A.C.; Koch, K.T.; Piek, J.J.; Henriques, J.P.; Mulder, K.J.; Ploegmakers, J.P.; Meesterman, M.; de Winter, R.J. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: A pathological thrombectomy study in primary percutaneous coronary intervention. Circulation 2005, 111, 1160–1165. [Google Scholar] [CrossRef]
- Kramer, M.C.; Rittersma, S.Z.; de Winter, R.J.; Ladich, E.R.; Fowler, D.R.; Liang, Y.-H.; Kutys, R.; Carter-Monroe, N.; Kolodgie, F.D.; van der Wal, A.C.; et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J. Am. Coll. Cardiol. 2010, 55, 122–132. [Google Scholar] [CrossRef]
- Sobel, B.E.; Schneider, D.J. Platelet function, coagulopathy, and impaired fibrinolysis in diabetes. Cardiol. Clin. 2004, 22, 511–526. [Google Scholar] [CrossRef]
- Hung, J.; Lam, J.Y.; Lacoste, L.; Letchacovski, G. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation 1995, 92, 2432–2436. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Dai, J.; Fang, C.; Zhang, S.; Hou, J.; Xing, L.; Li, L.; Wang, Y.; Wang, J.; Wang, Y.; Tu, Y.; et al. Not All Plaque Erosions Are Equal: Novel Insights from 1,660 Patients with STEMI: A Clinical, Angiographic, and Intravascular OCT Study. JACC Cardiovasc Imaging. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 516–518. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Flugelman, M.Y.; Virmani, R.; Correa, R.; Yu, Z.X.; Farb, A.; Leon, M.B.; Elami, A.; Fu, Y.M.; Casscells, W.; Epstein, S.E. Smooth muscle cell abundance and fibroblast growth factors in coronary lesions of patients with nonfatal unstable angina. A clue to the mechanism of transformation from the stable to the unstable clinical state. Circulation 1993, 88, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Hou, J.; Yu, H.; Jia, H.; Du, H.; Lee, H.; Yu, B.; Tian, J.; Jang, I.-K. Patterns of coronary plaque progression: Phasic versus gradual. A combined optical coherence tomography and intravascular ultrasound study. Coron. Artery Dis. 2016, 27, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Souteyrand, G.; Arbustini, E.; Motreff, P.; Gatto, L.; Di Vito, L.; Marco, V.; Amabile, N.; Chisari, A.; Kodama, T.; Romagnoli, E.; et al. Serial optical coherence tomography imaging of ACS-causing culprit