Experimental Evidence of Caffeic Acid’s Neuroprotective Activity in Alzheimer’s Disease: In Vitro, In Vivo, and Delivery-Based Insights
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. In Vitro Studies
3.1.1. Antioxidant and Redox Modulation
3.1.2. Anti-Amyloid and Anti-Tau Effects
3.2. In Vivo Studies
3.2.1. Pharmacological Models of AD
3.2.2. Transgenic and Genetic Models
3.2.3. Dietary and Metabolic Models
3.3. Delivery Systems
3.4. Nanoformulations for Bioavailability Enhancement
3.5. Targeted Delivery and Surface Modification
3.6. Derivatives
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| APP | Amyloid precursor protein |
| BBB | Blood–brain barrier |
| BChE | Butyrylcholinesterase |
| BDNF | Brain-derived neurotrophic factor |
| CA | Caffeic acid |
| CAPE | Caffeic acid phenethyl ester |
| CAT | Catalase |
| ChAT | Choline acetyltransferase |
| COX | Cyclooxygenase |
| CREB | cAMP response element-binding protein |
| CRP | C-reactive protein |
| CUPRAC | Cupric ion reducing antioxidant capacity |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| eNOS | Endothelial nitric oxide synthase |
| GSH | Glutathione |
| IL | Interleukin |
| MAO | Monoamine oxidase |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| PI3K | Phosphoinositide 3-kinase |
| ROS | Reactive oxygen species |
| SIRT1 | Sirtuin 1 |
| SLNs | Solid lipid nanoparticles |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| Tf | Transferrin |
| TNF-α | Tumor necrosis factor alpha |
References
- Fanlo-Ucar, H.; Picón-Pagès, P.; Herrera-Fernández, V.; ILL-Raga, G.; Muñoz, F.J. The dual role of amyloid beta-peptide in oxidative stress and inflammation: Unveiling their connections in Alzheimer’s disease etiopathology. Antioxidants 2024, 13, 1208. [Google Scholar] [CrossRef]
- Majdi, A.; Sadigh-Eteghad, S.; Rahigh Aghsan, S.; Farajdokht, F.; Vatandoust, S.M.; Namvaran, A.; Mahmoudi, J. Amyloid-β, tau, and the cholinergic system in Alzheimer’s disease: Seeking direction in a tangle of clues. Rev. Neurosci. 2020, 31, 391–413. [Google Scholar] [CrossRef]
- Jurcău, M.C.; Andronie-Cioara, F.L.; Jurcău, A.; Marcu, F.; Ţiț, D.M.; Pașcalău, N.; Nistor-Cseppentö, D.C. The Link between oxidative stress, mitochondrial dysfunction and neuroinflammation in the pathophysiology of Alzheimer’s disease: Therapeutic implications and future perspectives. Antioxidants 2022, 11, 2167. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Saeedi, F.M. Challenges and approaches of drugs such as memantine, donepezil, rivastigmine, and aducanumab in the treatment, control and management of Alzheimer’s disease. Recent Pat. Biotechnol. 2022, 16, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Vitek, G.E.; Decourt, B.M.N.S. Lecanemab (BAN2401): An anti–beta-amyloid monoclonal antibody for the treatment of Alzheimer disease. Expert Opin. Investig. Drugs 2023, 32, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic acids and prevention of cognitive decline: Polyphenols with a neuroprotective role in cognitive disorders and Alzheimer’s disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Khan, A.; Park, J.S.; Kang, M.H.; Lee, H.J.; Ali, J.; Tahir, M.; Choe, K.; Kim, M.O. Caffeic acid, a polyphenolic micronutrient rescues mice brains against Aβ-induced neurodegeneration and memory impairment. Antioxidants 2023, 12, 1284. