An Evaluation of the Peri-Implant Tissue in Patients Starting Antiresorptive Agent Treatment After Implant Placement: A Nested Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design and Participants
2.2. Criteria for Case Selection
2.3. Diagnosis of Periodontitis
2.4. Clinical Examinations
2.5. Diagnosis of Peri-Implantitis
2.6. Data Sources
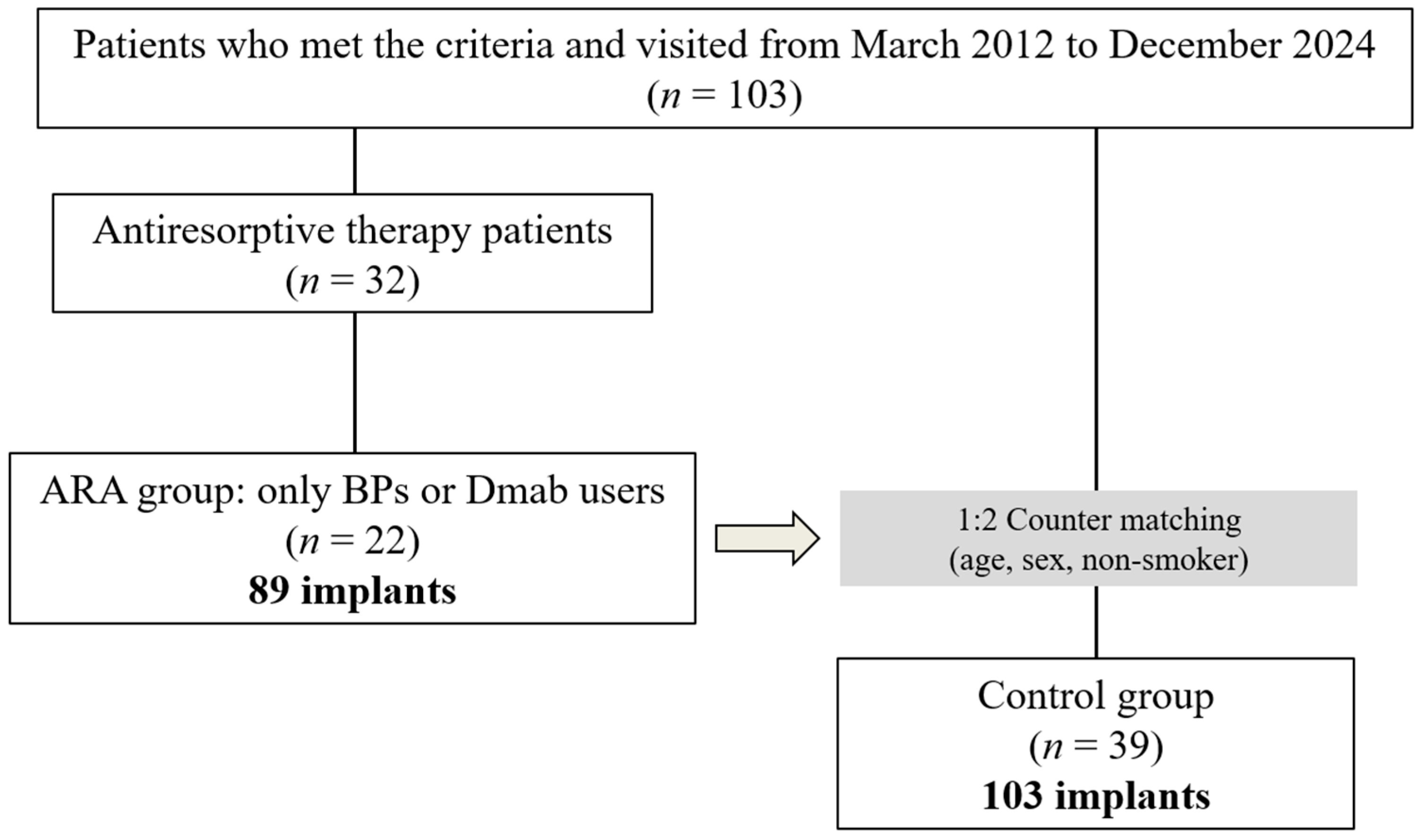
2.7. Group Selection and Matching
2.8. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Implant-Based Data Evaluation
3.3. Assessment of Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARAs | Antiresorptive agents |
| MRONJ | Medication-related osteonecrosis of the jaw |
| BPs | Bisphosphonates |
| Dmab | Denosumab |
| iPPD | Implant probing depth |
| iBoP | Implant bleeding on probing |
| MBL | Marginal bone loss |
| MCI | Mandibular cortical index |
| aOR | Adjusted odds ratio |
| CI | Confidence interval |
| AOMs | Anti-osteoporosis medications |
| PICO | Patients, Intervention, Comparison, Outcome |
| DPR | Digital panoramic radiography |
| SERMs | Selective estrogen receptor modulators |
| AAP | American Academy of Periodontology |
| EFP | European Federation of Periodontology |
| BMI | Body mass index |
| OR | Odds ratio |
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Grbic, J.T.; Black, D.M.; Lyles, K.W.; Reid, D.M.; Orwoll, E.; McClung, M.; Bucci-Rechtweg, C.; Su, G. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: Data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. Am. Dent. Assoc. 2010, 141, 1365–1370. [Google Scholar] [CrossRef]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Kunihara, T.; Tohmori, H.; Tsukamoto, M.; Kobayashi, M.; Okumura, T.; Teramoto, H.; Hamasaki, T.; Yamasaki, T.; Nakagawa, T.; Okimoto, N.; et al. Incidence and trend of antiresorptive agent-related osteonecrosis of the jaw from 2016 to 2020 in Kure, Japan. Osteoporos. Int. 2023, 34, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ position paper on medication-related osteonecrosis of the jaws–2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Pepe, J.; Napoli, N.; Palermo, A.; Magopoulos, C.; Khan, A.A.; Zillikens, M.C.; Body, J.J. Osteonecrosis of the jaw and antiresorptive agents in benign and malignant diseases: A critical review organized by the ECTS. J. Clin. Endocrinol. Metab. 2022, 107, 1441–1460. [Google Scholar] [CrossRef]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-related osteonecrosis of jaws (MRONJ) prevention and diagnosis: Italian consensus update 2020. Int. J. Envir. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef]
