The Evolving Role of Extracorporeal In Situ Perfusion Technology in Organ Donor Recovery with Donation After Circulatory Determination of Death Organ Donors
Abstract
1. Introduction
2. ECMO
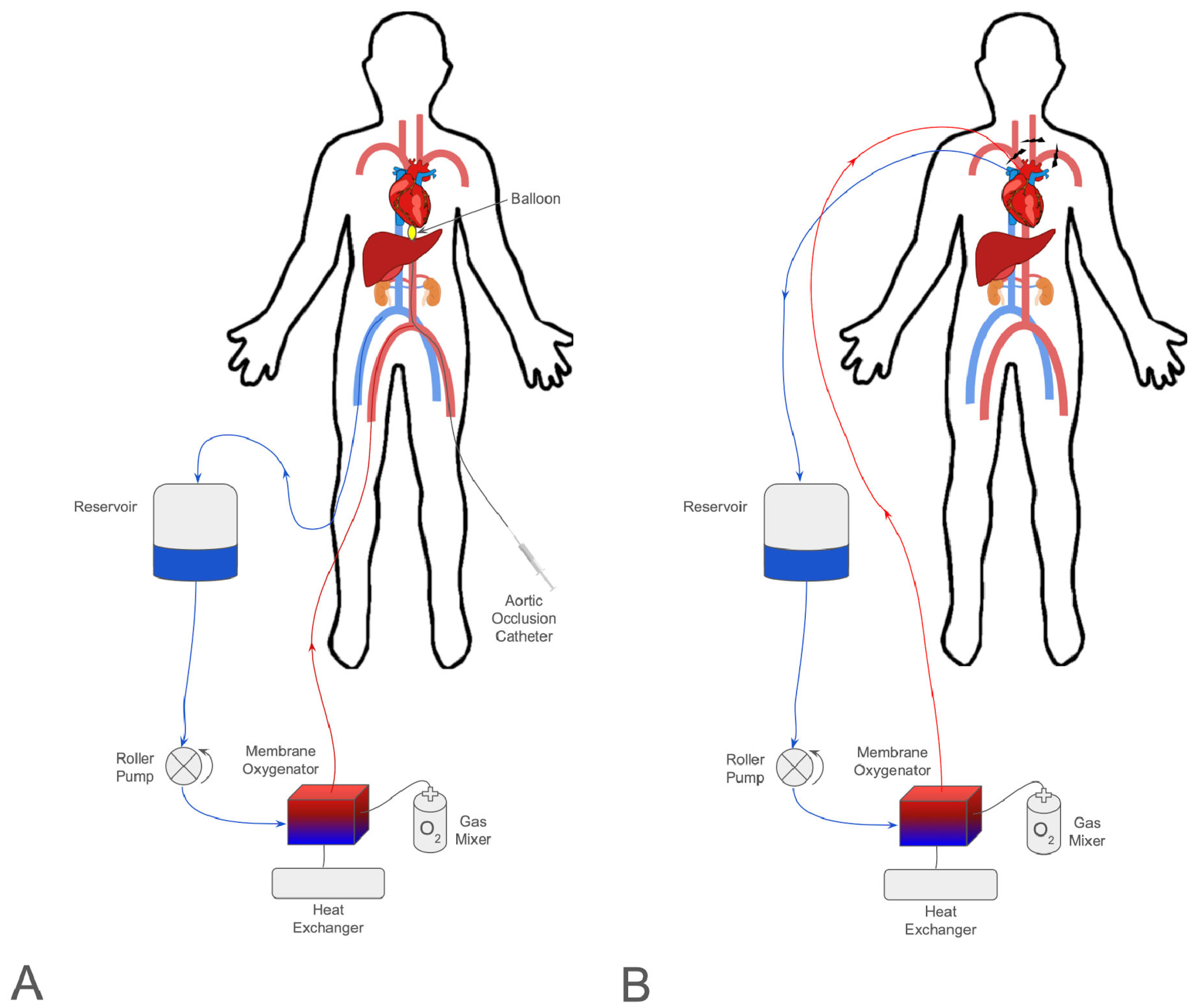
3. Abdominal Normothermic Regional Perfusion
4. Thoracoabdominal Normothermic Regional Perfusion
5. Ethical Concerns Surrounding ECMO, NRP, and TA-NRP in DCD Donation
6. Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Health Resources and Service Administration, United States Department of Health and Human Services. Organ Donation Statistics. Available online: https://www.organdonor.gov/learn/organ-donation-statistics (accessed on 19 May 2025).