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rapposelli, S.; Sestito, S.; Herrera-Bravo, J.; Arancibia-Diaz, A.; Salazar, L.A.; Yeskaliyeva, B.; Beyatli, A.; Leyva-Gómez, G.; González-Contreras, C.; et al. Multi-target mechanisms of phytochemicals in Alzheimer’s disease: Effects on oxidative stress, neuroinflammation and protein aggregation. J. Pers. Med. 2022, 12, 1515. [Google Scholar] [CrossRef]
- Gomes, T.M.; Sousa, P.; Campos, C.; Perestrelo, R.; Câmara, J.S. Secondary bioactive metabolites from foods of plant origin as theravention agents against neurodegenerative disorders. Foods 2024, 13, 2289. [Google Scholar] [CrossRef]
- Carecho, R.; Carregosa, D.; dos Santos, C.N. Low molecular weight (poly)phenol metabolites across the blood-brain barrier: The underexplored journey. Brain Plast. 2021, 6, 193–214. [Google Scholar] [CrossRef]
- Grabska-Kobylecka, I.; Kaczmarek-Bak, J.; Figlus, M.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Glabinski, A.; Nowak, D. The Presence of caffeic acid in cerebrospinal fluid: Evidence that dietary polyphenols can cross the blood-brain barrier in humans. Nutrients 2020, 12, 1531. [Google Scholar] [CrossRef]
- Marques, D.; Moura-Louro, D.; Silva, I.P.; Matos, S.; dos Santos, C.N.; Figueira, I. Unlocking the potential of low-molecular-weight (Poly)phenol metabolites: Protectors at the blood-brain barrier frontier. Neurochem. Int. 2024, 179, 105836. [Google Scholar] [CrossRef]
- Le Sayec, M.; Carregosa, D.; Khalifa, K.; de Lucia, C.; Aarsland, D.; Santos, C.N.; Rodriguez-Mateos, A. Identification and quantification of (poly)phenol and methylxanthine metabolites in human cerebrospinal fluid: Evidence of their ability to cross the BBB. Food Funct. 2023, 14, 8893–8902. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Jiménez, D.; Corella-Salazar, D.A.; Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Montiel-Herrera, M.; Salazar-López, N.J.; Rodrigo-Garcia, J.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Phenolic compounds that cross the blood-brain barrier exert positive health effects as central nervous system antioxidants. Food Funct. 2021, 12, 10356–10369. [Google Scholar] [CrossRef] [PubMed]
- Silakari, P.; Piplani, P.; Kumar, A.; Grewal, A.K. Novel piperazine-benzoquinone derivative as a possible lead molecule selectively targeting AChE for the management of dementia in Alzheimer ’s disease. Alzheimer’s Dement. 2024, 20, e087881. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Yu, C.; Dai, C.; Chen, J.; Zhong, J.; Yang, Y.; Huiling Lin, H.; Chen, X.; Zhang, Q.; et al. A novel carbamate-based hybrid derivative with anti-neuroinflammatory properties as a selective butyrylcholinesterase inhibitor for Alzheimer’s disease therapy. Bioorg. Chem. 2025, 161, 108551. [Google Scholar] [CrossRef]