- Leslie, W.D.; Lix, L.M.; Binkley, N. Osteoporosis treatment considerations based upon fracture history, fracture risk assessment, vertebral fracture assessment, and bone density in Canada. Arch. Osteoporos. 2020, 15, 93. [Google Scholar] [CrossRef]
- Johnston, C.B.; Dagar, M. Osteoporosis in older adults. Med. Clin. North Am. 2020, 104, 873–884. [Google Scholar] [CrossRef]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in dental implant use in the U.S., 1999–2016, and Projections to 2026. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Holzinger, D.; Seemann, R.; Matoni, N.; Ewers, R.; Millesi, W.; Wutzl, A. Effect of dental implants on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2014, 72, 1937.e1–1937.e8. [Google Scholar] [CrossRef]
- Gelazius, R.; Poskevicius, L.; Sakavicius, D.; Grimuta, V.; Juodzbalys, G. Dental implant placement in patients on bisphosphonate therapy: A systematic review. J. Oral Maxillofac. Res. 2018, 9, e2. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.I.; Kim, H.Y.; Kwon, Y.D. Is implant surgery a risk factor for osteonecrosis of the jaw in older adult patients with osteoporosis? A national cohort propensity score-matched study. Clin. Oral Implants Res. 2021, 32, 437–447. [Google Scholar] [CrossRef]
- Sher, J.; Kirkham-Ali, K.; Luo, J.D.; Miller, C.; Sharma, D. Dental implant placement in patients with a history of medications related to osteonecrosis of the jaws: A systematic review. J. Oral Implantol. 2021, 47, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Namaki, S.; Kamimoto, A.; Hagiwara, Y. Medication-related osteonecrosis of the jaw subsequent to periimplantitis: A case report and literature review. J. Oral Implantol. 2021, 47, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Hagiwara, Y. Peri-implant medication-related osteonecrosis of the jaw developed during long-term maintenance: A case report. J. Dent. Sci. 2022, 17, 576–577. [Google Scholar] [CrossRef]
- Yoshino, T.; Seki, K.; Nagasaki, M.; Kamimoto, A. Peri-implant osteonecrosis of the jaw in a patient prescribed selective estrogen receptor modulators. J. Dent. Sci. 2023, 18, 1939–1940. [Google Scholar] [CrossRef]
- Escobedo, M.F.; Cobo, J.L.; Junquera, S.; Milla, J.; Olay, S.; Junquera, L.M. Medication-related osteonecrosis of the jaw. Implant presence-triggered osteonecrosis: Case series and literature review. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 40–48. [Google Scholar] [CrossRef]