- Merion, R.M.; Goodrich, N.P.; Feng, S. How can we define expanded criteria for liver donors? J. Hepatol. 2006, 45, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Manara, A.R.; Murphy, P.G.; O’Callaghan, G. Donation after circulatory death. Br. J. Anaesth. 2012, 108, i108–i121. [Google Scholar] [CrossRef] [PubMed]
- Donation after Circulatory Death Steering Group. Donation after Circulatory Death. British Transplantation Society and Intensive Care Society. 2010. Available online: https://bts.org.uk/wp-content/uploads/2016/09/OD_Circulatory_D.pdf (accessed on 26 May 2025).
- Joffe, A.R.; Carcillo, J.; Anton, N.; deCaen, A.; Han, Y.Y.; Bell, M.J.; Maffei, F.A.; Sullivan, J.; Thomas, J.; Garcia-Guerra, G. Donation after cardiocirculatory death: A call for a moratorium pending full public disclosure and fully informed consent. Philos. Ethics Humanit. Med. 2011, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Goussous, N.; Alvarez-Casas, J.; Dawany, N.; Xie, W.; Malik, S.; Gray, S.H.; Barth, R.N.; LaMattina, J.C. Ischemic Cholangiopathy Postdonation After Circulatory Death Liver Transplantation: Donor Hepatectomy Time Matters. Transplant. Direct 2021, 8, e1277. [Google Scholar] [CrossRef]
- Callaghan, C.J.; Charman, S.C.; Muiesan, P.; Powell, J.J.; Gimson, A.E.; van der Meulen, J.H.P.; on behalf of the UK Liver Transplant Audit. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: A cohort study. BMJ Open 2013, 3, e003287. [Google Scholar] [CrossRef]
- Bellingham, J.M.; Santhanakrishnan, C.; Neidlinger, N.; Wai, P.; Kim, J.; Niederhaus, S.; Leverson, G.E.; Fernandez, L.A.; Foley, D.P.; Mezrich, J.D.; et al. Donation after cardiac death: A 29-year experience. Surgery 2011, 150, 692–702. [Google Scholar] [CrossRef]
- Cho, P.D.; Kim, S.T.; Zappacosta, H.; White, J.P.; McKay, S.; Biniwale, R.; Ardehali, A. Severe primary graft dysfunction in heart transplant recipients using donor hearts after circulatory death: United States experience. J. Heart Lung Transplant. 2025, 44, 760–769. [Google Scholar] [CrossRef]
- Organ Procurement and Transplantation Netwok, US Department of Health and Human Services. Continued Increase in Organ Donation Drives New Records in 2023. New Milestones Exceeded. Available online: https://optn.transplant.hrsa.gov/news/continued-increase-in-organ-donation-drives-new-records-in-2023-new-milestones-exceeded/ (accessed on 19 May 2025).
- Hobeika, M.J.; Glazner, R.; Foley, D.P.; Hanish, S.; Loss, G.; Quintini, C.; Eidbo, E.; Zollinger, C.; Ruterbories, J.; Lebovitz, D.J.; et al. A Step toward Standardization: Results of two National Surveys of Best Practices in Donation after Circulatory Death Liver Recovery and Recommendations from The American Society of Transplant Surgeons and Association of Organ Procurement Organizations. Clin. Transplant. 2020, 34, e14035. [Google Scholar] [CrossRef]
- Mosier, J.M.; Kelsey, M.; Raz, Y.; Gunnerson, K.J.; Meyer, R.; Hypes, C.D.; Malo, J.; Whitmore, S.P.; Spaite, D.W. Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: History, current applications, and future directions. Crit. Care 2015, 19, 431. [Google Scholar] [CrossRef]
- Fainberg, N.A.; Morrison, W.E.; West, S.; Hasz, R.; Kirschen, M.P. Organ Donation From Patients on Extracorporeal Membrane Oxygenation at the Time of Death. Crit. Care Explor. 2022, 4, e0812. [Google Scholar] [CrossRef]
- Hogen, R.; Sedra, A.H.; Motamed, A.; Emamaullee, J. The evolving role of ECMO in liver transplantation. Curr Opin Organ Transpl. 2021, 26, 333–338. [Google Scholar] [CrossRef]
