Association of Magnesium Deficiency and Reduction in Blood Pressure After Chemotherapy in Previously Hypertensive Cancer Patients: The Role of Chemotherapy and Magnesium Levels
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tini, G.; Sarocchi, M.; Tocci, G.; Arboscello, E.; Ghigliotti, G.; Novo, G.; Brunelli, C.; Lenihan, D.; Volpe, M.; Spallarossa, P. Arterial Hypertension in Cancer: The Elephant in the Room. Int. J. Cardiol. 2019, 281, 133–139. [Google Scholar] [CrossRef]
- Jain, M.; Townsend, R.R. Chemotherapy Agents and Hypertension: A Focus on Angiogenesis Blockade. Curr. Hypertens. Rep. 2007, 9, 320–328. [Google Scholar] [CrossRef]
- Adhikari, A.; Asdaq, S.M.B.; Al Hawaj, M.A.; Chakraborty, M.; Thapa, G.; Bhuyan, N.R.; Imran, M.; Alshammari, M.K.; Alshehri, M.M.; Harshan, A.A.; et al. Anticancer Drug-Induced Cardiotoxicity: Insights and Pharmacogenetics. Pharmaceuticals 2021, 14, 970. [Google Scholar] [CrossRef]
- Katsi, V.; Magkas, N.; Georgiopoulos, G.; Athanasiadi, E.; Virdis, A.; Masi, S.; Kliridis, P.; Hatziyanni, A.; Tsioufis, C.; Tousoulis, D. Arterial Hypertension in Patients under Antineoplastic Therapy: A Systematic Review. J. Hypertens. 2019, 37, 884–901. [Google Scholar] [CrossRef]
- Nakano, R.; Momo, K.; Matsuzaki, A.; Sakai, A.; Uchikura, T.; Tanaka, K.; Numazawa, S.; Sasaki, T. Irinotecan-Induced Severe Hypotension in a Patient with Lung Cancer. Clin. Case Rep. 2022, 10, e05718. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Santacruz, E.; García-Sancho, P.; Marengo, A.P.; Guerrero-Pérez, F.; Pian, H.; Fajardo, C.; Villabona, C.; Díez, J.J. Pheochromocytoma: A Three-Decade Clinical Experience in a Multicenter Study. Rev. Clin. Esp. 2021, 221, 18–25. [Google Scholar] [CrossRef]
- Castel, M.; Despas, F.; Modesto, A.; Gales, C.; Honton, B.; Galinier, M.; Senard, J.-M.; Pathak, A. Effets Indésirables Cardiaques Des Chimiothérapies. La Presse Médicale 2013, 42, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Angel-Korman, A.; Rapoport, V.; Leiba, A. The Relationship between Hypertension and Cancer. Isr. Med. Assoc. J. 2022, 24, 165–169. [Google Scholar]
