Metabolomic Profiling of Erector Spinae Plane Block for Breast Cancer Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Metabolomic Analysis
2.3. Statistical Analysis
3. Results
3.1. Demographic Data of Participants
3.2. Analgesic Outcomes of Groups
3.3. VEGF, Cortisol, and CRP Levels of Groups
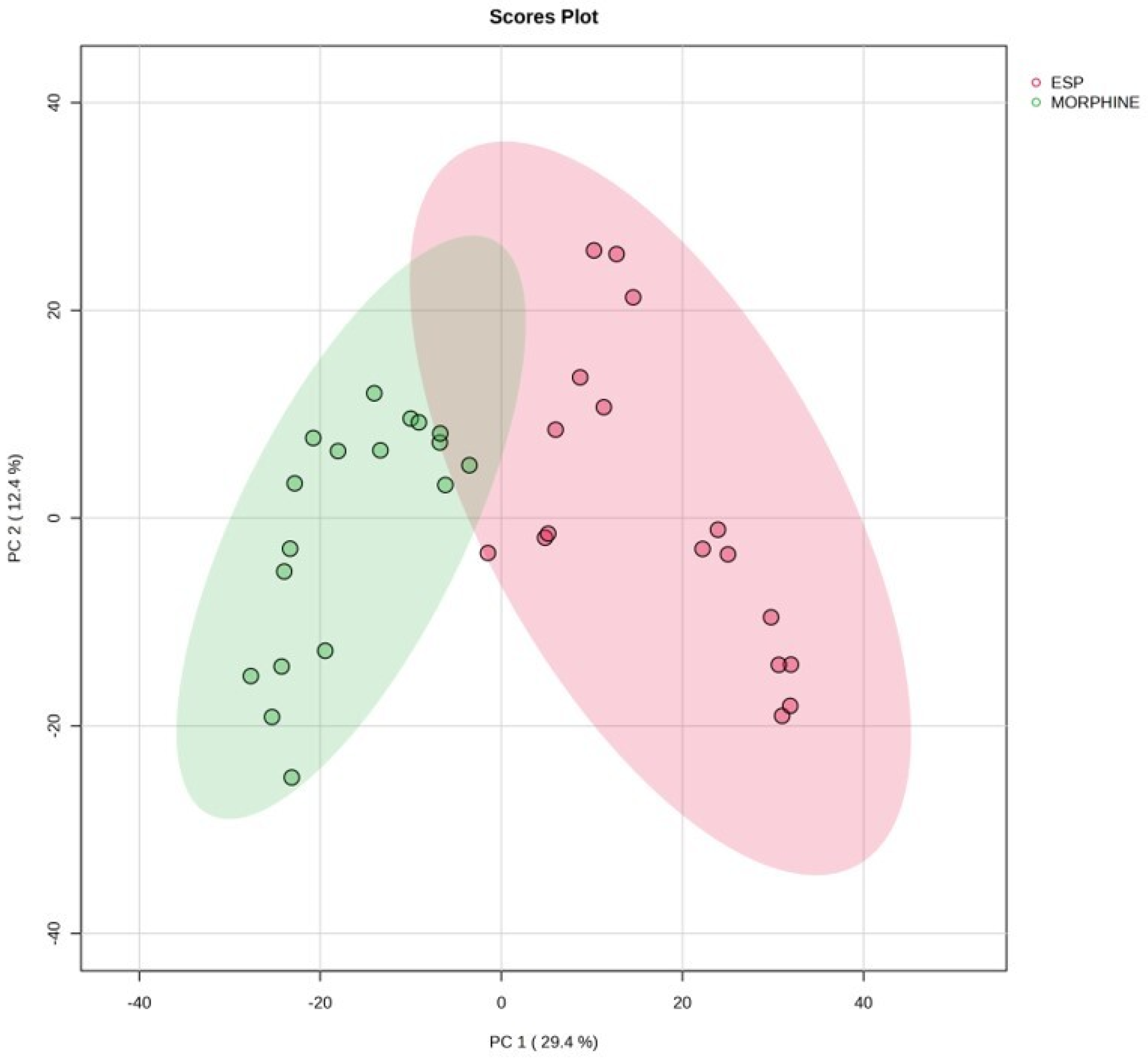
3.4. Metabolomic Profiles of ESP and Morphine Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| ASA | American Society of Anesthesiologists |
| ESP | Erector spinae plane block |
| VEGF | Vascular endothelial growth factor |
| CRP | C-reactive protein |
| PCA | Patient-controlled analgesia |
| Q-TOF-LC/MS | Quadrupole Time-of-Flight Mass Spectrometer |
| SD | Standard deviation |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Czajka, M.L.; Pfeifer, C. Breast Cancer Surgery. In StatPearls; StatPearls Publishing LLC.: Orlando, FL, USA, 2022. [Google Scholar]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Futami, R.; Miyashita, M.; Nomura, T.; Makino, H.; Matsutani, T.; Sasajima, K.; Tajiri, T. Increased serum vascular endothelial growth factor following major surgical injury. J. Nippon. Med. Sch. 2007, 74, 223–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neeman, E.; Zmora, O.; Ben-Eliyahu, S. A New Approach to Reducing Postsurgical Cancer Recurrence: Perioperative Targeting of Catecholamines and Prostaglandins. Clin. Cancer Res. 2012, 18, 4895–4902. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Neeman, E.; Ben-Eliyahu, S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav. Immun. 2013, 30, S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F.; Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Miller, M.C.; Matera, J.; Allard, W.J.; Doyle, G.V.; Terstappen, L.W. Circulating Tumor Cells at Each Follow-Up Time Point During Therapy of Metastatic Breast Cancer Patients Predict Progression-Free and Overall Survival. Clin. Cancer Res. 2006, 12, 4218–4224. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, A.; Sharma, S.; Ford, J.; Durieux, M.E.; Tiouririne, M. The Role of the Perioperative Period in Recurrence After Cancer Surgery. Anesth. Analg. 2010, 110, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eliyahu, S. The promotion of tumor metastasis by surgery and stress: Immunological basis and implications for psychoneuroimmunology. Brain Behav. Immun. 2003, 17 (Suppl. 1), S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Wall, T.; Sherwin, A.; Ma, D.; Buggy, D.J. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: A narrative review. Br. J. Anaesth. 2019, 123, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Desborough, J. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Baxevanis, C.N.; Papilas, K.; Dedoussis, G.V.; Pavlis, T.; Papamichail, M. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin. Immunol. Immunopathol. 1994, 71, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Xia, W.; Zheng, M.; Lu, C.; Han, X.; Sun, Y. Surgical excision promotes tumor growth and metastasis by promoting expression of MMP-9 and VEGF in a breast cancer model. Exp. Oncol. 2008, 30, 60–64. [Google Scholar] [PubMed]
