Abstract
Background and Objectives: Chronic obstructive pulmonary disease (COPD) significantly impacts morbidity and mortality, often due to cardiovascular comorbidities that are frequently overlooked. This study examines the prevalence of left ventricular dysfunction in COPD patients and its association with disease severity, hypoxemia, and exacerbation frequency. Materials and Methods: COPD patients (n = 114) were evaluated using spirometry and transthoracic echocardiography. Statistical analysis utilized Student’s t-test, chi-square test, and multivariable logistic regression with 1000 bootstrapping iterations, considering p < 0.05 as significant differences. Results: Most patients were classified as Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage III (40.4%) and stage IV (44.7%). Diastolic dysfunction was present in 67.5% of the patients (Grade 1: 53.5%, Grade 2: 13. 2%, Grade 3: 0.0.9%), while 18.4% exhibited systolic dysfunction (LVEF < 50%). The prevalence of diastolic dysfunction increased significantly, from 41.2% in GOLD stage II to 92. 2% in GOLD stage IV (p < 0.001). Independent predictors of diastolic dysfunction included GOLD stage IV (Odds Ratio [OR]: 5.39, 95% Confidence Interval [CI]: 1. 42–23.35, p < 0.001), older age (OR: 1.02 per year, 95% CI: 1.01–1.04, p = 0.025), and a history of frequent exacerbations (OR: 1.09 per event, 95% CI: 1.01–1.17, p = 0.039). Systolic dysfunction correlated significantly with GOLD stage IV (OR: 1.83, p = 0.014), oxygen saturation below 88% (OR: 3.12, p = 0.036), and having three or more exacerbations (OR: 4.18, p = 0.008). Conclusions: This study reveals a high prevalence of left ventricular dysfunction in COPD patients, linked to disease severity, hypoxemia, and frequent exacerbations. It supports incorporating complementary echocardiographic assessments in managing advanced COPD, especially for those with frequent exacerbations or oxygen desaturation.
1. Introduction
Chronic obstructive pulmonary disease (COPD) represents a significant global health burden, currently ranking as the third leading cause of mortality worldwide [1]. Beyond the characteristic airflow limitation, COPD is increasingly recognized as a complex multisystem disorder with significant extrapulmonary manifestations, particularly cardiovascular comorbidities [2,3]. These comorbidities substantially worsen clinical outcomes and contribute to increased hospitalization rates and mortality.
The cardiovascular manifestations of COPD extend beyond shared risk factors such as aging and tobacco exposure. Current evidence suggests that systemic inflammation, oxidative stress, chronic hypoxemia, and autonomic dysregulation directly contribute to myocardial dysfunction in COPD [4,5]. A recent study showed that a substantial number of patients with COPD had cardiac abnormalities that had not been identified before. In addition to a simultaneous burden of coronary artery disease and atrial fibrillation, the investigators found undiscovered left ventricular systolic dysfunction, indicating an underestimated overlap between cardiovascular and respiratory pathology in that cohort [5].
The pathophysiological impact of COPD on cardiac function involves multiple mechanisms. Chronic hypoxemia and pulmonary vascular remodeling impair myocardial oxygenation and promote ventricular remodeling [6,7]. Alterations in autonomic regulation, as evidenced by impaired heart rate variability, may precede overt structural cardiac changes [7]. Left ventricular diastolic and systolic dysfunction represent clinically significant consequences, associated with reduced exercise capacity, increased exacerbation frequency, and higher mortality [8,9,10]. Despite these established associations, cardiac abnormalities frequently remain undetected in COPD due to overlapping symptomatology and the absence of routine cardiovascular assessment in current management protocols [11,12]. Transthoracic echocardiography provides a non-invasive, widely accessible tool for evaluating left ventricular function; however, it still remains underexplored in COPD populations [13,14,15]. Recently, a hybrid ensemble method for predicting cardiovascular risk—HeartEnsembleNet—reported an accuracy of 92.95% and a precision of 93.08%. When considering similar predictive models for diseases like COPD, the methodological framework of this hybrid ensemble method may be worth comparing with the existing models for COPD, considering its potential implications in clinical practice [16].