- Yong, C.W.; Sng, T.J.H.; Choo, S.H.J.; Chew, J.R.J.; Islam, I. Implant presence-triggered osteonecrosis: A scoping review. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101759. [Google Scholar] [CrossRef]
- Nisi, M.; Gennai, S.; Graziani, F.; Barone, A.; Izzetti, R. Clinical and radiologic treatment outcomes of implant presence tirggered-MRONJ: Systematic review of literature. Oral Dis. 2024, 30, 5255–5267. [Google Scholar] [CrossRef]
- Seki, K.; Tamagawa, T.; Yasuda, H.; Manaka, S.; Akita, D.; Kamimoto, A.; Hagiwara, Y. A study of peri-implant tissue clinical parameters in patients starting anti-osteoporosis medication after existing implant function: A prospective cohort study. Int. J. Implant Dent. 2024, 10, 50. [Google Scholar] [CrossRef]
- Kuller, L.H.; Goldstein, B.D. Suggestions for STROBE recommendations. Epidemiology 2007, 18, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, 159–172. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Sanz, M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Klemetti, E.; Kolmakow, S. Morphology of the mandibular cortex on panoramic radiographs as an indicator of bone quality. Dentomaxillofac. Radiol. 1997, 26, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef]
- Schwartz, F.; Becker, J. Peri-Implant Infection: Etiology, Diagnosis and Treatment; Quintessence: Berlin, Germany, 2007. [Google Scholar]
- Kanis, J.A.; Johansson, H.; Odén, A.; Harvey, N.C.; Gudnason, V.; Sanders, K.M.; Sigurdsson, G.; Siggeirsdottir, K.; Fitzpatrick, L.A.; Borgström, F.; et al. Characteristics of recurrent fracture. Osteoporos. Int. 2018, 29, 1747–1757. [Google Scholar] [CrossRef]
- NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy, March 7–29, 2000: Highlights of the conference. South Med. J. 2001, 94, 569–573.
- Humphrey, M.B.; Russell, L.; Danila, M.I.; Fink, H.A.; Guyatt, G.; Cannon, M.; Caplan, L.; Gore, S.; Grossman, J.; Hansen, K.E.; et al. 2022 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023, 75, 2088–2102. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Penoni, D.C.; Fidalgo, T.K.S.; Torres, S.R.; Varela, V.M.; Masterson, D.; Leão, A.T.T.; Maia, L.C. Bone density and clinical periodontal attachment in postmenopausal women: A systematic review and meta-analysis. J. Dent. Res. 2017, 96, 261–269. [Google Scholar] [CrossRef]
- Taguchi, A.; Tsuda, M.; Ohtsuka, M.; Kodama, I.; Sanada, M.; Nakamoto, T.; Inagaki, K.; Noguchi, T.; Kudo, Y.; Suei, Y.; et al. Use of dental panoramic radiographs in identifying younger postmenopausal women with osteoporosis. Osteoporos. Int. 2006, 17, 387–394. [Google Scholar] [CrossRef]