- Calvo-Soto, P.; Martíanez-Contreras, A.; Trujillo-Hernández, B.; Peraza-Garay, F.; Vásquez, C. Spinal—General anaesthesia decreases neuroendocrine stress response in laparoscopic cholecystectomy. J. Int. Med. Res. 2012, 40, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, D.; Arita, T.; Nakanishi, M.; Kuriu, Y.; Murayama, Y.; Kudou, M.; Konishi, H.; Komatsu, S.; Shiozaki, A.; Otsuji, E. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Muthubharathi, B.C.; Gowripriya, T.; Balamurugan, K. Metabolomics: Small molecules that matter more. Mol. Omics 2021, 17, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Mock-Ohnesorge, J.; Mock, A.; Hackert, T.; Fröhling, S.; Schenz, J.; Poschet, G.; Jäger, D.; Büchler, M.W.; Uhle, F.; Weigand, M.A. Perioperative changes in the plasma metabolome of patients receiving general anesthesia for pancreatic cancer surgery. Oncotarget 2021, 12, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, Y.; Okada, M.; Ishii, N.; Kiyooka, K. Vascular endothelial growth factor increased by pulmonary surgery accelerates the growth of micrometastases in metastatic lung cancer. Chest 1998, 114, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, W.; Yang, X.; Chen, L.; Fan, L.; Liu, H.; Liu, K.; Zhang, Y.; Jiang, J. Less micrometastatic risk related to circulating tumor cells after endoscopic breast cancer surgery compared to open surgery. BMC Cancer 2019, 19, 1070. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Agresti, R.; Carcangiu, M.L.; Ghirelli, C.; Morelli, D.; Campiglio, M.; Martel, M.; Giovanazzi, R.; Greco, M.; Balsari, A.; et al. Role of HER2 in wound-induced breast carcinoma proliferation. Lancet 2003, 362, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Desmedt, C.; Retsky, M.; Sotiriou, C.; Piccart, M.; Biganzoli, E. Late effects of adjuvant chemotherapy adumbrate dormancy complexity in breast cancer. Breast 2020, 52, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Hwang, W. Anesthetic Approaches and Their Impact on Cancer Recurrence and Metastasis: A Comprehensive Review. Cancers 2024, 16, 4269. [Google Scholar] [CrossRef] [PubMed]
- Exadaktylos, A.K.; Buggy, D.J.; Moriarty, D.C.; Mascha, E.; Sessler, D.I. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 2006, 105, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, E.; Awad, A.E.; Rahman, M.; Feng, J.; Lou, P.H.; Zhang, L.; Ionescu, L.; Lemieux, H.; Thébaud, B.; Zaugg, M. Antiproliferative effects of local anesthetics on mesenchymal stem cells: Potential implications for tumor spreading and wound healing. Anesthesiology 2012, 116, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Hönemann, C.W.; Heyse, T.J.; Möllhoff, T.; Hahnenkamp, K.; Berning, S.; Hinder, F.; Linck, B.; Schmitz, W.; van Aken, H. The inhibitory effect of bupivacaine on prostaglandin E(2) (EP(1)) receptor functioning: Mechanism of action. Anesth. Analg. 