plaques. EuroIntervention 2015, 11, 319–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prati, F.; Romagnoli, E.; La Manna, A.; Burzotta, F.; Gatto, L.; Marco, V.; Fineschi, M.; Fabbiocchi, F.; Versaci, F.; Trani, C.; et al. Long-term consequences of optical coherence tomography findings during percutaneous coronary intervention: The Centro Per La Lotta Contro L’infarto—Optimization Of Percutaneous Coronary Intervention (CLI-OPCI) LATE study. EuroIntervention 2018, 14, e443–e451. [Google Scholar] [CrossRef] [PubMed]


| Variable | Culprit HCP-Thrombus (n = 27) | Culprit HCP-Only (n = 51) | p-Value |
|---|---|---|---|
| Age, years | 59.4 ± 11.9 | 60.6 ± 10.1 | 0.641 |
| Male gender | 18 (66.7) | 30 (58.8) | 0.5 |
| Diagnosis | 0.356 | ||
| NSTE-ACS | 18 (66.7) | 39 (76.5) | |
| STEMI | 9 (33.3) | 12 (23.5) | |
| Risk factors | |||
| Hypertension | 22 (81.5) | 42 (82.4) | 0.924 |
| Diabetes mellitus | 12 (44.4) | 10 (19.6) | 0.021 |
| Previous hyperCHOL | 18 (66.6) | 39 (76.4) | 0.353 |
| Smoking habit | 18 (66.7) | 21 (41.2) | 0.033 |
| Overweight | 9 (33.3) | 25 (49.0) | 0.186 |
| Severe * CKD | 3 (11.1) | 2 (3.9) | 0.217 |
| Clinical history | |||
| Previous MI | 8 (29.6) | 18 (35.3) | 0.615 |
| Previous PCI | 6 (22.2) | 11 (21.6) | 0.947 |
| Previous stroke | 5 (18.5) | 2 (3.9) | 0.033 |
| Previous CHF | 2 (7.4) | 8 (15.7) | 0.301 |
| Atrial fibrillation | 2 (7.4) | 6 (11.8) | 0.548 |
| Laboratory data | |||
| LDL-C, mg/dL | 109.1 ± 33.7 | 121.7 ± 40.2 | 0.171 |
| HDL-C, mg/dL | 40 (34.5–47.2) | 42 (32.5–48.7) | 0.841 |
| Triglycerides, mg/dL | 125 (89.2–203.7) | 140 (91.2–210) | 0.559 |
| Creatinine, mg/dL | 1 (0.7–1.3) | 0.9 (0.8–1.1) | 0.776 |
| Peak CK-MB, U/L | 23 (12.7–70.2) | 25.8 (15–62) | 0.92 |
| Peak hs-cTnI, ng/L | 72 (38–4665.7 | 55 (30.5–3580) | 0.394 |
| NT-proBNP, pg/mL | 233 (51.5–942) | 133 (44.2–410.7) | 0.341 |
| Leucocytes, 109/L | 9.3 (6.8–10.8) | 8.2 (6.3–9.2) | 0.115 |
| Ne-Ly ratio | 2.9 (2.3–4.3) | 3.1 (2.3–4.2) | 0.979 |
| CRP, mg/dL | 0.4 (0.3–1.2) | 0.4 (0.1–1.9) | 0.389 |
| Hemoglobin, g/dL | 13.9 (12.3–14.8) | 14.1 (12.9–14.6) | 0.360 |
| Echocardiography data | |||
| LVEF, % | 50 (40.7–50) | 50 (48–55) | 0.382 |
| LVEDD, mm | 48 (46.25–51.7) | 48 (44.2–51) | 0.720 |