- Darreh-Shori, T.; Baidya, A.T.K.; Brouwer, M.; Kumar, A.; Kumar, R. Repurposing duloxetine as a potent butyrylcholinesterase inhibitor: Potential cholinergic enhancing benefits for elderly individuals with depression and cognitive impairment. ACS Omega 2024, 9, 37299–37309. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; Maaty, D.A.M.; Al-Saraireh, Y.M. Qualitative chemical compounds analysis and in vitro estimation of antiproliferative, antidiabetic and anti-Alzheimer’s disease effects of Ononis natrix (L.) family Fabaceae. Pharmacia 2024, 71, 1–11. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Anti-Helicobacter pylori, antioxidant, antidiabetic, and anti-Alzheimer’s activities of laurel leaf extract treated by moist heat and molecular docking of its flavonoid constituent, naringenin, against acetylcholinesterase and butyrylcholinesterase. Life 2023, 13, 1512. [Google Scholar] [CrossRef]
- Karageili, H.; Yilmaz, M.A.; Erturk, A.; Kiziltas, H.; Cuven, L.; Alwasel, S.H.; Gulcin, I. Comprehensive metabolite profiling of Berdav propolis using LC-MS/MS: Determination of antioxidant, anticholinergic, antiglaucoma, and antidiabetic effects. Molecules 2023, 28, 1739. [Google Scholar] [CrossRef]
- Ojo, O.A.; Gyebi, G.A.; Ezenabor, E.H.; Iyobhebhe, M.; Emmanuel, D.A.; Adelowo, O.A.; Olujinmi, F.E.; Ogunwale, T.E.; Babatunde, D.E.; Ogunlakin, A.D.; et al. Exploring beetroot (Beta vulgaris L.) for diabetes mellitus and Alzheimer’s disease dual therapy: In vitro and computational studies. RSC Adv. 2024, 14, 19362–19380. [Google Scholar] [CrossRef]
- Gürbüz, P.; Dokumacı, A.H.; Gündüz, M.G.; Perez, C.; Göger, F.; Paksoy, M.Y.; Yerer, M.B.; Demirezer, L.Ö. In vitro biological activity of Salvia fruticosa Mill. infusion against amyloid β-peptide-induced toxicity and inhibition of GSK-3β, CK-1δ, and BACE-1 enzymes relevant to Alzheimer’s disease. Saudi Pharm. J. 2021, 29, 236–243. [Google Scholar] [CrossRef]
- Deshmukh, R.; Kaundal, M.; Bansal, V. Samardeep Caffeic acid attenuates oxidative stress, learning and memory deficit in intra-cerebroventricular streptozotocin induced experimental dementia in rats. Biomed. Pharmacother. 2016, 81, 56–62. [Google Scholar] [CrossRef]
- Rezaee, N.; Hone, E.; Sohrabi, H.R.; Johnson, S.; Zhong, L.; Chatur, P.; Gunzburg, S.; Martins, R.N.; Binosha Fernando, W.M.A.D. Sorghum grain polyphenolic extracts demonstrate neuroprotective effects related to Alzheimer’s disease in cellular assays. Foods 2024, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Swaraz, A.M.; Sultana, F.; Bari, M.W.; Ahmed, K.S.; Hasan MIslam, M.M.; Islam, M.A.; Satter, M.A.; Hossain, M.d.H.; Islam, M.d.S.; Khan, M.d.I.; et al. Phytochemical profiling of Blumea laciniata (Roxb.) DC. and its phytopharmaceutical potential against diabetic, obesity, and Alzheimer’s. Biomed. Pharmacother. 2021, 141, 111859. [Google Scholar] [CrossRef] [PubMed]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The effect of steaming and fermentation on nutritive values, antioxidant activities, and inhibitory properties of tea leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Zhang, R.; Yu, H.; Zhang, L. Network pharmacology mechanism of Rosmarinus officinalis L.