2001, 93, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, Y.; Aksu, C.; Kuş, A.; Yörükoğlu, U.H.; Kılıç, C.T. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: A randomized controlled study. J. Clin. Anesth. 2018, 50, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Jafra, A.; Sharma, S.; Arora, S.; Singh, G. Efficacy of erector spinae plane block for postoperative analgesia in total mastectomy and axillary clearance: A randomized controlled trial. Saudi J. Anaesth. 2020, 14, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Romero, A.; Fajardo-Perez, M. Function of the sympathetic supply in the erector spinae plane block. Can. J. Anaesth. 2021, 68, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, M.; Li, J.; Lyu, Q.; Jiang, R.; Du, Y. Effects of ultrasound-guided erector spinae plane block on the immune function and postoperative recovery of patients undergoing radical mastectomy. Gland. Surg. 2021, 10, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.; Sheta, M.; El Mohsen, M.A. Elevated serum and tissue VEGF associated with poor outcome in breast cancer patients. Alex. J. Med. 2011, 47, 217–224. [Google Scholar] [CrossRef]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999, 13, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ohm, J.E.; Carbone, D.P. VEGF as a mediator of tumor-associated immunodeficiency. Immunol. Res. 2001, 23, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Yoshii, H.; Sato, A.; Kuroda, K.; Asakuma, J.; Horiguchi, A.; Sumitomo, M.; Asano, T. Impact of Postoperative C-Reactive Protein Level on Recurrence and Prognosis in Patients with N0M0 Clear Cell Renal Cell Carcinoma. J. Urol. 2011, 186, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, J.; James, R.L.; Grimes, J. Structure-affinity relationships and stereospecificity of several homologous series of local anesthetics for the beta2-adrenergic receptor. Anesth. Analg. 1997, 85, 336–342. [Google Scholar] [PubMed]
- Hellstrand, K.; Czerkinsky, C.; Ricksten, A.; Jansson, B.; Asea, A.; Kylefjord, H.; Hermodsson, S. Role of serotonin in the regulation of interferon-gamma production by human natural killer cells. J. Interf. Res. 1993, 13, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Saeed, I.; La Caze, A.; Hollmann, M.W.; Shaw, P.N.; Parat, M.-O. New Insights on Tramadol and Immunomodulation. Curr. Oncol. Rep. 2021, 23, 123. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, C.C.; Mabvuure, N.T.; Ali, A.; Kozar, R.A.; Herndon, D.N. The surgically induced stress response. J. Parenter Enter. Nutr. 2013, 37 (Suppl. 5), 21S–29S. [Google Scholar] [CrossRef] [PubMed]