- Sun, X.; Qin, K.; Dong, J.; Li, H.; Huang, Y.; Cao, S.; Li, M.; Lan, L.; Kuang, X.; Wen, N.; et al. The Extra Corporeal Membrane Oxygenation in the Deceased Donors After Brain Death With Severe Hemodynamic Instability Allow to Optimize the Viability of Organs Procured for Transplantation. Transplantation 2017, 101, S175. [Google Scholar] [CrossRef]
- Yumoto, T.; Tsukahara, K.; Obara, T.; Hongo, T.; Nojima, T.; Naito, H.; Nakao, A. Organ donation after extracorporeal cardiopulmonary resuscitation: A nationwide retrospective cohort study. Crit. Care 2024, 28, 160. [Google Scholar] [CrossRef]
- Sandroni, C.; D’Arrigo, S.; Callaway, C.W.; Cariou, A.; Dragancea, I.; Taccone, F.S.; Antonelli, M. The rate of brain death and organ donation in patients resuscitated from cardiac arrest: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Taran, S.; Steel, A.; Healey, A.; Fan, E.; Singh, J.M. Organ donation in patients on extracorporeal membrane oxygenation: Considerations for determination of death and withdrawal of life support. Can. J. Anaesth. 2020, 67, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Rajsic, S.; Treml, B.; Innerhofer, N.; Eckhardt, C.; Radovanovic Spurnic, A.; Breitkopf, R. Organ Donation from Patients Receiving Extracorporeal Membrane Oxygenation: A Systematic Review. J. Cardiothorac. Vasc. Anesth. 2024, 38, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Casadio, M.C.; Coppo, A.; Vargiolu, A.; Villa, J.; Rota, M.; Avalli, L.; Citerio, G. Organ donation in cardiac arrest patients treated with extracorporeal CPR: A single centre observational study. Resuscitation 2017, 118, 133–139. [Google Scholar] [CrossRef]
- Raphalen, J.-H.; Soumagnac, T.; Blanot, S.; Bougouin, W.; Bourdiault, A.; Vimpere, D.; Ammar, H.; Dagron, C.; An, K.; Mungur, A.; et al. Kidneys recovered from brain dead cardiac arrest patients resuscitated with ECPR show similar one-year graft survival compared to other donors. Resuscitation 2023, 190, 109883. [Google Scholar] [CrossRef]
- Gregorini, M.; Ticozzelli, E.; Abelli, M.; Grignano, M.A.; Pattonieri, E.F.; Giacomoni, A.; De Carlis, L.; Dell’Acqua, A.; Caldara, R.; Socci, C.; et al. Kidney Transplants From Donors on Extracorporeal Membrane Oxygenation Prior to Death Are Associated With Better Long-Term Renal Function Compared to Donors After Circulatory Death. Transpl. Int. 2021, 35, 10179. [Google Scholar] [CrossRef]
- Wall, A.; Testa, G. The ethics surrounding normothermic regional perfusion in donors following circulatory death. Clin. Liver Dis. 2024, 23, e0193. [Google Scholar] [CrossRef]
- Sellers, M.T.; Philip, J.L.; Brubaker, A.L.; Cauwels, R.L.; Croome, K.P.; Hoffman, J.R.; Neidlinger, N.A.; Reynolds, A.M.; Wall, A.E.; Edwards, J.M. Normothermic Regional Perfusion Experience of Organ Procurement Organizations in the US. JAMA Netw. Open 2024, 7, e2440130. [Google Scholar] [CrossRef] [PubMed]
- Hessheimer, A.J.; de la Rosa, G.; Gastaca, M.; Ruíz, P.; Otero, A.; Gómez, M.; Alconchel, F.; Ramírez, P.; Bosca, A.; López-Andújar, R.; et al. Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: Outcomes and risk factors for graft loss. Am. J. Transplant. 2022, 22, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Bababekov, Y.J.; Ha, A.H.; Nydam, T.L.; Goncalves, C.; Choudhury, R.; Shinsako, J.; Baimas-George, M.; Reynolds, D.M.; Yoshida, C.; Racke, C.A.; et al. Thoracoabdominal Normothermic Regional Perfusion: Real-world Experience and Outcomes of DCD Liver Transplantation. Transpl. Direct 2025, 11, e1767. [Google Scholar] [CrossRef] [PubMed]