(Rosemary) to improve cell viability and reduces apoptosis in treating Alzheimer’s disease. BMC Complement. Med. Ther. 2025, 25, 94. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Dubey, R.; Gomatam, A.; Chakor, R.; Kshirsagar, A.; Lohidasan, S. Deciphering the multi-functional role of Indian propolis for the management of Alzheimer’s disease by integrating LC–MS/MS, network pharmacology, molecular docking, and in-vitro studies. Mol. Divers. 2024, 28, 4325–4342. [Google Scholar] [CrossRef]
- Sul, D.; Kim, H.S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009, 84, 257–262. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Saha, S.; Dutta, S.; Mahalanobish, S.; Sil, P.C. Nutraceuticals: An emerging therapeutic approach against the pathogenesis of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 100–114. [Google Scholar] [CrossRef]
- Rezaee, N.; Hone, E.; Sohrabi, H.; Abdulraheem, R.; Johnson, S.K.; Gunzburg, S.; Martins, R.N.; Binosha Fernando, W.M.A.D. Investigating the impact of sorghum on tau protein phosphorylation and mitochondrial dysfunction modulation in Alzheimer’s disease: An in vitro study. Nutrients 2025, 17, 516. [Google Scholar] [CrossRef]
- Touati, I.; Abdalla, M.; Ali, N.H.; Alruwaili, R.; Alruwaili, M.; Britel, M.R.; Maurady, A. Constituents of Stachys plants as potential dual inhibitors of AChE and NMDAR for the treatment of Alzheimer’s disease: A molecular docking and dynamic simulation study. J. Biomol. Struct. Dyn. 2024, 42, 2586–2602. [Google Scholar] [CrossRef]
- Zhang, F.; Li, S.; Liu, C.; Fang, K.; Jiang, Y.; Zhang, J.; Lan, J.; Zhu, L.; Pang, H.; Wang, G. Rapid screening for acetylcholinesterase inhibitors in Selaginella doederleinii Hieron by using functionalized magnetic Fe3O4 nanoparticles. Talanta 2022, 243, 123284. [Google Scholar] [CrossRef]
- Fatima, I.; Safdar, N.; Akhtar, W.; Munir, A.; Saqib, S.; Ayaz, A.; Bahadur, S.; Alrefaei, A.F.; Ullah, F.; Zaman, W. Evaluation of potential inhibitory effects on acetylcholinesterase, pancreatic lipase, and cancer cell lines using raw leaves extracts of three fabaceae species. Heliyon 2023, 9, e15909. [Google Scholar] [CrossRef]
- Callizot, N.; Campanari, M.L.; Rouvière, L.; Jacquemot, G.; Henriques, A.; Garayev, E.; Poindron, P. Huperzia serrata Extract ‘NSP01’ with neuroprotective effects-potential synergies of huperzine a and polyphenols. Front. Pharmacol. 2021, 12, 681532. [Google Scholar] [CrossRef]
- Işık, M.; Beydemir, Ş. The impact of some phenolic compounds on serum acetylcholinesterase: Kinetic analysis of an enzyme/inhibitor interaction and molecular docking study. J. Biomol. Struct. Dyn. 2021, 39, 6515–6523. [Google Scholar] [CrossRef]
- Shamsi, A.; Shahwan, M.; Das Gupta, D.; Abdullah, K.M.; Khan, M.S. Implication of caffeic acid for the prevention and treatment of Alzheimer’s disease: Understanding the binding with human transferrin using in silico and in vitro approaches. Mol. Neurobiol. 2024, 61, 2176–2185. [Google Scholar] [CrossRef]