- Buyukkocak, U.; Daphan, C.; Caglayan, O.; Aydinuraz, K.; Kaya, T.; Saygun, O.; Agalar, F. Effects of different anesthetic techniques on serum leptin, C-reactive protein, and cortisol concentrations in anorectal surgery. Croat. Med. J. 2006, 47, 862–868. [Google Scholar] [PubMed]
- Shirzad, H.; Shahrani, M.; Rafieian-Kopaei, M. Comparison of morphine and tramadol effects on phagocytic activity of mice peritoneal phagocytes in vivo. Int. Immunopharmacol. 2009, 9, 968–970. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Guo, M.; Liu, Z.; Fu, Y.; Wu, H.; Wang, C.; Cao, M. Morphine Promotes the Angiogenesis of Postoperative Recurrent Tumors and Metastasis of Dormant Breast Cancer Cells. Pharmacology 2019, 104, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Kshirsagar, S.; Chang, L.; Schwartz, R.; Law, P.-Y.; Yee, D.; Hebbel, R.P. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002, 62, 4491–4498. [Google Scholar] [PubMed]
- Bortsov, A.V.; Millikan, R.C.; Belfer, I.; Boortz-Marx, R.L.; Arora, H.; McLean, S.A. μ-Opioid receptor gene A118G polymorphism predicts survival in patients with breast cancer. J. Am. Soc. Anesthesiol. 2012, 116, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Bianchi, M.; Manfredi, B.; E Panerai, A. Effects of tramadol on immune responses and nociceptive thresholds in mice. Pain 1997, 72, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Won, S.J. Effects of tramadol on T lymphocyte proliferation and natural killer cell activity in rats with sciatic constriction injury. Pain 2001, 92, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, J.-R.; Kim, K.-J.; Jun, J.H.; Hwang, H.J.; Lee, W.; Nam, S.H.; Oh, J.E.; Yoo, Y.C. Identification for antitumor effects of tramadol in a xenograft mouse model using orthotopic breast cancer cells. Sci. Rep. 2021, 11, 22113. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Bianchi, M.; Gaspani, L.; Manfredi, B.; Maucione, A.; Terno, G.; Ammatuna, M.; Panerai, A.E. The Effects of Tramadol and Morphine on Immune Responses and Pain After Surgery in Cancer Patients. Anesth. Analg. 2000, 90, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, M.; Xu, W.; Yu, S. Research progress of bile acids in cancer. Front. Oncol. 2022, 11, 778258. [Google Scholar] [CrossRef] [PubMed]
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.-A.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G.; et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-D.; Garibaldi, S.; Gopal, S.; Polak, K.; Palla, G.; Spina, S.; Mannella, P.; Genazzani, A.D.; Simoncini, T. Dydrogesterone exerts endothelial anti-inflammatory actions decreasing expression of leukocyte adhesion molecules. Mol. Hum. Reprod. 