- Heuchert, J.; Kozieł, S.; Spinek, A.E. Radiomorphometric indices of the mandible as indicators of decreased bone mineral density and osteoporosis—meta-analysis and systematic review. Osteoporos. Int. 2024, 35, 401–412. [Google Scholar] [CrossRef]
- Seki, K.; Hasuike, A.; Hagiwara, Y. Clinical evaluation of the relationship between systemic disease and the time of onset of peri-implantitis: A retrospective cohort study. J. Oral Implantol. 2023, 49, 55–61. [Google Scholar] [CrossRef]
- Jung, J.; Shim, G.J.; Park, J.S.; Kwon, Y.D.; Ryu, J.I. Effect of anti-resorptive therapy on implant failure: A systematic review and meta-analysis. J. Periodontal Implant Sci. 2025, 55, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Kobayashi, A.; Takahashi, N.; Kamimoto, A. Medication-related osteonecrosis of the jaw in a patient with rheumatoid arthritis with suspected involvement of methotrexate and tocilizumab. J. Dent. Sci. 2024, 19, 2428–2429. [Google Scholar] [CrossRef] [PubMed]
- Turri, A.; Rossetti, P.H.; Canullo, L.; Grusovin, M.G.; Dahlin, C. Prevalence of peri-implantitis in medically compromised patients and smokers: A systematic review. Int. J. Oral Maxillofac. Implants 2016, 31, 111–118. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Giro, G.; Chambrone, L.; Goldstein, A.; Rodrigues, J.A.; Zenóbio, E.; Feres, M.; Figueiredo, L.C.; Cassoni, A.; Shibli, J.A. Impact of osteoporosis in dental implants: A systematic review. World J. Orthop. 2015, 6, 311–315. [Google Scholar] [CrossRef]
- Basson, A.A.; Mann, J.; Findler, M.; Chodick, G. Correlates of early dental implant failure: A retrospective study. Int. J. Oral Maxillofac. Implants 2023, 38, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Balshi, T.J.; Wolfinger, G.J. Management of the posterior maxilla in the compromised patient: Historical, current, and future perspectives. Periodontol. 2000 2003, 33, 67–81. [Google Scholar] [CrossRef] [PubMed]
| ARA (n = 22) | Control (n = 39) | p Value | Significant Difference | |||
|---|---|---|---|---|---|---|
| a | Sex | |||||
| Female | 19 (86.4) | 33 (84.6) | 1.000000 | ns | ||
| b | Age (years) | 78.4 ± 6.8 | 70.9 ± 8.4 | 0.000694 | ** | |
| b | Body mass index (kg/m2) | 22.0 ± 2.6 | 21.3 ± 2.7 | 0.352000 | ns | |
| a | History of insufficiency fracture | 9 (40.9) | 1 (2.6) | 0.000222 | ** | |
| a | Smoking | 3 (13.6) | 5 (12.8) | 1.000000 | ns | |
| a | History of periodontitis | 17 (77.3) | 18 (46.2) | 0.030100 | * | |
| c | Stage 2 Grade A | 4 | 3 | 0.240000 | ns | |
| c | Stage 3 Grade A | 4 | 5 | 0.710000 | ns | |