- Bluhme, E.; Gäbel, M.; Martinez de la Maza, L.; Nilsén, V.; Hildebrand, K.; Jarsäter, J.; Bååth, C.; Proos, M.; Romano, A.; Villard, C.; et al. Normothermic regional perfusion in controlled DCD liver procurement: Outcomes of the Swedish national implementation protocol. Liver Transpl. 2024, 30, 1132–1144. [Google Scholar] [CrossRef]
- Brubaker, A.L.; Taj, R.; Jackson, B.; Lee, A.; Tsai, C.; Berumen, J.; Parekh, J.R.; Mekeel, K.L.; Gupta, A.R.; Gardner, J.M.; et al. Early patient and liver allograft outcomes from donation after circulatory death donors using thoracoabdominal normothermic regional: A multi-center observational experience. Front Transpl. 2023, 2, 1184620. [Google Scholar] [CrossRef]
- Oniscu, G.C.; Mehew, J.; Butler, A.J.; Sutherland, A.; Gaurav, R.; Hogg, R.; Currie, I.; Jones, M.; Watson, C.J.E. Improved Organ Utilization and Better Transplant Outcomes With In Situ Normothermic Regional Perfusion in Controlled Donation After Circulatory Death. Transplantation 2023, 107, 438–448. [Google Scholar] [CrossRef]
- Rodriguez, R.P.; Perez, B.S.; Daga, J.A.P.; Diaz, F.J.L.; Aguilar, J.L.F.; Muñoz, M.A.S.; Casado, M.C.M.; Narvaez, J.M.A.; Santoyo, J.S. Outcome of Liver Transplants Using Donors After Cardiac Death With Normothermic Regional Perfusion. Transpl. Proc. 2022, 54, 37–40. [Google Scholar] [CrossRef]
- Pearson, R.; Geddes, C.; Mark, P.; Clancy, M.; Asher, J. Transplantation of kidneys after normothermic perfusion: A single center experience. Clin Transpl. 2021, 35, e14431. [Google Scholar] [CrossRef]
- Sellers, M.T.; Nassar, A.; Alebrahim, M.; Sasaki, K.; Lee, D.D.; Bohorquez, H.; Cannon, R.M.; Selvaggi, G.; Neidlinger, N.; McMaster, W.G.; et al. Early United States experience with liver donation after circulatory determination of death using thoraco-abdominal normothermic regional perfusion: A multi-institutional observational study. Clin Transpl. 2022, 36, e14659. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, A.; Dorsey, M.; James, L.; Hussain, S.; Kadosh, B.; Goldberg, R.; Reyentovich, A.; Moazami, N.; Smith, D. Outcomes of Donation After Circulatory Death Heart Transplantation Using Normothermic Regional Perfusion. Ann. Thorac. Surg. Short Rep. 2025, 3, 229–234. [Google Scholar] [CrossRef]
- Siddiqi Hasan, K.; Trahanas, J.; Xu, M.; Wells, Q.; Farber-Eger, E.; Pasrija, C.; Amancherla, K.; Debose-Scarlett, A.; Brinkley, D.M.; Lindenfeld, J.; et al. Outcomes of Heart Transplant Donation After Circulatory Death. JACC 2023, 82, 1512–1520. [Google Scholar] [CrossRef]
- Alderete, I.S.; Pontula, A.; Halpern, S.E.; Patel, K.J.; Klapper, J.A.; Hartwig, M.G. Thoracoabdominal Normothermic Regional Perfusion and Donation After Circulatory Death Lung Use. JAMA Netw. Open 2025, 8, e2460033. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.L.; Leng, A.; Ruck, J.M.; Akbar, A.F.; Desai, N.M.; King, E.A. Kidney Donation After Circulatory Death Using Thoracoabdominal Normothermic Regional Perfusion: The Largest Report of the United States Experience. Transplantation 2024, 108, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Motter, J.D.; Jaffe, I.S.; Moazami, N.; Smith, D.E.; Kon, Z.N.; Piper, G.L.; Sommer, P.M.; Reyentovich, A.; Chang, S.H.; Aljabban, I.; et al. Single center utilization and post-transplant outcomes of thoracoabdominal normothermic regional perfusion deceased cardiac donor organs. Clin. Transpl. 2024, 38, e15269. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, A.L.; Sellers, M.T.; Abt, P.L.; Croome, K.P.; Merani, S.; Wall, A.; Abreu, P.; Alebrahim, M.; Baskin, R.; Bohorquez, H.; et al. US Liver Transplant Outcomes After Normothermic Regional Perfusion vs Standard Super Rapid Recovery. JAMA Surg. 2024, 159, 677–685. [Google Scholar] [CrossRef]