2011, 18, 44–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanderson, J.T. The Steroid Hormone Biosynthesis Pathway as a Target for Endocrine-Disrupting Chemicals. Toxicol. Sci. 2006, 94, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Capper, C.P.; Rae, J.M.; Auchus, R.J. The Metabolism, Analysis, and Targeting of Steroid Hormones in Breast and Prostate Cancer. Discov. Oncol. 2016, 7, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J. Steroids in Cancer: Mechanisms, Therapies, and Challenges in Hormone-Driven Malignancies; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–38. [Google Scholar]
- Acconcia, F.; Marino, M. Steroid Hormones: Synthesis, Secretion, and Transport. In Principles of Endocrinology and Hormone Action; Belfiore, A., LeRoith, D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–31. [Google Scholar]
- Dutra, F.F.; Bozza, M.T. Heme on innate immunity and inflammation. Front. Pharmacol. 2014, 5, 115. [Google Scholar] [CrossRef] [PubMed]
- Aftab, H.; Donegan, R.K. Regulation of heme biosynthesis via the coproporphyrin dependent pathway in bacteria. Front. Microbiol. 2024, 15, 1345389. [Google Scholar] [CrossRef] [PubMed]
- Consoli, V.; Sorrenti, V.; Pittalà, V.; Greish, K.; D’amico, A.G.; Romeo, G.; Intagliata, S.; Salerno, L.; Vanella, L. Heme oxygenase modulation drives ferroptosis in TNBC cells. Int. J. Mol. Sci. 2022, 23, 5709. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Page, T.H.; Smolinska, M.; Gillespie, J.; Urbaniak, A.M.; Foxwell, B.M. Tyrosine kinases and inflammatory signalling. Curr. Mol. Med. 2009, 9, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, Y.; Ding, J.-H.; Jin, X.; Ma, D.; Li, D.-Q.; Shi, J.-X.; Huang, W.; Wang, Y.-P.; Jiang, Y.-Z.; et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2022, 35, 84–100.e8. [Google Scholar] [CrossRef] [PubMed]
- Didžiapetrienė, J.; Kazbarienė, B.; Tikuišis, R.; Dulskas, A.; Dabkevičienė, D.; Lukosevičienė, V.; Kontrimavičiūtė, E.; Sužiedėlis, K.; Ostapenko, V. Oxidant/Antioxidant Status of Breast Cancer Patients in Pre- and Post-Operative Periods. Medicina 2020, 56, 70. [Google Scholar] [CrossRef] [PubMed]


| Morphine (n = 29) | ESP (n = 29) | Control (n = 29) | p Value | |
|---|---|---|---|---|
| Age (year), mean ± SD | 51.20 ± 9.60 | 50.83 ± 10.70 | 54.13 ± 9.95 | 0.39 a |
| Body weight (kg), mean ± SD | 74.26 ± 11.6 | 72.86 ± 10.64 | 70.46 ± 8.93 | 0.90 a |
| Height (cm), mean ± SD | 160.5 ± 8.32 | 161.7± 9.33 | 159.8 ± 11.27 | 0.29 a |
| BMI (kg/m2), mean ± SD | 27.91 ± 3.10 | 27.79 ± 3.63 | 28.712 ± 2.96 | 0.50 a |
| ASA classification, n (%) | ||||
| I | 8 (27.5) | 9 (31.1) | 7 (24.2) | 0.42 b |
| II | 21 (72.5) | 20 (68.9) | 22 (73.8) | |
| Comorbidities | 16 (55.1) | 17 (58.6) | 15 (51.7) | 0.9 a |
| Surgery type, n (%) | ||||
| Segmental mastectomy | 21 (72.4) | 18 (62.0) | 17 (58.6) | 0.50 a |
| Modified radical mastectomy | 8 (27.5) | 11 (37.9) | 12 (41.3) | 0.50 a |