The present study aimed to investigate echocardiographic evidence of left ventricular dysfunction—both systolic and diastolic—in patients with COPD attending a tertiary care hospital in Silchar, India. The population, characterized by a high prevalence of advanced disease stages and significant biomass fuel exposure, offers a valuable opportunity to examine the relationship between COPD severity, hypoxemia, exacerbation frequency, and cardiac function. The goal of this investigation is to identify key clinical and pathophysiological factors associated with cardiac issues. It is expected that the findings will support the need to include regular cardiac examinations in the management of COPD, allowing for the early detection and treatment of cardiovascular comorbidities in this high-risk group.
2. Materials and Methods
2.1. Study Design and Setting
This prospective observational study was conducted at the Department of Medicine, Silchar Medical College and Hospital, Silchar, India, between 1 March 2023 and 29 February 2024. The Institutional Human Ethics Committee (IHEC) of Silchar Medical College and Hospital approved the study protocol. All participants provided written informed consent before enrollment. The study was conducted in accordance with the Declaration of Helsinki and local regulatory standards.
2.2. Patient Selection
Patients were consecutively recruited from the inpatient and outpatient services of the Department of Medicine. A diagnosis of COPD was established based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 criteria [2], which incorporated clinical symptoms, exposure history (including smoking or biomass fuel), and spirometric evidence of persistent airflow limitation, i.e., post-bronchodilator forced expiratory volume in 1 s/forced vital capacity ratio <0.70 (FEV1/FVC ratio <0.70).
Inclusion criteria comprised age ≥40 years, confirmed COPD diagnosis as per GOLD 2023 guidelines, adequate spirometry performance, and provision of written informed consent. Exclusion criteria included asthma or other primary respiratory disorders, known cardiac diseases (valvular heart disease, congenital defects, cardiomyopathies), recent acute coronary syndrome (within the past 3 months), severe systemic illness affecting cardiac function, pregnancy or lactation, inability to perform spirometry, and declined consent.
2.3. Clinical and Pulmonary Assessment
Each participant underwent a comprehensive clinical evaluation, which included demographic data, comorbidities, respiratory symptoms, smoking status (in terms of pack-years), and biomass fuel exposure (among female participants). Vital parameters were documented, including respiratory rate, blood pressure, and resting oxygen saturation (measured via pulse oximeter on room air). The participants’ exacerbation history over the preceding 12 months was recorded.
Pulmonary function testing was performed using a calibrated spirometer (Spirolab III, MIR, Italy) [17]. Post-bronchodilator spirometry (after 400 µg salbutamol via MDI with spacer) was used to assess FEV1, FVC, FEV1/FVC ratio, and FEV1% predicted.
Disease severity was classified according to GOLD stages:—GOLD I: FEV1 ≥ 80%, GOLD II: 50–79%, GOLD III: 30–49%, and GOLD IV: < 30%.
2.4. Laboratory Investigations
Peripheral blood samples were analyzed for complete blood count, erythrocyte sedimentation rate (ESR), alanine transaminase (ALT), aspartate aminotransferase (AST), random blood sugar, serum creatinine, and blood urea to assess systemic comorbidities and ensure eligibility.
2.5. Echocardiographic Evaluation
All patients underwent transthoracic echocardiography performed by a single experienced cardiologist blinded to clinical data, using a Philips EPIQ 7C system. Standard parasternal and apical views were obtained in accordance with the American Society of Echocardiography (ASE) guidelines [18].
Left ventricular systolic function was assessed using Simpson’s biplane method. Left ventricular systolic dysfunction (LVSD) was defined as left ventricular ejection fraction (LVEF) <50%. Diastolic function was evaluated according to ASE criteria [19], including: mitral inflow (E/A ratio and deceleration time), tissue doppler (septal and lateral e’ velocities), and average E/e’ ratio (with grading as Grade 1: E/A ≤ 0.8, E/e’ < 10; Grade 2: E/A 0.8–2.0, E/e’ 10–14; and Grade 3: E/A ≥ 2.0, E/e’ >14).