- Gardiner, D.; McGee, A. Normothermic Regional Perfusion for Organ Donation in the US-The Dangers of Unregulated Adoption. JAMA Netw. Open 2024, 7, e2440017. [Google Scholar] [CrossRef]
- Dalle Ave, A.L.; Shaw, D.M.; Bernat, J.L. Ethical Issues in the Use of Extracorporeal Membrane Oxygenation in Controlled Donation After Circulatory Determination of Death. Am. J. Transpl. 2016, 16, 2293–2299. [Google Scholar] [CrossRef]
- Halpern, S.D.; Truog, R.D. Organ donors after circulatory determination of death: Not necessarily dead, and it does not necessarily matter. Crit. Care Med. 2010, 38, 1011–1012. [Google Scholar] [CrossRef]
- Glannon, W. The moral insignificance of death in organ donation. Camb. Q. Heal. Ethics 2013, 22, 192–202. [Google Scholar] [CrossRef]
- Frontera, J.A.; Lewis, A.; James, L.; Melmed, K.; Parent, B.; Raz, E.; Hussain, S.T.; Smith, D.E.; Moazami, N. Thoracoabdominal normothermic regional perfusion in donation after circulatory death does not restore brain blood flow. J. Heart Lung Transpl. 2023, 42, 1161–1165. [Google Scholar] [CrossRef]
- Royo-Villanova, M.; Miñambres, E.; Sánchez, J.M.; Torres, E.; Manso, C.; Ballesteros, M.; Parrilla, G.; de Paco Tudela, G.; Coll, E.; Pérez-Blanco, A.; et al. Maintaining the permanence principle of death during normothermic regional perfusion in controlled donation after the circulatory determination of death: Results of a prospective clinical study. Am. J. Transpl. 2024, 24, 213–221. [Google Scholar] [CrossRef]
- Royo-Villanova, M.; Sánchez, J.M.; Moreno-Monsalve, T.; Contreras, J.; Ortín, A.; Vargas, H.; Murcia, C.M.; Llosa, M.M.; Coll, E.; Pérez-Blanco, A.; et al. A scintigraphic look at the dead donor rule in donation after the circulatory determination of death with the use of normothermic regional perfusion: A single-center interventional trial. Am. J. Transplant. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Subramanian, V.; Mathur, A.K.; Aqel, B.; Mao, S.A.; Clendenon, J.N.; Perry, D.K.; Dhanireddy, K.; Taner, C.B. Outcomes of DCD Liver Transplant Using Sequential Normothermic Regional Perfusion and Normothermic Machine Perfusion or NRP Alone Versus Static Cold Storage. Transplantation 2025, 109, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Zanierato, M.; Vergano, M.; Magaton, C.; Diale, E.; Rizza, G.; Catalano, S.; Mirabella, S.; Cocchis, D.; Potenza, R.; et al. Normothermic Regional Perfusion and Hypothermic Oxygenated Machine Perfusion for Livers Donated After Controlled Circulatory Death With Prolonged Warm Ischemia Time: A Matched Comparison With Livers From Brain-Dead Donors. Transpl. Int. 2022, 35, 10390. [Google Scholar] [CrossRef] [PubMed]
- De Carlis, R.; Lauterio, A.; Centonze, L.; Buscemi, V.; Schlegel, A.; Muiesan, P.; De Carlis, L. Current practice of normothermic regional perfusion and machine perfusion in donation after circulatory death liver transplants in Italy. Updates Surg. 2022, 74, 501–510. [Google Scholar] [CrossRef]
- Bery, A.; Ali, A.; Cypel, M.; Kreisel, D. Centralized Organ Recovery and Reconditioning Centers. Thorac. Surg. Clin. 2022, 32, 167–174. [Google Scholar] [CrossRef]
- Marklin, G.F.; Brockmeier, D.; Spector, K. The 20-year paradigm shift toward organ recovery centers: 2500 donors at Mid-America Transplant and broader adoption across the United States. Am. J. Transpl. 2023, 23, 891–903. [Google Scholar] [CrossRef]
- Dor, F.; Porte, R.J.; Manas, D.M. The future of organ donation in Europe: Is there a role for organ recovery centers? Am. J. Transpl. 2023, 23, 873–874. [Google Scholar] [CrossRef]