- Gums, J.G. Magnesium in Cardiovascular and Other Disorders. Am. J. Health Syst. Pharm. 2004, 61, 1569–1576. [Google Scholar] [CrossRef]
- Lajer, H.; Daugaard, G. Cisplatin and Hypomagnesemia. Cancer Treat. Rev. 1999, 25, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Whang, R.; Ryder, K.W. Frequency of Hypomagnesemia and Hypermagnesemia. Requested vs. Routine. JAMA 1990, 263, 3063–3064. [Google Scholar] [CrossRef]
- Kuruppath, J.; Patel, P. Hypomagnesemia and Hypokalemia: Considerations for Cancer Care. Clin. J. Oncol. Nurs. 2022, 26, 313–317. [Google Scholar] [CrossRef]
- Altura, B.M.; Altura, B.T. Role of Magnesium in Patho-Physiological Processes and the Clinical Utility of Magnesium Ion Selective Electrodes. Scand. J. Clin. Lab. Investig. 1996, 224, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Liamis, G.; Hoorn, E.J.; Florentin, M.; Milionis, H. An Overview of Diagnosis and Management of Drug-Induced Hypomagnesemia. Pharmacol. Res. Perspect. 2021, 9, e00829. [Google Scholar] [CrossRef] [PubMed]
- Tanzawa, S.; Yoshioka, H.; Misumi, T.; Miyauchi, E.; Ninomiya, K.; Murata, Y.; Takeshita, M.; Kinoshita, F.; Fujishita, T.; Sugawara, S.; et al. Clinical Impact of Hypomagnesemia Induced by Necitumumab plus Cisplatin and Gemcitabine Treatment in Patients with Advanced Lung Squamous Cell Carcinoma: A Subanalysis of the NINJA Study. Ther. Adv. Med. Oncol. 2025, 17, 1–16. [Google Scholar] [CrossRef]
- Workeneh, B.T.; Uppal, N.N.; Jhaveri, K.D.; Rondon-Berrios, H. Hypomagnesemia in the Cancer Patient. Kidney360 2021, 2, 154–166. [Google Scholar] [CrossRef]
- Liu, W.; Qdaisat, A.; Soliman, P.T.; Ramondetta, L.; Lopez, G.; Narayanan, S.; Zhou, S.; Cohen, L.; Bruera, E.; Yeung, S.-C.J. Hypomagnesemia and Survival in Patients with Ovarian Cancer Who Received Chemotherapy with Carboplatin. Oncologist 2019, 24, e312–e317. [Google Scholar] [CrossRef] [PubMed]
- Karlstaedt, A.; Moslehi, J.; de Boer, R.A. Cardio-Onco-Metabolism: Metabolic Remodelling in Cardiovascular Disease and Cancer. Nat. Rev. Cardiol. 2022, 19, 414–425. [Google Scholar] [CrossRef]