| c | Stage 3 Grade B | 3 | 6 | 1.000000 | ns | |
| c | Stage 4 Grade B | 6 | 3 | 0.059600 | ns | |
| c | Stage 4 Grade C | 0 | 1 | 1.000000 | ns | |
| Mandibular cortical index | ||||||
| c | Class 1 | 0 (0) | 9 (23.1) | 0.020300 | * | |
| a | Class 2 | 11 (50.0) | 23 (59.0) | 0.595000 | ns | |
| a | Class 3 | 11 (50.0) | 7 (17.9) | 0.017700 | * | |
| Antiresorptive therapy *overlapping | ||||||
| BPs (oral 18, injection 1) | 21 | |||||
| Denosumab | 3 | |||||
| Duration of antiresorptive therapy (years) | 4.1 ± 3.6 (1–16) | |||||
| 1–3 years | 13 | |||||
| over 4 years | 11 | |||||
| ARA (n = 89) | Control (n = 103) | p Value | Significant Difference | |||
|---|---|---|---|---|---|---|
| a | Implant site | |||||
| Maxilla | 41 (46.1) | 56 (54.4) | 0.311000 | ns | ||
| Mandible | 48 (53.9) | 47 (45.6) | ||||
| Implant dimensions | ||||||
| b | Diameter | 4.0 ± 0.5 | 4.2 ± 0.4 | 0.054700 | ns | |
| (range, median) | (3.3–5, 4) | (3.3–5, 4.1) | ||||
| b | Length | 10.2 ± 1.6 | 10.4 ± 1.5 | 0.294000 | ns | |
| (range, median) | (7–16, 10) | (7–13, 10) | ||||
| a | Additional surgery | 2 (2.3) | 15 (14.6) | 0.003810 | * | |
| a | Sinus floor elevation | 2 (2.3) | 8 (7.8) | 0.110000 | ns | |
| a | Split crest technique | 0 (0) | 7 (6.8) | 0.015600 | * | |
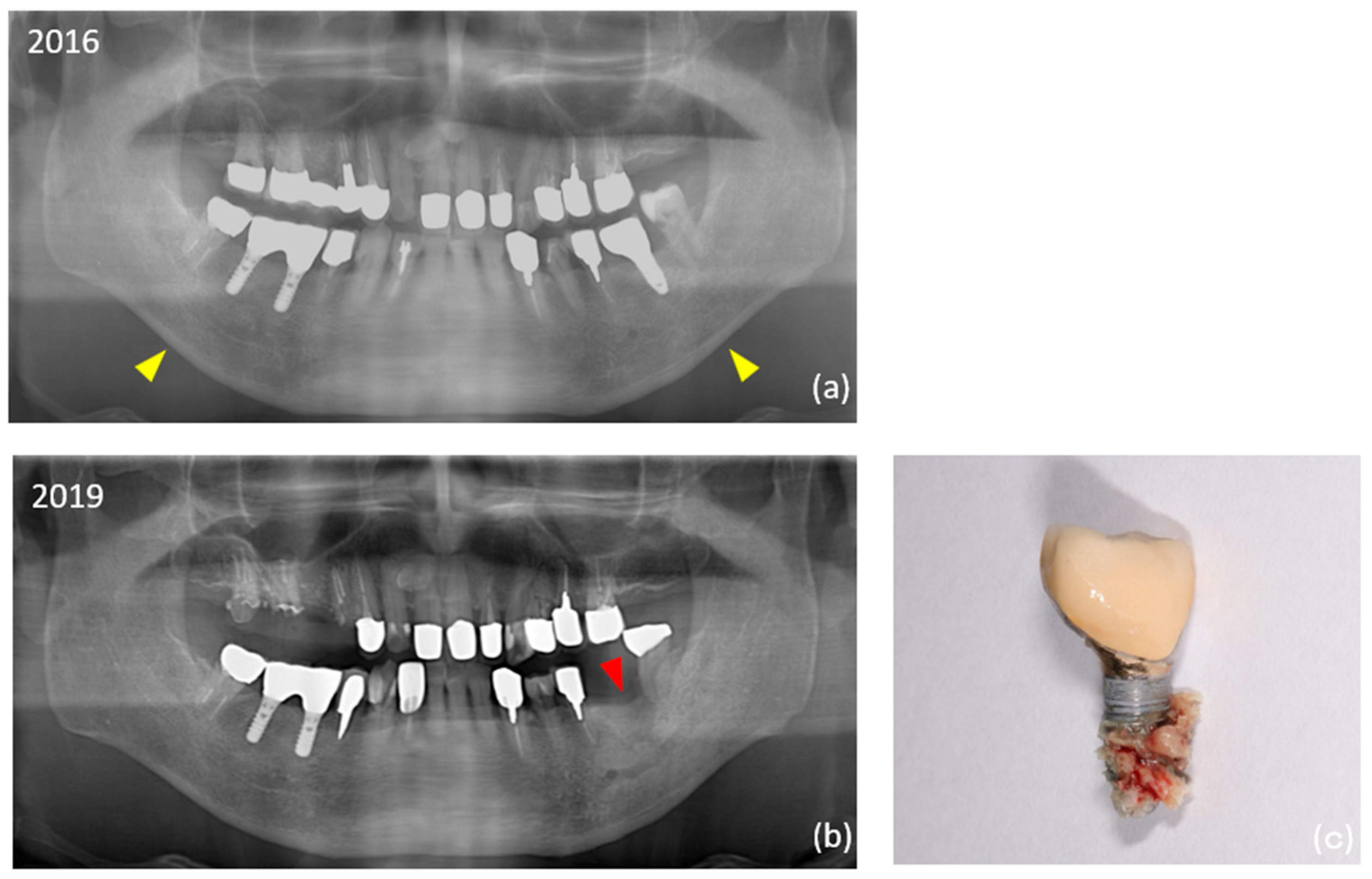
| a | Peri-implantitis | 25 (28.1) | 10 (9.7) | 0.001290 | ** | |
| a | Failure | 10 (11.2) | 3 (2.9) | 0.045400 | * | |
| a | Peri-implant MRONJ | 4 (4.5) | 0 (0) | 0.044500 | * | |
| Superstructure | ||||||
| a | Cement retention | 29 (32.6) | 38 (36.9) | 0.548000 | ns | |
| Screw retention (including side-screw system) | 60 (67.4) | 65 (63.1) | ||||
| b | Mean maintenance duration (years) | 14.7 ± 6.4 | 11.8 ± 6.0 | 0.004330 | ** | |
| (range, median) | (5–27, 13) | (2–20, 14) | ||||
| Case | Age | Sex | Periodontitis | Medication | Type | Interval | Duration (Years) | Type of Implant (n) | ONJ (Location: FDI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Stage | Grade | |||||||||
| 1 | 86 | Female | 3 | A | Alendronate | Oral | Daily | 4 | Srerioss (4) | |
| 2 | 85 | Female | 4 | B | Denosumab | Subcutaneous injection | 6 month | 7 | Astra (14), Straumann (1) | |
| 3 | 82 | Female | Minodronate | Oral | 4 weeks | 5 | Nobel Biocare (2) | |||
| 4 | 80 | Female | 4 | B | Minodronate | Oral | 4 weeks | 11 | 3i (8), Straumann (1) | |
| 5 | 79 | Female | 4 | B | Risedronate | Oral | 1 week | 1 | Nobel Biocare (5), Zimmer (3) | |
| GC (2), 3i (1), Straumann (1) | ||||||||||
| 6 | 72 | Female | Risedronate | Oral | Daily | 2 | Nobel Biocare (2) | MRONJ (near #35) | ||