| Previous medication | |||
| Aspirin | 10 (37) | 17 (33.3) | 0.745 |
| Statin | 20 (74.1) | 37 (72.5) | 0.885 |
| ACEI/ARB | 20 (74.1) | 36 (70.6) | 0.746 |
| Angiographic data | |||
| Culprit vessel | 0.225 | ||
| LM | 2 (7.4) | 1 (2.0) | |
| LAD | 23 (85.2) | 46 (90.2) | |
| LCX | 0 (0) | 3 (5.9) | |
| RCA | 2 (7.4) | 1 (2) | |
| Multivessel disease * | 12 (44.4) | 19 (37.3) | 0.539 |
| Lesion severity | 0.05 | ||
| Non-significant | 3 (11.1) | 17 (33.3) | |
| Borderline | 4 (14.8) | 12 (23.5) | |
| Significant | 17 (63) | 20 (39.2) | |
| Occlusive | 3 (11.1) | 2 (3.9) | |
| OCT data | |||
| Initial lesion | 0.711 | ||
| Plaque erosion | 8 (29.6) | 16 (31.4) | |
| Plaque rupture | 16 (59.3) | 32 (62.7) | |
| CN (protrusive/eruptive) | 3 (11.1) | 3 (5.9) | |
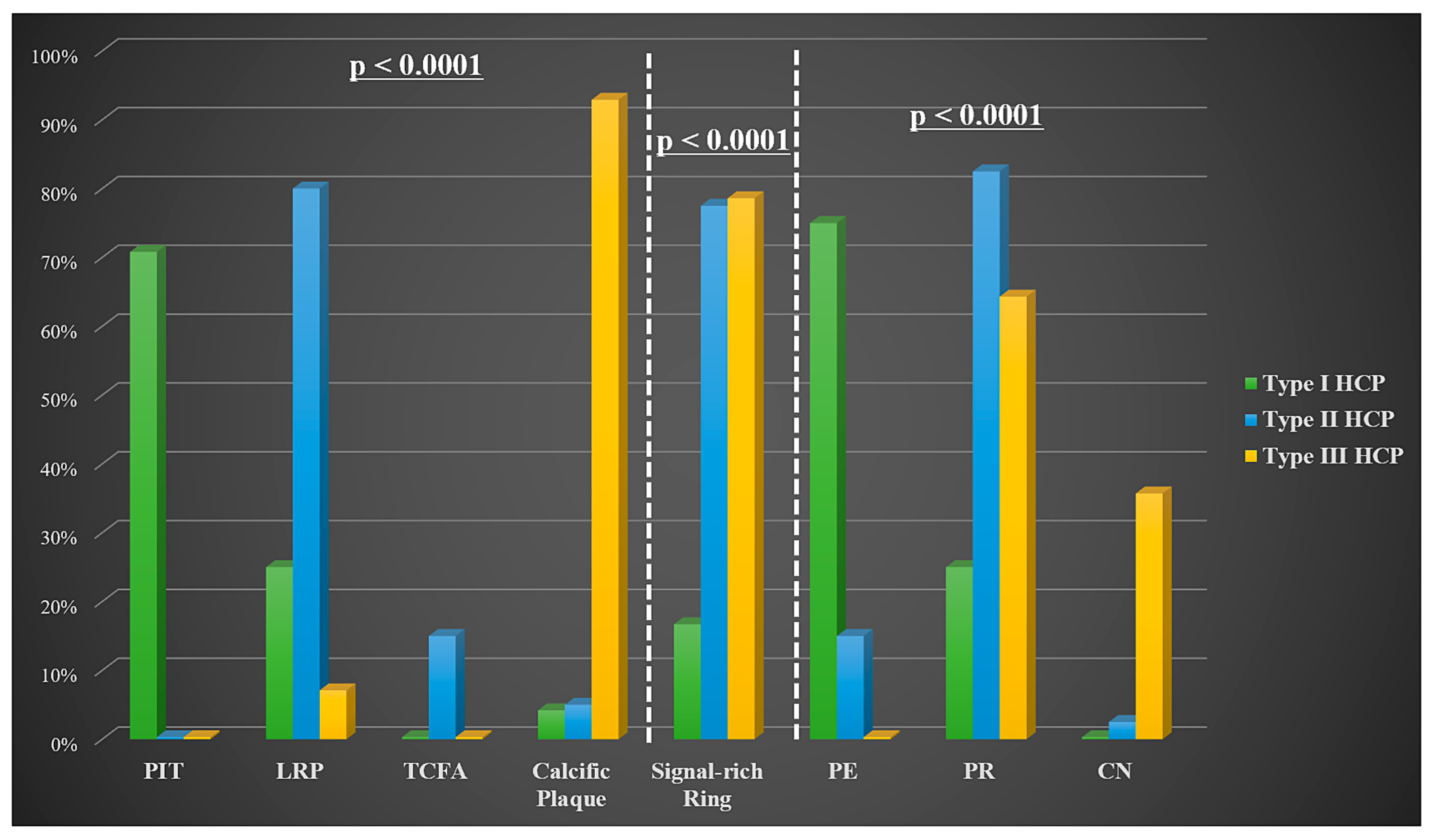
| Underlying plaque morphology | 0.992 | ||
| PIT | 6 (22.2) | 11 (21.6) | |
| LRP | 13 (48.1) | 26 (51) | |
| TCFA | 2 (7.4) | 4 (7.8) | |
| Calcific plaque | 6 (22.2) | 10 (19.6) | |
| Signal-rich arch at tissue interface | 14 (51.9) | 32 (62.7) | 0.355 |
| Ostial HCP involvement | 7 (25.9) | 8 (15.7) | 0.278 |
| Bifurcation HCP involvement | 19 (70.4) | 32 (62.7) | 0.503 |
| RLA, mm2 | 7.0 (5.4–8.4) | 7.5 (6.12–8.7) | 0.756 |
| MLA, mm2 | 1.2 (0.9–2.1) | 2.1 (1.3–3.9) | 0.013 |
| Area stenosis, % | 83 (71.7–83.7) | 70 (53.2–79.7) | 0.001 |
| Lesion length, mm | 26.0 ± 8.8 | 22.3 ± 10.8 | 0.128 |
| Non-culprit HCP on culprit vessel | 7 (25.9) | 18 (35.3) | 0.402 |
| Variable | HCP Type I (n = 24) | HCP Type II (n = 40) | HCP Type III (n = 14) | p-Value |
|---|---|---|---|---|
| Age, years | 57.3 ± 11.3 | 60.8 ± 10.3 | 63.5 ± 10.5 | 0.204 |
| Male gender | 14 (58.3) | 24 (60) | 10 (71.4) | 0.696 |
| Diagnosis | 0.01 | |||
| NSTE-ACS | 13 (54.2) | 32 (80) | 13 (92.9) | |
| STEMI | 11 (45.8) | 8 (20) | 1 (7.1) | |
| Risk factors | ||||
| Hypertension | 20 (83.3) | 32 (80) | 12 (85.7) | 0.874 |
| Diabetes mellitus | 9 (37.5) | 10 (25) | 3 (21.4) | 0.462 |
| Previous hyperCHOL | 15 (62.5) | 28 (70) | 10 (71.4) | 0.786 |
| Smoking habit | 11 (45.8) | 21 (52.5) | 6 (42.9) | 0.778 |
| Overweight | 7 (29.2) | 17 (42.5) | 10 (71.4) | 0.039 |
| Severe * CKD | 1 (4.3) | 3 (7.5) | 1 (7.1) | 0.882 |