- Yin, Y.; Zhou, Y.; Zhou, J.; Zhao, L.; Hu, H.; Xiao, M.; Niu, B.; Peng, J.; Dai, Y.; Tang, Y. Cisplatin Causes Erectile Dysfunction by Decreasing Endothelial and Smooth Muscle Content and Inducing Cavernosal Nerve Senescence in Rats. Front. Endocrinol 2023, 14, 1096723. [Google Scholar] [CrossRef]
- Hanson, M.; Gluckman, P. Endothelial Dysfunction and Cardiovascular Disease: The Role of Predictive Adaptive Responses. Heart 2005, 91, 864–866. [Google Scholar] [CrossRef]
- Stelwagen, J.; Lubberts, S.; Steggink, L.C.; Steursma, G.; Kruyt, L.M.; Donkerbroek, J.W.; van Roon, A.M.; van Gessel, A.I.; van de Zande, S.C.; Meijer, C.; et al. Vascular Aging in Long-Term Survivors of Testicular Cancer More than 20 Years after Treatment with Cisplatin-Based Chemotherapy. Br. J. Cancer 2020, 123, 1599–1607. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Niemann Nejsum, L.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with Secondary Hypocalcemia Is Caused by Mutations in TRPM6, a New Member of the TRPM Gene Family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Renigunta, A.; Konrad, M.; Gomes, A.S.; Schneeberger, E.E.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and Claudin-19 Interact and Form a Cation-Selective Tight Junction Complex. J. Clin. Investig. 2008, 118, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Steyger, P.S. An Integrated View of Cisplatin-Induced Nephrotoxicity and Ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Suppadungsuk, S.; Phitakwatchara, W.; Reungwetwattana, T.; Pathumarak, A.; Phakdeekitcharoen, B.; Kitiyakara, C.; Srisuwarn, P.; Davenport, A.; Nongnuch, A. Preloading Magnesium Attenuates Cisplatin-Associated Nephrotoxicity: Pilot Randomized Controlled Trial (PRAGMATIC Study). ESMO Open 2022, 7, 100351. [Google Scholar] [CrossRef]
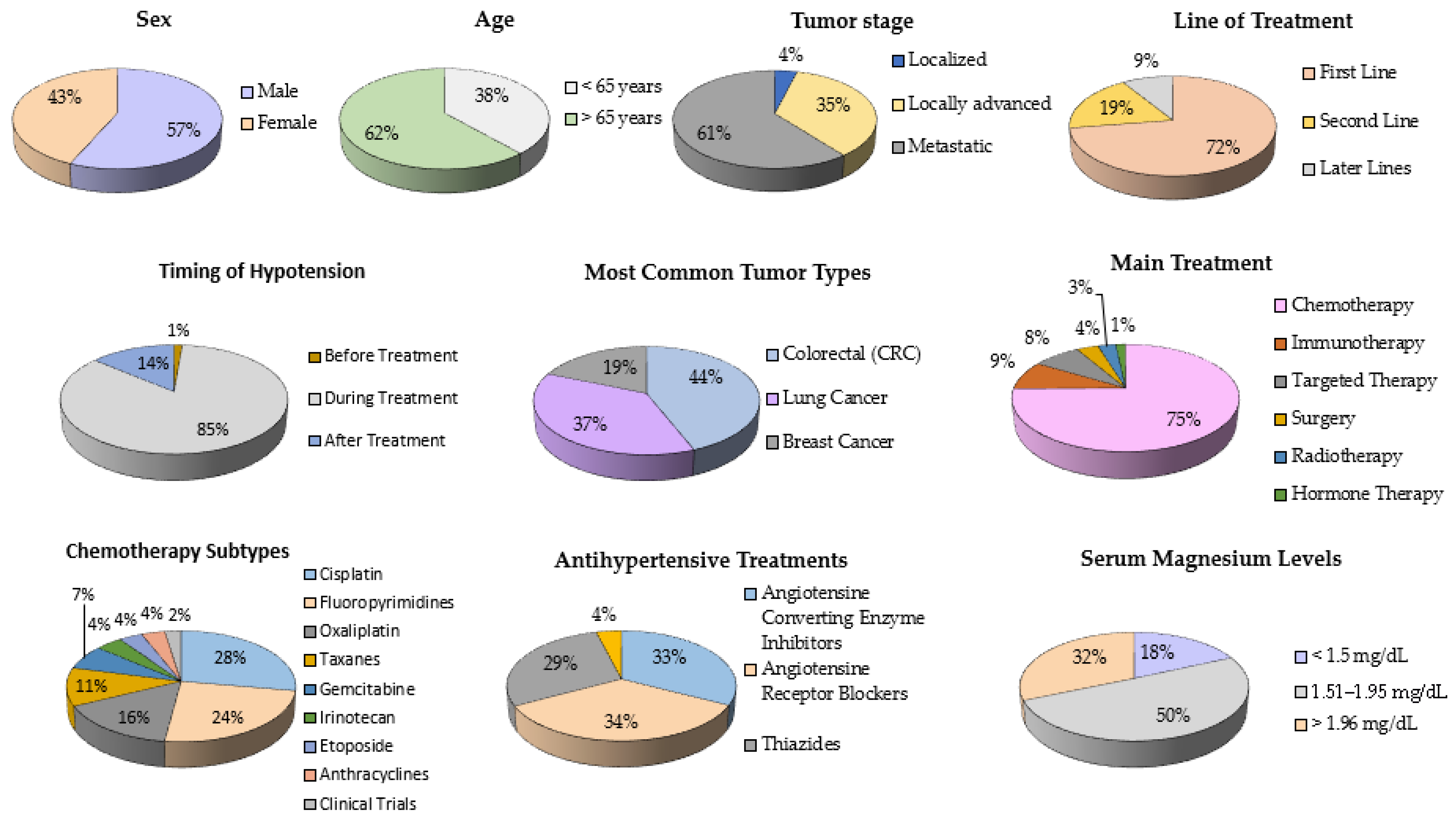
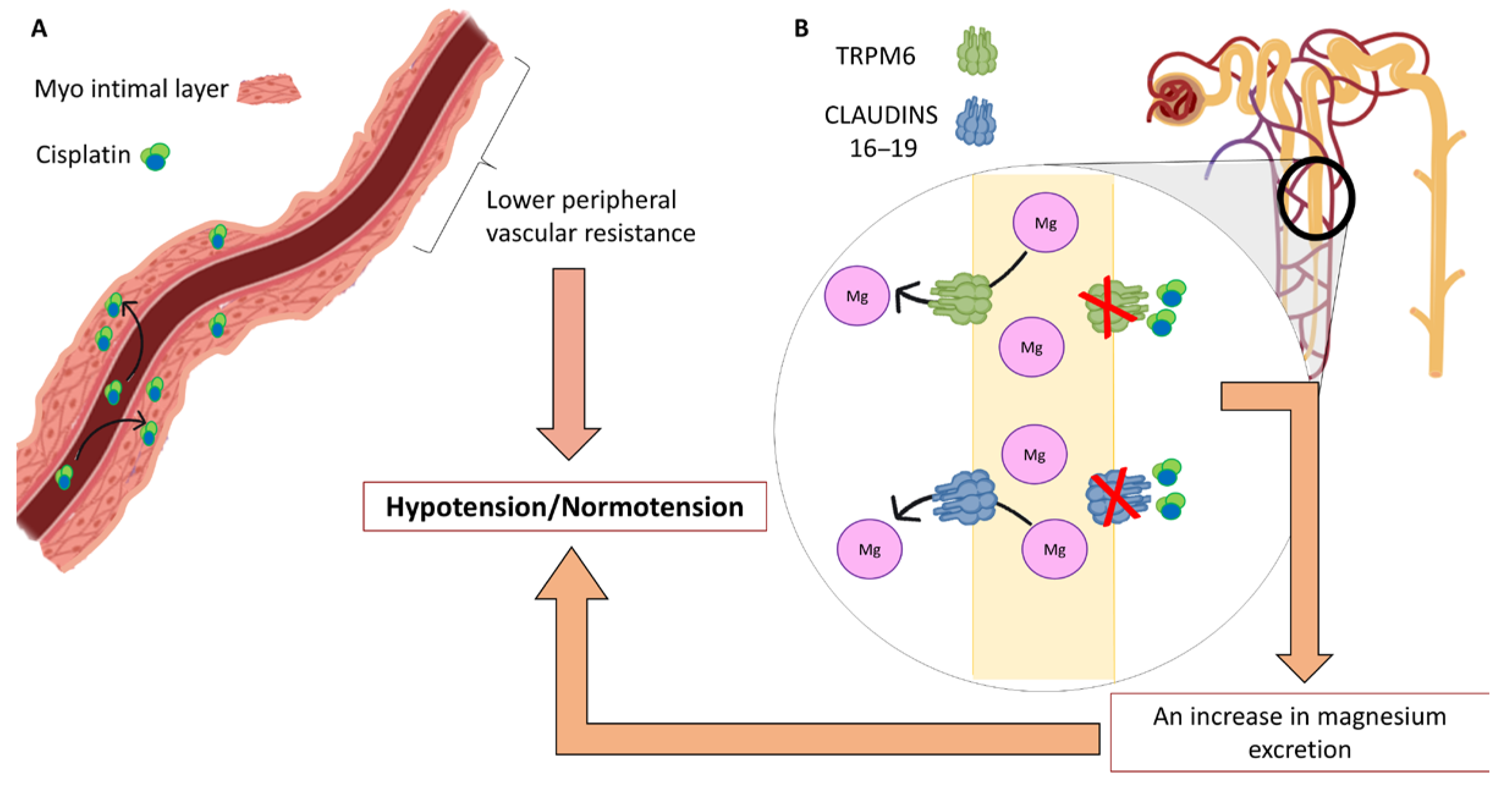
| Variable | Cases (Yes) | Cases (No) | Controls (Yes) | Controls (No) | OR | IC | Z | p |
|---|---|---|---|---|---|---|---|---|
| Sex (male) | 95 | 61 | 76 | 70 | 1.422 | (0.890–2.27) | 1.61 | 0.11 |
| Age (up to 65 years) | 66 | 90 | 49 | 95 | 1.43 | (0.908–2.265) | 2.1 | 0.035 |