| Numeric Rating Scale (0–10) | Morphine (n = 29) | ESP (n = 29) | Control (n = 29) | p |
|---|---|---|---|---|
| Preoperative (mean ± SD) | 0.5 ± 0.72 | 0.4 ± 0.56 | 0.7 ± 0.64 | 0.16 a |
| Postop hour 1 (mean ± SD) | 3.37 ± 0.66 | 1.6 ± 0.56 | 3.65 ± 0.72 | <0.01 a |
| Postop hour 2 (mean ± SD) | 3.06 ± 0.69 | 1.53 ± 0.57 | 3.89 ± 0.85 | <0.01 a |
| Postop hour 12 (mean ± SD) | 2.3 ± 0.65 | 2.63 ± 0.66 | 3.103 ± 0.77 | <0.01 a |
| Postop hour 24 (mean ± SD) | 2.66 ± 0.71 | 2.8 ± 0.71 | 2.53 ± 0.93 | 0.43 a |
| Opioid consumption (mg/24 h) | ||||
| Morphine (mean ± SD) | 11.1 ± 4.1 | N/A | N/A | N/A |
| Tramadol (mean ± SD) | N/A | 57 ± 38 | 166 ± 69 | <0.01 b |
| Morphine (n = 29) | ESP (n = 29) | Control (n = 29) | p | |
|---|---|---|---|---|
| VEGF (pg/mL), mean ± SD | ||||
| T0 | 1219 ± 634 | 1092 ± 516 | 1312 ± 722 | 0.42 a |
| T24 | 1373 ± 627 | 1191 ± 518 | 1656 ± 845 | 0.025 b |
| Cortisol (mcg/dL), mean ± SD | ||||
| T0 | 13.6 ± 6.2 | 14.6 ± 6.4 | 14.1 ± 6.6 | 0.74 a |
| T1 | 21.2 ± 10.1 | 19.3 ± 12.2 | 23.7 ± 8.1 | 0.35 a |
| T24 | 19.3 ± 9.1 | 14.2 ± 6.1 | 20.0 ± 8.3 | 0.041 b |
| CRP (mg/L), mean ± SD | ||||
| T0 | 3.8 ± 4.0 | 2.2 ± 1.9 | 2.9 ± 3.0 | 0.36 a |
| T1 | 4.5 ± 5.6 | 1.7 ± 1.5 | 2.3 ± 2.0 | 0.028 c |
| T24 | 29.2 ± 17.8 | 19.3 ± 10.7 | 18.3 ± 12.1 | 0.022 a |
| Metabolite | Log2 FC | p-Value | On ESP Group | Biological Class | Biological Sub-Class |
|---|---|---|---|---|---|
| Homogentisic acid | 2.50 | 1.58 × 10−2 | ↑ | Benzene and substituted derivatives | Phenylacetic acids |
| Benzamide | 10.63 | 3.22 × 10−17 | ↑ | Benzene and substituted derivatives | Benzoic acid derivative; elevated in inflammation |
| Creatine | 10.34 | 1.32 × 10−16 | ↓ | Carboxylic acids and derivatives | Energy and muscle metabolism; altered in stress and inflammation |
| Glutathione | 5.06 | 4.80 × 10−6 | ↑ | Carboxylic acids and derivatives | Amino acids, peptides, and analogs |
| L-Tyrosine | 3.82 | 4.44 × 10−4 | ↑ | Carboxylic acids and derivatives | Amino acids, peptides, and analogs |
| Sarcosine | 7.24 | 3.32 × 10−10 | ↓ | Carboxylic acids and derivatives | Amino acids, peptides, and analogs |
| Leukotriene C4 | 6.97 | 1.15 × 10−9 | ↓ | Carboxylic acids and derivatives | Amino acids, peptides, and analogs |
| N-Formyl-L-glutamic acid | 5.62 | 4.65 × 10−7 | ↓ | Carboxylic acids and derivatives | Amino acids, peptides, and analogs |
| S-(PGA1)-glutathione | 3.75 | 5.46 × 10−4 | ↑ | Carboxylic acids and derivatives | Amino acids, peptides, and analogs |
| Isobutyryl-CoA | 5.55 | 6.24 × 10−7 | ↑ | Fatty Acyls | Fatty acyl thioesters |
| Acrylyl-CoA | 8.06 | 7.17 × 10−12 | ↓ | Fatty Acyls | Fatty acyl thioesters |
| 3-Oxotetradecanoyl-CoA | 3.65 | 7.56 × 10−4 | ↓ | Fatty Acyls | Fatty acyl thioesters |
| Palmitoylcarnitine | 5.83 | 1.87 × 10−7 | ↑ | Fatty Acyls | Pyrimidine ribonucleotides |