- Shaji, D.; Das, A.; Suzuki, R.; Nagura, Y.; Sabishiro, H.; Kurita, N. Proposal of novel ApoE4 inhibitors from the natural spice Cinnamon for the treatment of Alzheimer’s disease: Ab initio molecular simulations. Biophys. Chem. 2023, 296, 106990. [Google Scholar] [CrossRef]
- Akomolafe, S.F. The effects of caffeine, caffeic acid, and their combination on acetylcholinesterase, adenosine deaminase and arginase activities linked with brain function. J. Food Biochem. 2017, 41, e12401. [Google Scholar] [CrossRef]
- Khan, K.; Emad, N.A.; Sultana, Y. Inducing agents for Alzheimer’s disease in animal models. J. Explor. Res. Pharmacol. 2024, 9, 169–179. [Google Scholar] [CrossRef]
- Ye, M.; Jang, D.; Lee, S.Y.; Kim, K.R.; Rhie, S.J.; Oh, J.K.; Shim, I. Neuroprotective effect of Ixeris dentata extract on trimethyltin-induced memory impairment in rats. Curr. Issues Mol. Biol. 2024, 46, 11772–11782. [Google Scholar] [CrossRef]
- Balkrishna, A.; Bhattacharya, K.; Shukla, S.; Varshney, A. Neuroprotection by polyherbal medicine divya-medha-vati against scopolamine-induced cognitive impairment through modulation of oxidative stress, acetylcholine activity, and cell signaling. Mol. Neurobiol. 2024, 61, 1363–1382. [Google Scholar] [CrossRef]
- Saadullah, M.; Batool, J.A.; Rashad, M.; Asif, M.; Chauhdary, A.B.Z. Exploration of neuroprotective and cognition boosting effects of Mazus pumilus in Alzheimer’s disease model. J. Complement. Integr. Med. 2024, 21, 461–471. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Ashraf, M.; Alhasaniah, A.H.; Ullah, H.; Zeb, A.; Ghufran, M.; Fahad, S.; Ayaz, M.; Daglia, M. Polyphenol-enriched Desmodium elegans DC. ameliorate scopolamine-induced amnesia in animal model of Alzheimer’s disease: In vitro, in vivo and in silico approaches. Biomed. Pharmacother. 2023, 165, 115144. [Google Scholar] [CrossRef]
- Teng, Y.; Yuan, Q.; Wu, Y.; Wu, S.; Su, J.; Zhang, P.; Zhang, Y. Research on the chemical constituents against Alzheimer’s Disease of the fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino. Chem. Biodivers. 2023, 20, e202301075. [Google Scholar]
- Sun, R.; Wu, T.; Xing, S.; Wei, S.; Bielicki, J.K.; Pan, X.; Zhou, M.; Jianbin Chen, J. Caffeic acid protects against atherosclerotic lesions and cognitive decline in ApoE−/−mice. J. Pharmacol. Sci. 2023, 151, 110–118. [Google Scholar] [CrossRef]
- Zhuo, Y.; Fu, X.; Jiang, Q.; Lai, Y.; Gu, Y.; Fang, S.; Chen, H.; Liu, C.; Pan, H.; Wu, Q.; et al. Systems pharmacology-based mechanism exploration of Acanthopanax senticosusin for Alzheimer’s disease using UPLC-Q-TOF-MS, network analysis, and experimental validation. Eur. J. Pharmacol. 2023, 954, 175895. [Google Scholar] [CrossRef]
- Ogunsuyi, O.B.; Olasehinde, T.A.; Oboh, G. Neuroprotective properties of solanum leaves in transgenic Drosophila melanogaster model of Alzheimer’s disease. Biomarkers 2022, 27, 587–598. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Li, C.; Ma, L.; Zhao, Z.; Guan, S.; Wang, L. Caffeic acid protects against Aβ toxicity and prolongs lifespan in: Caenorhabditis elegans models. Food Funct. 2021, 12, 1219–1231. [Google Scholar] [CrossRef]