| Fluoropirimidines | 50 | 106 | 37 | 109 | 1.39 | (0.841–2.296) | 1.34 | 0.177 |
| Taxanes | 20 | 136 | 20 | 126 | 0.926 | (0.476–1.802) | 0.31 | 0.75 |
| Etoposide | 10 | 146 | 3 | 143 | 3.265 | (0.880–12.108) | 1.75 | 0.072 |
| Irinotecan | 10 | 146 | 5 | 141 | 1.93 | (0.644–5.792) | 1.062 | 0.28 |
| Pemetrexed | 12 | 144 | 6 | 140 | 1.94 | (0.710–5.324) | 1.28 | 0.29 |
| Gemcitabine | 16 | 140 | 9 | 137 | 1.74 | (0.744–4.070) | 1.2 | 0.22 |
| Carboplatin | 18 | 138 | 20 | 126 | 0.822 | (0.416–1.624) | 0.25 | 0.82 |
| Oxaliplatin | 33 | 123 | 23 | 123 | 1.44 | (0.78–2.58) | 1.12 | 0.27 |
| Cisplatin | 69 | 87 | 30 | 116 | 3.06 | (1.82–5.11) | 4.45 | 0.0001 |
| Anthracyclines | 7 | 149 | 6 | 140 | 1.09 | (0.36–3.34) | 0.17 | 0.86 |
| Immunotherapy | 9 | 147 | 9 | 137 | 0.932 | (0.36–2.41) | 0.11 | 0.91 |
| Biologicals | 19 | 137 | 17 | 129 | 1.052 | (0.524–2.11) | 0.13 | 0.89 |
| Clinical trials | 3 | 153 | 6 | 140 | 0.46 | (0.112–1.86) | 0.64 | 0.552 |
| Surgery | 4 | 152 | 8 | 138 | 0.45 | (0.13–1.54) | 1.08 | 0.28 |
| Angiotensine Converting Enzyme Inhibitors | 84 | 72 | 70 | 76 | 1.26 | (0.806–1.991) | 1.04 | 0.89 |
| Angiotensine Receptor Blockers | 79 | 77 | 86 | 60 | 0.716 | (0.454–1.128) | 1.56 | 0.11 |
| Thiazides | 69 | 87 | 67 | 79 | 0.935 | (0.594–1.472) | 0.34 | 0.72 |
| Calcium antagonists | 10 | 146 | 9 | 137 | 1.043 | (0.411–2.643) | 0.147 | 0.88 |
| Effect | Log-Likelihood | Chi-Square | p |
|---|---|---|---|
| Age | 163.493 | 0.354 | 0.552 |
| Sex | 163.158 | 0.018 | 0.893 |
| Type Of Tumor | 170.174 | 7.034 | 0.071 |
| Stage | 170.815 | 7.676 | 0.053 |
| Carboplatin | 167.255 | 4.115 | 0.043 |
| Fluoropyrimidines | 166.669 | 3.529 | 0.060 |
| Taxanes | 166.358 | 3.218 | 0.073 |
| Immunotherapy | 167.327 | 4.187 | 0.041 |
| Etoposide | 164.207 | 1.067 | 0.302 |
| Biologicals | 165.636 | 2.496 | 0.114 |
| Irinotecan | 163.513 | 0.373 | 0.541 |
| Pemetrexed | 167.403 | 4.264 | 0.039 |
| Gemcitabine | 171.445 | 8.305 | 0.004 |
| Clinical Trial | 166.839 | 3.700 | 0.054 |
| Radiotherapy | 163.500 | 0.361 | 0.548 |
| Surgery | 167.575 | 4.435 | 0.035 |
| Oxaliplatin | 166.436 | 3.296 | 0.069 |
| Anthracyclines | 168.191 | 5.051 | 0.025 |