| Octanoylcarnitine | 5.31 | 1.68 × 10−6 | ↓ | Fatty Acyls | Fatty acid esters |
| 3-Hydroxy-OPC6-CoA | 5.06 | 4.77 × 10−6 | ↑ | Fatty Acyls | Fatty acid esters |
| 3-Methylthiopropionic acid | 4.40 | 6.02 × 10−5 | ↑ | Fatty Acyls | Fatty acids and conjugates |
| Prostaglandin D2 | 3.83 | 4.19 × 10−4 | ↓ | Fatty Acyls | Prostaglandins and related compounds |
| LysoPC(O-18:0/0:0) | 4.79 | 1.37 × 10−5 | ↑ | Glycerophospholipids | Glycerophosphocholines |
| LysoPC(18:1/0:0) | 7.36 | 1.95 × 10−10 | ↑ | Glycerophospholipids | Glycerophosphocholines |
| 6-Hydroxyhexanoic acid | 6.71 | 3.86 × 10−9 | ↑ | Hydroxy acids | Medium-chain hydroxy acids and derivatives |
| Xanthine | 2.21 | 2.88 × 10−2 | ↑ | Imidazopyrimidines | Purines and purine derivative |
| 2-Oxoarginine | 5.32 | 1.67 × 10−6 | ↓ | Keto acids and derivatives | Short-chain keto acids and derivatives |
| 2-Acetolactate | 3.12 | 3.51 × 10−3 | ↓ | Keto acids and derivatives | Short-chain keto acids and derivatives |
| Sphingosine | 2.58 | 1.32 × 10−2 | ↑ | Organonitrogen compounds | Amines |
| Putrescine | 4.16 | 1.41 × 10−4 | ↑ | Organonitrogen compounds | Amines |
| 2-Phospho-D-glyceric acid | 7.03 | 8.83 × 10−10 | ↑ | Organooxygen compounds | Carbohydrates and carbohydrate conjugates |
| 2,5-Diamino-6-(5′-triphosphoryl-3′,4′-trihydroxy-2′-oxopentyl)-amino-4-oxopyrimidine | 2.43 | 1.82 × 10−2 | ↑ | Organooxygen compounds | Carbohydrates and carbohydrate conjugates |
| cis-Melilotoside | 4.22 | 1.13 × 10−4 | ↑ | Organooxygen compounds | Carbohydrates and carbohydrate conjugates |
| 3-Dehydrosphinganine | 4.29 | 8.93 × 10−5 | ↑ | Organooxygen compounds | Quinone and hydroquinone lipids |
| Quinone | 3.60 | 8.79 × 10−4 | ↑ | Organooxygen compounds | Quinone and hydroquinone lipids |
| Normetanephrine | 3.35 | 1.88 × 10−3 | ↑ | Phenols | Catecholamine metabolite; reflects sympathetic activation |
| Chlordecone alcohol | 3.02 | 4.57 × 10−3 | ↑ | Prenol lipids | Monoterpenoids |
| Perillic acid | 5.73 | 2.88 × 10−7 | ↑ | Prenol lipids | Monoterpenoids |
| Vitamin K1 | 5.48 | 8.51 × 10−7 | ↓ | Prenol lipids | Quinone and hydroquinone lipids |
| Deoxyadenosine | 2.98 | 5.07 × 10−3 | ↓ | Purine nucleosides | Purine 2′-deoxyribonucleosides |
| Inosine triphosphate | 3.44 | 1.44 × 10−3 | ↓ | Purine nucleotides | Purine ribonucleotides |
| Phosphoribosyl-ATP | 3.65 | 7.43 × 10−4 | ↑ | Purine nucleotides | Purine ribonucleotides |
| 4-Pyridoxic acid | 9.62 | 4.20 × 10−15 | ↑ | Pyridines and derivatives | Pyridinecarboxylic acids and derivatives, Vitamin B6 catabolite; oxidative stress marker |
| Orotidylic acid | 6.55 | 7.78 × 10−9 | ↓ | Pyrimidine nucleotides | |
| Taurocholic acid | 4.08 | 1.82 × 10−4 | ↑ | Steroids and steroid derivatives | Bile acid conjugate; immunological relevance |