- Banu, Z.; Poduri, R.R.; Bhattamisra, S.K. Phytochemical profiling, in silico molecular docking and ADMET prediction of alkaloid rich fraction of Elaeocarpus angustifolius blume seeds against Alzheimer’s disease. Nat. Prod. Res. 2025, 11, 1–9. [Google Scholar] [CrossRef]
- Mohamed, R.S.; Fouda, K.; Salama, A.; Akl, E.M. Peanut meal-derived bioactive compounds: Extraction, co-extrusion encapsulation and neuroprotection against aluminum-induced Alzheimer’s disease via in silico and in vivo studies. Phytomed. Plus 2024, 4, 100588. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Szwajgier, D.; Baranowska-Wójcik, E.; Pérez-Sánchez, H.; Carmena-Bargueño, M.; Sosnowska, B.; Budryn, G. Effect of inhibiting butyrylcholinesterase activity using fractionated coffee extracts digested in vitro in gastrointestinal tract: Docking simulation and calorimetric and studies. Nutrients 2023, 15, 2366. [Google Scholar] [CrossRef]
- Kadar, M.A.N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Comparable benefits of stingless bee honey and caffeic acid in mitigating the negative effects of metabolic syndrome on the brain. Antioxidants 2022, 11, 2154. [Google Scholar] [CrossRef]
- Rajkumar, M.; Govindaraj, P.; Vimala, K.; Thangaraj, R.; Kannan, S. Chitosan/PLA-loaded magnesium oxide nanocomposite to attenuate oxidative stress, neuroinflammation and neurotoxicity in rat models of Alzheimer’s disease. Metab. Brain Dis. 2024, 39, 487–508. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Abbas, H.; Zewail, M.; Noureldin, M.H.; Ali, M.M.; Shamaa, M.M.; Khattab, M.A.; Ibrahim, N. Neuroprotective effect of Artichoke-based nanoformulation in sporadic Alzheimer’s disease mouse model: Focus on antioxidant, anti-inflammatory, and amyloidogenic pathways. Pharmaceuticals 2022, 15, 1202. [Google Scholar] [CrossRef]
- Hu, Y.; Cui, J.; Sun, J.; Liu, X.; Gao, S.; Mei, X.; Wu, C.; Tian, H. A novel biomimetic nanovesicle containing caffeic acid-coupled carbon quantum dots for the the treatment of Alzheimer’s disease via nasal administration. J. Nanobiotechnol. 2024, 22, 642. [Google Scholar] [CrossRef]
- Andrade, S.; Pereira, M.C.; Loureiro, J.A. Caffeic acid loaded into engineered lipid nanoparticles for Alzheimer’s disease therapy. Colloids Surf. B Biointerfaces 2023, 225, 113270. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Fang, J.; He, Y.; Liu, M.; Hong, Z.; Chai, Y. Simultaneous determination of seven lipophilic and hydrophilic components in Salvia miltiorrhiza Bunge by LC-MS/MS method and its application to a transport study in a blood-brain-barrier cell model. Molecules 2022, 27, 657. [Google Scholar] [CrossRef]
- Luo, Z.; Wan, Q.; Han, Y.; Li, Z.; Li, B. CAPE-pNO2 ameliorates diabetic brain injury through modulating Alzheimer’s disease key proteins, oxidation, inflammation and autophagy via a Nrf2-dependent pathway. Life Sci. 2021, 287, 119929. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
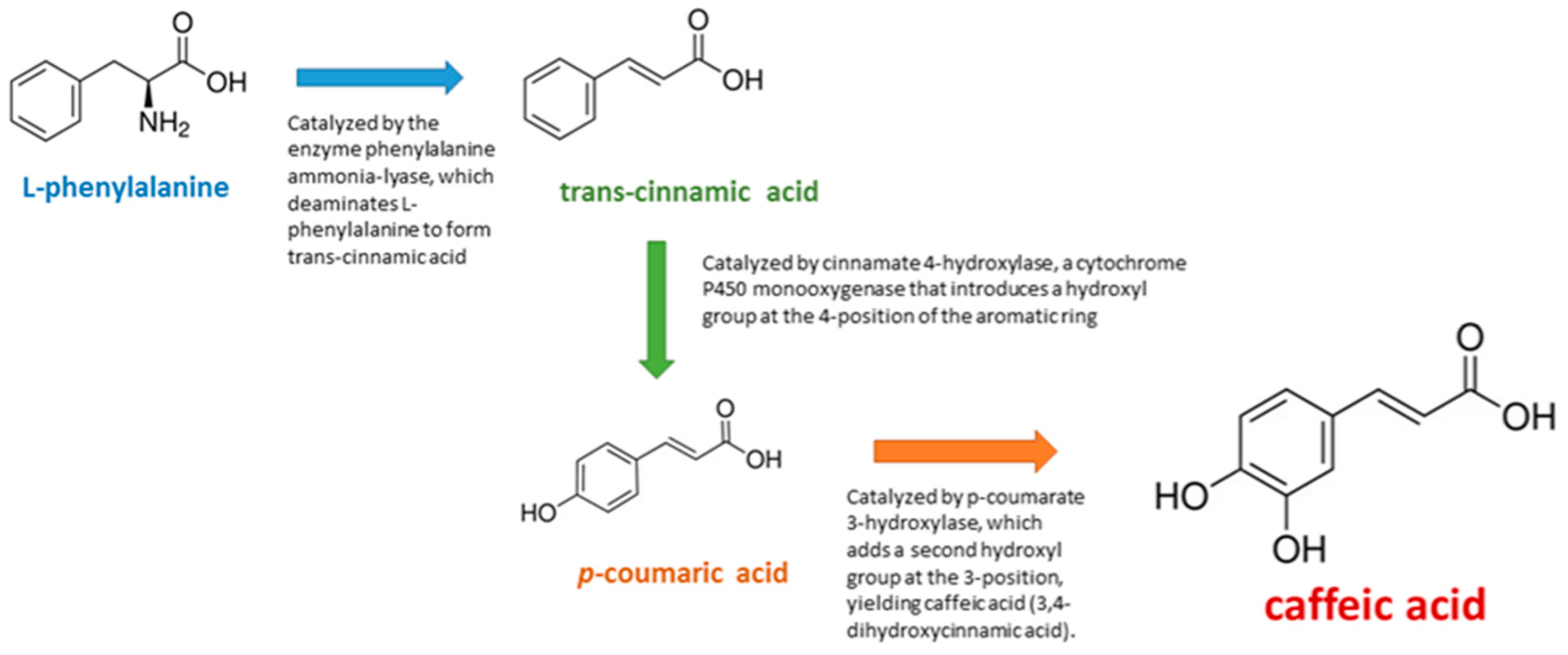
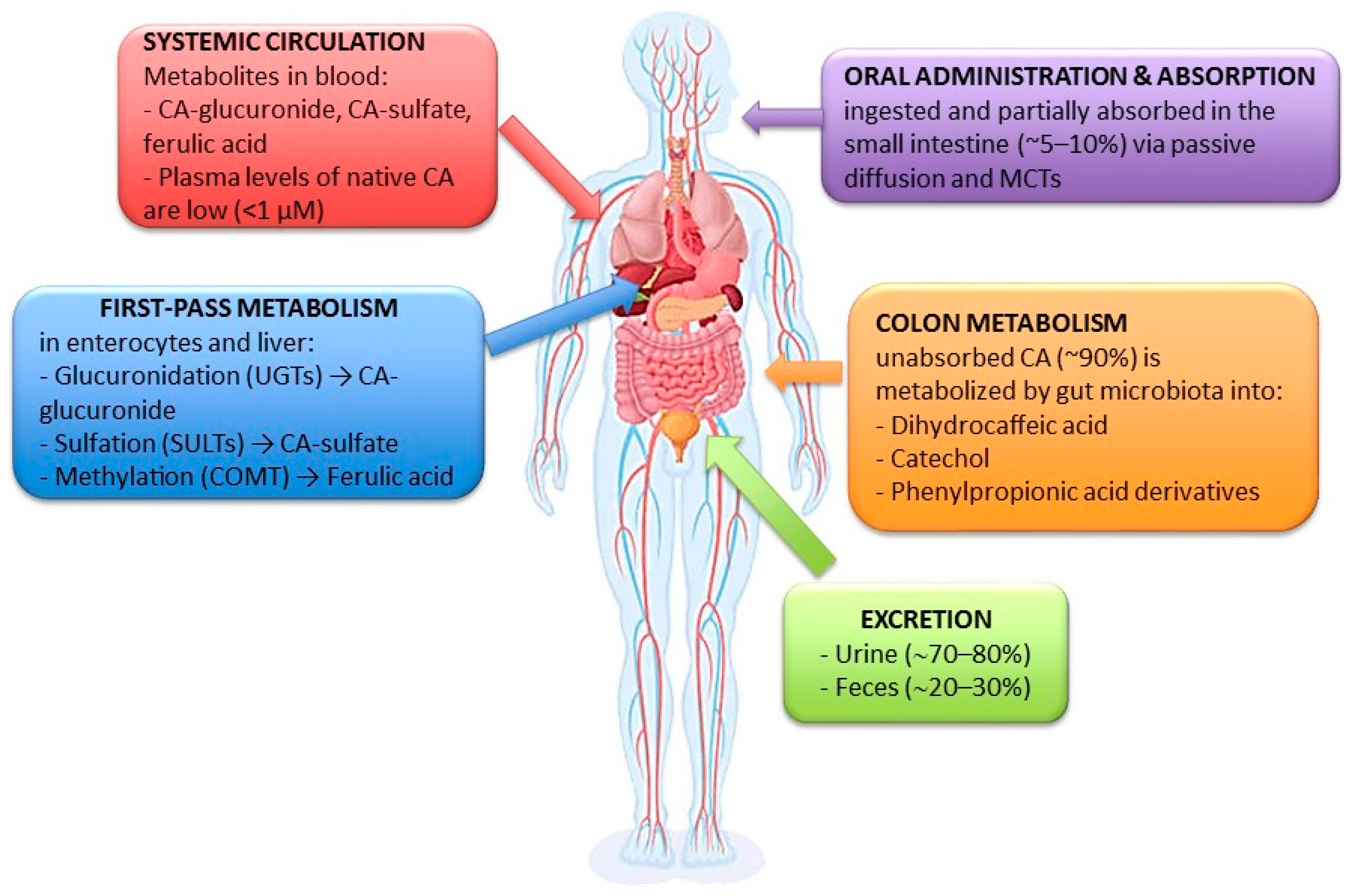

| Reference (No.) | Experimental Model/Method | Biological Target/Mechanism | Main Outcomes/Findings | Study Type |