| Technique | Organ | n | Outcomes | References |
|---|---|---|---|---|
| NRP | Liver | n = 162 NRP = 97 SCS = 79 | Comparable 12 mo allograft and recipient survival for NRP and SCS, despite higher risk donor–recipient pairing in NRP group. | Bababekov et al. [26] |
| DCD NRP = 18 DBD = 28 | Comparable recipient and graft survival, early allograft dysfunction, and AKI between DCD NRP and DBD groups. | Bluhme et al. [27] | ||
| n = 242 NRP = 106 SRR = 136 | Reduced rates of ischemic cholangiopathy, biliary complications, and early allograft dysfunction in NRP group. | Brubaker et al. [28] | ||
| n = 1165 NRP = 775 SRR = 390 | NRP cohort showed reduced rates of complications | Hessheimer et al. [25] | ||
| Ntotal = 4716 Non-NRP = 4553 NRP = 163 | NRP increased odds of liver being transplanted 3-fold. Improved 1-year survival | Oniscu et al. [29] | ||
| n = 117 DCD NRP = 39 DBD = 78 | Comparable survival, early allograft dysfunction and primary non-function between NRP and DBD groups. | Rodriguez et al. [30] | ||
| Kidney | Ntotal = 4716 Non-NRP = 4553 NRP = 163 | NRP increased odds of kidney being transplanted by 1.5-fold; 35% lower chance of DGF in NRP cohort | Oniscu et al. [29] | |
| n = 632 DCD = 229 NRP = 29 | Delayed graft function rate lower in NRP group. One-year graft loss rate was lower in NRP group. | Pearson, et al. [31] | ||
| Pancreas | Ntotal = 4716 Non-NRP = 4553 NRP = 163 | NRP increased odds of pancreas being transplanted 1.6-fold | Oniscu et al. [29] | |
| TA-NRP | Liver | n = 43 | Good early allograft and recipient outcomes in small cohort. | Brubaker et al. [28] |
| n = 13 | Observation study with small cohort; 92% of cohort alive with good liver function at median follow-up of 439 days. | Sellers, et al. [32] | ||
| Heart | n = 32 | Observational study. Cohort had 100% 1-year survival. | Kumar et al. [33] | |
| n = 385 DCD = 122 | DCD heart graft outcomes were non-inferior to DBD outcomes. | Siddiqi et al. [34] | ||
| Lungs | Ntot = 24,431 TA-NRPcardiac = 325 | Improved 90-day and overall survival. | Alderete et al. [35] | |
| Kidney | Ntot = 16,140 TA-NRP = 306 | Similar survival and all-cause graft failure between TA-NRP and direct-recovery groups. Lower likelihood of delayed graft function with TA-NRP. | Zhou et al. [36] | |
| Multi | TA-NRP = 22 | Comparable recipient and graft survival between TA-NRP group and DBD group | Motter et al. [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammond, V.R.; Franklin, M.E.; Franklin, G.A. The Evolving Role of Extracorporeal In Situ Perfusion Technology in Organ Donor Recovery with Donation After Circulatory Determination of Death Organ Donors. Medicina 2025, 61, 1276. https://doi.org/10.3390/medicina61071276
Hammond VR, Franklin ME, Franklin GA. The Evolving Role of Extracorporeal In Situ Perfusion Technology in Organ Donor Recovery with Donation After Circulatory Determination of Death Organ Donors. Medicina. 2025; 61(7):1276. https://doi.org/10.3390/medicina61071276
Chicago/Turabian StyleHammond, Victoria R., Marisa E. Franklin, and Glen A. Franklin. 2025. "The Evolving Role of Extracorporeal In Situ Perfusion Technology in Organ Donor Recovery with Donation After Circulatory Determination of Death Organ Donors" Medicina 61, no. 7: 1276. https://doi.org/10.3390/medicina61071276
APA StyleHammond, V. R., Franklin, M. E., & Franklin, G. A. (2025). The Evolving Role of Extracorporeal In Situ Perfusion Technology in Organ Donor Recovery with Donation After Circulatory Determination of Death Organ Donors. Medicina, 61(7), 1276. https://doi.org/10.3390/medicina61071276