| Chenodeoxycholic acid glycine conjugate | 11.31 | 1.16 × 10−18 | ↓ | Steroids and steroid derivatives | Bile acids, alcohols, and derivatives, Immune-modulatory bile acid; anti-inflammatory role |
| Glycocholic acid | 5.25 | 2.20 × 10−6 | ↑ | Steroids and steroid derivatives | Bile acids, alcohols, and derivatives |
| 7 alpha,26-Dihydroxy-4-cholesten-3-one | 2.80 | 8.03 × 10−3 | ↑ | Steroids and steroid derivatives | Bile acids, alcohols, and derivatives |
| Taurochenodesoxycholic acid | 9.85 | 1.38 × 10−15 | ↑ | Steroids and steroid derivatives | Secondary bile acid; immune and hormonal modulation |
| 20alpha-Dihydroprogesterone | 3.93 | 3.10 × 10−4 | ↑ | Steroids and steroid derivatives | Progesterone metabolite; anti-inflammatory effects |
| 21-Deoxycortisol | 2.75 | 8.96 × 10−3 | ↓ | Steroids and steroid derivatives | Pregnane steroids |
| 3a-Hydroxy-5b-pregnane-20-one | 3.45 | 1.38 × 10−3 | ↑ | Steroids and steroid derivatives | Pregnane steroids |
| Testosterone glucuronide | 3.31 | 2.10 × 10−3 | ↓ | Steroids and steroid derivatives | Steroidal glycosides |
| 2-Methoxy-estradiol-17b 3-glucuronide | 4.81 | 1.25 × 10−5 | ↓ | Steroids and steroid derivatives | Steroidal glycosides |
| Estradiol | 10.97 | 6.13 × 10−18 | ↑ | Steroids and steroid derivatives | Estrane steroids, Steroid hormone; related to hormone-sensitive breast cancer |
| 7a,12a-Dihydroxy-5a-cholestan-3-one | 2.57 | 1.37 × 10−2 | ↓ | Steroids and steroid derivatives | Cholestane steroids |
| Coproporphyrin I | 3.70 | 6.45 × 10−4 | ↓ | Tetrapyrroles and derivatives | Porphyrins |
| Coproporphyrin III | 2.75 | 9.07 × 10−3 | ↓ | Tetrapyrroles and derivatives | Porphyrins |
| Heme | 9.12 | 4.64 × 10−14 | ↓ | Tetrapyrroles and derivatives | Porphyrin-related; linked to ferroptosis and oxidative stress |
| Ecgonine methyl ester | 4.78 | 1.43 × 10−5 | ↑ | Tropane alkaloids | Fatty acid esters |
| Se-Adenosylselenohomocysteine | 2.38 | 2.04 × 10−2 | ↑ | 5′-deoxyribonucleosides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guran, E.; Kaplan, O.; Savlı, S.; Sonmez, C.; Dogan, L.; Unver, S. Metabolomic Profiling of Erector Spinae Plane Block for Breast Cancer Surgery. Medicina 2025, 61, 1294. https://doi.org/10.3390/medicina61071294
Guran E, Kaplan O, Savlı S, Sonmez C, Dogan L, Unver S. Metabolomic Profiling of Erector Spinae Plane Block for Breast Cancer Surgery. Medicina. 2025; 61(7):1294. https://doi.org/10.3390/medicina61071294
Chicago/Turabian StyleGuran, Ekin, Ozan Kaplan, Serpil Savlı, Cigdem Sonmez, Lutfi Dogan, and Suheyla Unver. 2025. "Metabolomic Profiling of Erector Spinae Plane Block for Breast Cancer Surgery" Medicina 61, no. 7: 1294. https://doi.org/10.3390/medicina61071294
APA StyleGuran, E., Kaplan, O., Savlı, S., Sonmez, C., Dogan, L., & Unver, S. (2025). Metabolomic Profiling of Erector Spinae Plane Block for Breast Cancer Surgery. Medicina, 61(7), 1294. https://doi.org/10.3390/medicina61071294