When indices were discordant, left atrium (LA) volume, tricuspid regurgitation velocity (TRV), and pulmonary vein flow were used for classification. Additional parameters included LA volume index, left ventricular mass index (LVMi), right ventricular (RV) function, and pulmonary artery systolic pressure.
2.6. Statistical Analysis
The data were analyzed using R software version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were expressed as mean ± standard deviation (SD) or median (IQR) and compared using Student’s t-test or Mann-Whitney U test as appropriate. Categorical variables were expressed as frequencies and percentages and analyzed using the Chi-square or Fisher’s exact test. Correlations were assessed using Pearson or Spearman coefficients.
Multivariable logistic regression was performed to identify independent predictors of LV dysfunction. Bootstrapping (1000 iterations) was employed for model stability [20]. Benjamini–Hochberg correction was applied to account for multiple comparisons [21], and unadjusted as well as corrected p-values were reported. Statistical significance was set at p < 0.05. No imputation was performed, as there were no missing data.
3. Results
3.1. Demographic and Clinical Characteristics
A total of 114 patients with COPD were enrolled, with a mean age of 62.1 ± 9.5 years. The majority were male (70.2%) and belonged to the 51–70 age group (70.2%). Most patients were smokers (88.6%), with a mean smoking exposure of 25.0 ± 8.3 pack-years. Among female participants, 26.5% reported significant exposure to biomass fuel. Inhaler use was reported by 50.0% of patients. The most common symptoms were cough (79.8%), dyspnea (71.9%), fever (41.2%), and sputum production (38.6%). The mean respiratory rate was 27.1 ± 2.7 breaths/min, the mean oxygen saturation (SpO2) was 84.6 ± 5.0%, and the mean body mass index (BMI) was 19.3 ± 1.4 kg/m2. Exacerbation history in the previous year showed 29.8% participants had none, 27.2% had one, 21.1% had two, and 21.9% had three or more (Table 1).

Table 1.
Demographic and clinical characteristics of study participants (n = 114).
3.2. COPD Severity and Lung Function
No patients were identified as being classified in GOLD stage I. In the cohort under study, GOLD stage II comprised 14.9% (n = 17) participants, GOLD stage III accounted for 40.4% (n = 46), and GOLD stage IV represented 44.7% (n = 51). The mean FEV1/FVC ratio was 53.6 ± 7.8%, while the mean predicted FEV1 was 34.6 ± 10.4%, indicating a patient population with moderate to severe chronic obstructive pulmonary disease (Table 2).

Table 2.
Pulmonary Function and GOLD Classification (n = 114).
3.3. Prevalence of Left Ventricular Dysfunction
Diastolic dysfunction was identified in 67.5% (n = 77) patients, with Grade 1 being the most prevalent (53.5%, n = 61), followed by Grade 2 (13.2%, n = 15) and Grade 3 (0.9%, n = 1). The remaining 32.5% (n = 37) exhibited normal diastolic function. Systolic dysfunction (LVEF < 50%) was detected in 18.4% (n = 21), with a mean LVEF of 44.8 ± 3.2% for those affected, compared to 58.3 ± 4.7% in unaffected individuals.
3.4. Association Between COPD Severity and Cardiac Dysfunction
The current study revealed a notable link between the advancement of the GOLD stage and the prevalence of diastolic and systolic dysfunction. The prevalence of diastolic dysfunction increased from 41.2% in GOLD II to 92.2% in GOLD IV (χ2 = 24.32, p < 0.001). Similarly, systolic dysfunction rose from 5.9% to 27.5% within the same stages (χ2 = 9.62, p = 0.008) (Figure 1).