|---|---|---|---|---|
| [19] | Methanolic extract of Ononis natrix | BChE | Potent BChE inhibition (IC50 = 9 µg/mL); CA confirmed active | In vitro |
| [20] | Laurus nobilis extract with moist heat processing | BChE | Significant inhibition (IC50 = 17.3 µg/mL); CA enriched by processing | In vitro |
| [21] | Propolis extract from Berdav region | AChE | IC50 = 3.4 μg/mL; CA showed selective AChE inhibition | In vitro |
| [22] | Beta vulgaris extract and docking | AChE/BChE | Moderate dual inhibition; CA confirmed via docking | In vitro |
| [23] | Salvia fruticosa extract and GSK-3β inhibition | GSK-3β | Inhibition through kinase pathway modulation; docking with CA | In vitro |
| [25] | Sorghum bicolor extracts on BE(2)-M17 cells | ROS, Aβ aggregation | ↓ ROS, ↑ viability, anti-amyloid effect (up to 78%) | In vitro |
| [26] | Blumea laciniata extract (DPPH, ABTS) | Antioxidant enzymes | CA among compounds contributing to strong antioxidant activity | In vitro |
| [27] | Camellia sinensis (fermentation) | Antioxidant and AChE/BChE | Improved antioxidant and cholinesterase inhibition after fermentation | In vitro |
| [28] | Rosmarinus officinalis on HT22 cells | Aβ25–35, MMP9, MAPK14 | Restored mitochondria, ↓ apoptosis, confirmed CA binding | In vitro |
| [29] | Indian propolis (PC-12 cells) | PTGS2, Oxidative markers | Dose-dependent antioxidant effects; CA derivative with COX-2 affinity | In vitro |
| [32] | Sorghum extracts on BE(2)-M17 cells | Tau, Mitochondria | ↓ p-Tau, ↑ ATP; CA-rich extract reversed Aβ toxicity | In vitro |
| [33] | Stachys species screening | AChE, NMDAR | CA as dual ligand with stable dynamics; favorable ADMET | In silico |
| [34] | AChE-functionalized nanoparticles | AChE | CA as negative control; specificity validation | In vitro |
| [35] | Methanolic extract of Sophora mollis | AChE, Antioxidant | CA (45.2 ppm) showed IC50 = 75.96 μg/mL; neuroprotective via AChE inhibition and antioxidant activity | In vitro |
| [36] | Huperzia serrata formulation (NSP01) with HA, CA, ferulic acid | Glutamate, Aβ1–42, ERK1/2 | CA synergized with HA, preserved neurons, activated ERK1/2; no additional AChE inhibition | In vitro |
| [37] | CA kinetic and docking study | AChE | Moderate inhibition (IC50 = 16.80 µM; Kᵢ = 12.42 µM); binds AChE active site residues | In vitro |
| [38] | CA–transferrin interaction (molecular dynamics) | Iron homeostasis, Oxidative stress | CA binds Htf iron pocket; suggests role in metal-regulated oxidative stress in AD | In silico |
| [39] | CA docking to ApoE4 (molecular simulations) | ApoE4 | Strong binding (−101.9 kcal/mol) to ApoE4; potential for ApoE4-targeted AD therapy | In silico |
| [42] | TMT-induced rats + Ixeris dentata/CA | AChE, CREB, ChAT | ↑ memory, ↑ CREB/ChAT expression; CA effective | In vivo |
| [43] | Scopolamine-induced mouse model + Divya-Medha-Vati | AChE, ROS, BDNF | ↑ cognition, ↓ oxidative stress; CA among active compounds | In Vivo |
| [44] | Sodium azide rat model + Mazus pumilus | AChE, Aβ, Tau | ↑ cognition, ↓ plaques/tangles; CA present via HPLC | In vivo |