| Clinical history | ||||
| Previous MI | 9 (37.5) | 11 (27.5) | 5 (35.7) | 0.672 |
| Previous PCI | 7 (29.2) | 8 (20) | 2 (14.3) | 0.521 |
| Previous stroke | 1 (4.2) | 5 (12.5) | 1 (7.1) | 0.510 |
| Previous CHF | 3 (12.5) | 4 (10) | 3 (21.4) | 0.544 |
| Atrial fibrillation | 2 (8.3) | 3 (7.5) | 3 (21.4) | 0.312 |
| Laboratory data | ||||
| LDL-C, mg/dL | 115.0 ± 43.5 | 121.2 ± 34.9 | 110.4 ± 40.1 | 0.629 |
| HDL-C, mg/dL | 41 (29.5–46.5) | 39.5 (34.5–46) | 46 (33–55) | 0.516 |
| Triglycerides, mg/dL | 128 (85–217) | 131.5 (90.5–187.5) | 149.5 (125–293) | 0.302 |
| Creatinine, mg/dL | 0.8 (0.8–1.1) | 1.0 (0.8–1.1) | 0.9 (0.8–1.0) | 0.565 |
| Peak CK-MB, U/L | 36.5 (17.3–67) | 23 (11.5–55.5) | 18 (15–62) | 0.276 |
| Peak hs-cTnI, ng/L | 1866.5 (37–4088.5) | 44 (927–3688.5) | 42.5 (33–3570) | 0.353 |
| NT-proBNP, pg/mL | 271.5 (50–886.5) | 127.5 (43–366) | 151.5 (78–560) | 0.762 |
| Leucocytes, 109/L | 8.52 (6.3–11.0) | 8.3 (6.8–10.4) | 6.6 (5.7–8.4) | 0.086 |
| Ne-Ly ratio | 3.07 (1.7–4.4) | 2.9 (2.3–4.4) | 3.05 (2.3–3.8) | 0.716 |
| CRP, mg/dL | 0.47 (0.1–3.8) | 0.42 (0.2–1.1) | 0.4 (0.1–2.2) | 0.901 |
| Hemoglobin, g/dL | 13.8 (12.7–14.5) | 14.1 (12.9–14.6) | 14.05 (13.3–15) | 0.639 |
| Echocardiography data | ||||
| LVEF, % | 50 (47–50) | 50 (46–55) | 50 (50–55) | 0.588 |
| LVEDD, mm | 48 (44.5–54) | 48 (44–51) | 50 (48–52) | 0.057 |
| Previous medication | ||||
| Aspirin | 9 (37.5) | 13 (32.5) | 6 (42.9) | 0.770 |
| Statin | 15 (62.5) | 31 (77.5) | 11 (78.6) | 0.372 |
| ACEI/ARB | 15 (62.5) | 29 (72.5) | 12 (85.7) | 0.305 |
| Variable | HCP Type I (n = 24) | HCP Type II (n = 40) | HCP Type III (n = 14) | p-Value |
|---|---|---|---|---|
| Angiographic data | ||||
| Culprit vessel | 0.255 | |||
| LM | 0 (0) | 1 (2.5) | 2 (14.3) | |
| LAD | 21 (87.5) | 36 (90) | 12 (85.7) | |
| LCX | 1 (4.2) | 2 (5) | 0 (0) | |
| RCA | 2 (8.3) | 1 (2.5) | 0 (0) | |
| Multivessel disease * | 6 (25) | 17 (42.5) | 8 (57) | 0.130 |
| Lesion severity | 0.05 | |||
| Non-significant | 11 (45.8) | 8 (20) | 1 (7.1) | |
| Borderline | 3 (12.5) | 7 (17.5) | 6 (42.9) | |
| Significant | 10 (41.7) | 24 (60) | 7 (50) | |
| Occlusive | 0 (0) | 1 (2.5) | 0 (0) | |
| OCT data | ||||
| Ostial HCP involvement | 3 (12.5) | 9 (22.5) | 3 (21.4) | 0.6 |