| Ibandronate | Oral | Daily | 3 | |||||||
| 7 | 72 | Female | Ibandronate | Subcutaneous injection | 1 month | 7 | Astra (3) | |||
| 8 | 72 | Female | Risedronate | Oral | 1 week | 2 | Platon Japan (1) | |||
| 9 | 67 | Female | Risedronate | Oral | Daily | 1 | Nobel Biocare (4) | |||
| 10 | 86 | Female | 4 | B | Unknown BPs | Oral | Daily | 2 | Nobel Biocare (2) | |
| 11 | 83 | Female | 3 | A | Alendronate | Oral | 1 week | 1 | Nobel Biocare (3) | |
| 12 | 80 | Female | 3 | B | Alendronate | Oral | 1 week | 5 | Nobel Biocare (5) | |
| 13 | 79 | Female | 4 | B | Alendronate | Oral | 1 week | 4 | Astra (4) | PI-MRONJ (#14, #16, #36) |
| 14 | 79 | Female | 2 | A | Minodronate | Oral | 4 weeks | 8 | Straumann (2), Nobel Biocare (1) | |
| 15 | 76 | Female | Alendronate | Oral | Daily | 2 | Nobel Biocare (2), POI (1) | |||
| 16 | 75 | Female | 2 | A | Minodronate | Oral | 4 weeks | 2 | Nobel Biocare (1) | |
| 17 | 73 | Female | 2 | A | Alendronate | Oral | 1 week | 1 | Nobel Biocare (2), Endopore (2) | |
| Astra (1), Straumann (1) | ||||||||||
| 18 | 72 | Female | 2 | A | Alendronate | Oral | Daily | 2 | Nobel Biocare (1) | |
| 19 | 61 | Female | 3 | A | Alendronate | Oral | Daily | 5 | Nobel Biocare (1) | |
| 20 | 87 | Male | 3 | A | Minodronate | Oral | Daily | 2 | Nobel Biocare (1) | PI-MRONJ (#36) |
| Denosumab | Subcutaneous injection | 6 months | 2 | Straumann (2) | ||||||
| 21 | 85 | Male | 3 | B | Unknown BP | Oral | Daily | 4 | Nobel Biocare (1), Endopore (1) | |
| 22 | 73 | Male | 4 | B | Denosumab | Subcutaneous injection | 6 months | 16 | Astra (4) | |
| Baseline | Last visit | Variation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| iPPD (mm) | iBoP (0–1) | MBL (mm) | iPPD (mm) | iBoP (0–1) | MBL (mm) | iPPD (mm) | iBoP (0–1) | MBL (mm) | ||||
| Mean | Maximum | Mean | Maximum | Mean | Maximum | |||||||
| ARA group | 3.1 ± 0.6 | 3.5 ± 1.0 | 0.1 ± 0.2 | 1.2 ± 1.1 | 3.8 ± 2.0 | 4.6 ± 2.3 | 0.3 ± 0.3 | 2.2 ± 2.5 | 0.7 ± 1.8 | 1.1 ± 2.0 | 0.2 ± 0.3 | 1.0 ± 2.2 |
| (range, median) | (2.0–5.5, 3.2) | (2–8, 3) | (0–1, 0.2) | (0–4.5, 1.1) | (2.0–11.0, 3.2) | (2–12, 4) | (0–1, 0.2) | (0–11.0, 1.5) | (−1.0–8.0, 0.2) | (−2–8, 1) | (−1–0.8, 0) | (−1.6–10.8, 0.2) |
| Control group | 2.8 ± 0.5 | 3.4 ± 0.9 | 0.2 ± 0.2 | 1.2 ± 0.8 | 3.2 ± 1.6 | 4.0 ± 1.9 | 0.2 ± 0.3 | 1.9 ± 2.1 | 0.3 ± 1.4 | 0.6 ± 1.8 | 0 ± 0.3 | 0.8 ± 1.8 |
| (range, median) | (1.8–4.3, 2.8) | (2–8, 3) | (0–1, 0.2) | (0–2.9, 1.2) | (1.0–13.0, 2.7) | (2–13, 3) | (0–1, 0) | (0–13, 1.5) | (−1.5–8.7, 0) | (−5–7, 0) | (−0.8–0.8, 0) | (−1.3–11.5, 0.2) |