| [45] | Scopolamine-induced mouse model + Desmodium extract | AChE, BChE, ROS | ↑ cognition; docking supports CA action | In vivo |
| [46] | D-galactose + AlCl3 model + Physalis extract | Aβ, Tau, p38 MAPK | ↓ amyloid/tau, ↓ inflammation; CA among components | In vivo |
| [47] | ApoE−/− mice + CA | Aβ, Inflammation, ABCA1 | ↓ Aβ, ↓ cytokines, ↑ memory; dual action | In vivo |
| [48] | Scopolamine-induced mouse model + Acanthopanax extract | AChE, Apoptosis | ↑ cognition, ↓ AChE/apoptosis; CA interaction confirmed | In vivo |
| [49] | Drosophila APP/BACE1 model + Solanum extract | Aβ, AChE, MAO | ↓ Aβ, ↑ behavior; CA-linked effects | In vivo |
| [50] | C. elegans Aβ model + CA | DAF-16/FOXO, ROS | ↑ lifespan, ↓ Aβ, ↑ stress genes | In vivo |
| [51] | Elaeocarpus extract | TACE, AChE, APP | CA binds TACE; broad multitarget profile | In vivo |
| [52] | AlCl3-induced rats + peanut extract | AChE, TNF-α | ↓ AChE, ↓ neuroinflammation; CA bioavailability enhanced | In vivo |
| [8] | Aβ1–42 mice + CA (50 mg/kg) | BDNF, Aβ, PI3K/Akt | ↑ synaptic markers, ↓ inflammation | In vivo |
| [53] | Simulated digestion of coffee polyphenols | BChE | Green coffee → ↑ BChE inhibition; CA bioactive | In vivo |
| [54] | Metabolic syndrome rats + CA | TNF-α, IL-6, BDNF | ↓ cytokines, ↑ BDNF; partial reversal | In vivo |
| [55] | STZ rat + CH/PLA/MgONCs | AChE, ATP, TNF-α | ↑ memory, ↓ oxidative stress, CA binds Aβ42 | Nanoformulation |
| [56] | STZ mouse + ART-SLNs | Aβ, Tau, TNF-α | ↑ memory, ↓ Tau/Aβ; SLN ↑ brain delivery | Nanoformulation |
| [57] | 5xFAD mice + CDs-CA-MGs | Aβ, IL-6, TNF-α | ↓ Aβ, ↑ IL-10; CA release in inflammation | Targeted Delivery |
| [58] | Liposomal CA (Tf-conjugated) | Aβ aggregation | Sustained CA release, ↓ fibrils, ↑ BBB transport | Targeted Delivery |
| [59] | BBB model with CA transport assay | BBB permeability | ↑ CA permeability, low efflux | Derivative |
| [60] | Diabetic mice + CAPE-pNO2 | Aβ, p-Tau, Nrf2 | ↓ Aβ/Tau, ↑ Sirt1/Nrf2; multitarget protection | Derivative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Tuberoso, C.I.G.; Jerković, I. Experimental Evidence of Caffeic Acid’s Neuroprotective Activity in Alzheimer’s Disease: In Vitro, In Vivo, and Delivery-Based Insights. Medicina 2025, 61, 1428. https://doi.org/10.3390/medicina61081428
Kowalczyk A, Tuberoso CIG, Jerković I. Experimental Evidence of Caffeic Acid’s Neuroprotective Activity in Alzheimer’s Disease: In Vitro, In Vivo, and Delivery-Based Insights. Medicina. 2025; 61(8):1428. https://doi.org/10.3390/medicina61081428
Chicago/Turabian StyleKowalczyk, Adam, Carlo Ignazio Giovani Tuberoso, and Igor Jerković. 2025. "Experimental Evidence of Caffeic Acid’s Neuroprotective Activity in Alzheimer’s Disease: In Vitro, In Vivo, and Delivery-Based Insights" Medicina 61, no. 8: 1428. https://doi.org/10.3390/medicina61081428
APA StyleKowalczyk, A., Tuberoso, C. I. G., & Jerković, I. (2025). Experimental Evidence of Caffeic Acid’s Neuroprotective Activity in Alzheimer’s Disease: In Vitro, In Vivo, and Delivery-Based Insights. Medicina, 61(8), 1428. https://doi.org/10.3390/medicina61081428