| Bifurcation HCP involvement | 14 (58.3) | 27 (67.5) | 10 (71.4) | 0.659 |
| RLA, mm2 | 8.2 (6.5–9) | 7.0 (6.1–8.1) | 7.9 (5.4–9.4) | 0.454 |
| MLA, mm2 | 2.8 (1.0–4.3) | 1.4 (1.1–2.6) | 1.8 (1.1–3.0) | 0.586 |
| Area stenosis, % | 67 (51–82.5) | 76.5 (64.5–83) | 71.5 (67–83) | 0.404 |
| Lesion length, mm | 21.5 ± 9.5 | 23.1 ± 9.3 | 28.7 ± 13.0 | 0.098 |
| Non-culprit HCP on culprit vessel | 8 (33.3) | 16 (40) | 1 (7.1) | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homorodean, C.; Onea, H.-L.; Lazar, F.-L.; Ober, M.C.; Spinu, M.; Tataru, D.-A.; Olinic, M.; Popa Ilie, I.R.; Homorodean, R.; Leucuta, D.-C.; et al. OCT-Based Morphological Classification of Healed Coronary Plaques: Insights from Imaging of Fresh Thrombi at Different Stages of Healing and Implications for Post-Stenting Edge Dissections. Medicina 2025, 61, 1440. https://doi.org/10.3390/medicina61081440
Homorodean C, Onea H-L, Lazar F-L, Ober MC, Spinu M, Tataru D-A, Olinic M, Popa Ilie IR, Homorodean R, Leucuta D-C, et al. OCT-Based Morphological Classification of Healed Coronary Plaques: Insights from Imaging of Fresh Thrombi at Different Stages of Healing and Implications for Post-Stenting Edge Dissections. Medicina. 2025; 61(8):1440. https://doi.org/10.3390/medicina61081440
Chicago/Turabian StyleHomorodean, Calin, Horea-Laurentiu Onea, Florin-Leontin Lazar, Mihai Claudiu Ober, Mihail Spinu, Dan-Alexandru Tataru, Maria Olinic, Ioana Rada Popa Ilie, Romana Homorodean, Daniel-Corneliu Leucuta, and et al. 2025. "OCT-Based Morphological Classification of Healed Coronary Plaques: Insights from Imaging of Fresh Thrombi at Different Stages of Healing and Implications for Post-Stenting Edge Dissections" Medicina 61, no. 8: 1440. https://doi.org/10.3390/medicina61081440
APA StyleHomorodean, C., Onea, H.-L., Lazar, F.-L., Ober, M. C., Spinu, M., Tataru, D.-A., Olinic, M., Popa Ilie, I. R., Homorodean, R., Leucuta, D.-C., & Olinic, D.-M. (2025). OCT-Based Morphological Classification of Healed Coronary Plaques: Insights from Imaging of Fresh Thrombi at Different Stages of Healing and Implications for Post-Stenting Edge Dissections. Medicina, 61(8), 1440. https://doi.org/10.3390/medicina61081440