| Cisplatin | 179.145 | 16.006 | 0.0001 |
| Treatment Line | 168.695 | 5.555 | 0.235 |
| Number of Antihypertensives | 168.596 | 5.456 | 0.065 |
| Angiotensine Receptor Blockers | 164.856 | 1.717 | 0.190 |
| Thiazides | 163.140 | 0.000 | 0.994 |
| Calcium Antagonists | 166.019 | 2.879 | 0.090 |
| Angiotensine Converting Enzyme Inhibitors | 163.433 | 0.293 | 0.588 |
| Morphine Use | 168.131 | 4.991 | 0.025 |
| Time Of Hypotension | 200.444 | 37.304 | 0.000 |
| Variable | Patients Without Cisplatin (%) | Patients with Cisplatin (%) | Chi-Square (χ2) | p-Value |
|---|---|---|---|---|
| Mild/Moderate Hypomagnesemia | 34 (42%) | 43 (65.2%) | 8.2 | 0.017 |
| Normal Magnesium Levels | 29 (35.8%) | 16 (24.2%) | - | - |
| Severe Hypomagnesemia (<1.5 mg/dL) | 18 (22.2%) | 7 (10.6%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldado, A.; Doello, K.; Prados, J.; Mesas, C.; Melguizo, C. Association of Magnesium Deficiency and Reduction in Blood Pressure After Chemotherapy in Previously Hypertensive Cancer Patients: The Role of Chemotherapy and Magnesium Levels. Medicina 2025, 61, 1357. https://doi.org/10.3390/medicina61081357
Soldado A, Doello K, Prados J, Mesas C, Melguizo C. Association of Magnesium Deficiency and Reduction in Blood Pressure After Chemotherapy in Previously Hypertensive Cancer Patients: The Role of Chemotherapy and Magnesium Levels. Medicina. 2025; 61(8):1357. https://doi.org/10.3390/medicina61081357
Chicago/Turabian StyleSoldado, Aurora, Kevin Doello, Jose Prados, Cristina Mesas, and Consolacion Melguizo. 2025. "Association of Magnesium Deficiency and Reduction in Blood Pressure After Chemotherapy in Previously Hypertensive Cancer Patients: The Role of Chemotherapy and Magnesium Levels" Medicina 61, no. 8: 1357. https://doi.org/10.3390/medicina61081357
APA StyleSoldado, A., Doello, K., Prados, J., Mesas, C., & Melguizo, C. (2025). Association of Magnesium Deficiency and Reduction in Blood Pressure After Chemotherapy in Previously Hypertensive Cancer Patients: The Role of Chemotherapy and Magnesium Levels. Medicina, 61(8), 1357. https://doi.org/10.3390/medicina61081357