| Significant difference | ** | ns | ns | ns | ** | * | ** | ns | ns | * | ** | ns |
| p value | 0.00155 | 0.23500 | 0.07650 | 0.58400 | 0.00700 | 0.01880 | 0.00664 | 0.98100 | 0.17400 | 0.03430 | 0.00016 | 0.57000 |
| Test | Student’s t | MWUt | MWUt | Student’s t | MWUt | MWUt | MWUt | MWUt | MWUt | MWUt | MWUt | MWUt |
| Explanatory Variable | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | [95% CI] | p Value | Odds Ratio | [95% CI] | p Value | |
| ARA (yes; 1, no; 0) | 3.63 | [1.630–8.080] | ** | 3.91 | [1.290000–11.9000] | * |
| History of insufficiency fracture (yes; 1, no; 0) | 0.89 | [0.386–2.050] | ** | 0.53 | [0.172000–1.6000] | ns |
| History of periodontitis (yes; 1, no; 0) | 8.17 | [1.89000–35.400] | ** | 3.72 | [0.743000–18.6000] | ns |
| MCI (class3; 1, others; 0) | 1.97 | [0.9420–4.130] | ** | 1.63 | [0.574000–4.6500] | ns |
| Additional surgery (yes; 1, no; 0) | 0.57 | [0.125–2.630] | ** | 2.88 | [0.427000–19.4000] | ns |
| Observational duration (over 12 years; 1, others; 0) | 2.58 | [1.1800–5.620] | ** | 2.73 | [0.94000–7.9300] | ns |
| Variation iPPD maximum (yes; 1, no; 0) | 7.42 | [3.0400–18.100] | ** | 5.90 | [2.260000–15.4000] | ** |
| Variation iBoP mean ( yes; 1, no; 0 ) | 2.85 | [1.35000–6.030] | ** | 1.86 | [0.754–4.5800] | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seki, K.; Koyama, R.; Takayama, K.; Kobayashi, A.; Kamimoto, A.; Hagiwara, Y. An Evaluation of the Peri-Implant Tissue in Patients Starting Antiresorptive Agent Treatment After Implant Placement: A Nested Case–Control Study. Medicina 2025, 61, 1348. https://doi.org/10.3390/medicina61081348
Seki K, Koyama R, Takayama K, Kobayashi A, Kamimoto A, Hagiwara Y. An Evaluation of the Peri-Implant Tissue in Patients Starting Antiresorptive Agent Treatment After Implant Placement: A Nested Case–Control Study. Medicina. 2025; 61(8):1348. https://doi.org/10.3390/medicina61081348
Chicago/Turabian StyleSeki, Keisuke, Ryo Koyama, Kazuki Takayama, Atsushi Kobayashi, Atsushi Kamimoto, and Yoshiyuki Hagiwara. 2025. "An Evaluation of the Peri-Implant Tissue in Patients Starting Antiresorptive Agent Treatment After Implant Placement: A Nested Case–Control Study" Medicina 61, no. 8: 1348. https://doi.org/10.3390/medicina61081348
APA StyleSeki, K., Koyama, R., Takayama, K., Kobayashi, A., Kamimoto, A., & Hagiwara, Y. (2025). An Evaluation of the Peri-Implant Tissue in Patients Starting Antiresorptive Agent Treatment After Implant Placement: A Nested Case–Control Study. Medicina, 61(8), 1348. https://doi.org/10.3390/medicina61081348