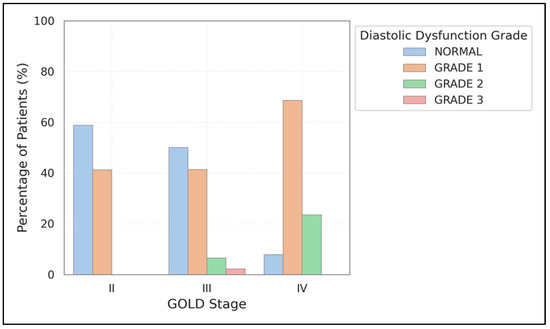
Figure 1.
Prevalence of diastolic dysfunction grades across GOLD stages. The graph shows increasing prevalence of diastolic dysfunction with worsening COPD severity, with Grade 1 and Grade 2 dysfunction becoming more common in advanced disease stages.
3.5. Subgroup Analyses
3.5.1. Smoking Status
Diastolic dysfunction was more frequently observed in smokers (72.5%) compared to non-smokers (55.9%), although this difference was not statistically significant (p = 0.077). Notably, Grade 2 dysfunction was almost twice as prevalent in smokers (15.0% versus 8.8%). Systolic dysfunction was found in 20.0% of smokers and 14.7% of non-smokers. Figure 2 reinforces these findings and emphasizes the trend toward increased severity of diastolic dysfunction among smokers. Further details of diastolic and systolic dysfunction by smoking status can be found in Supplementary Figure S1.
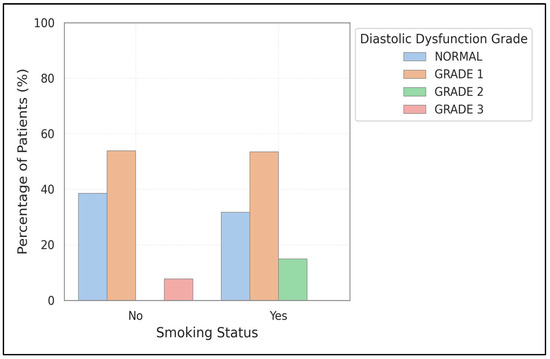
Figure 2.
Comparison of diastolic dysfunction prevalence and severity between smokers and non-smokers. The chart shows higher overall prevalence and greater severity of diastolic dysfunction in smokers compared to non-smokers.
3.5.2. Oxygen Saturation
Both forms of left ventricular dysfunction were linked to lower SpO2 levels. For diastolic dysfunction, the rates were 82.5 ± 6.1% compared to 86.9 ± 5.6% (p < 0.001); for systolic dysfunction, they were 80.2 ± 6.4% versus 84.7 ± 5.9% (p = 0.003). Figure 3 illustrates a decreasing trend in oxygen saturation as diastolic dysfunction progresses from Grade 1 to Grade 3, with a critical threshold at SpO2 < 88% highlighted.
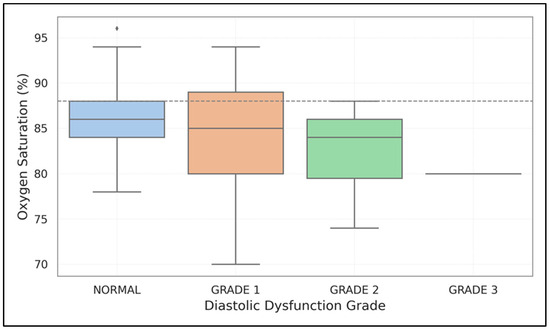
Figure 3.
Relationship between oxygen saturation and diastolic dysfunction grades. The box plot demonstrates progressively lower SpO2 values with increasing severity of diastolic dysfunction, with a threshold of SpO2 < 88% highlighted.
3.5.3. Exacerbation Frequency
The occurrence of systolic dysfunction increased from 8.8% (no exacerbations) to 36.0% (≥3 exacerbations) (χ2 = 7.95, p = 0.047). In contrast, diastolic dysfunction rose from 55.9% to 79.2%, although the statistical significance diminished after adjustment. Figure 4 depicts this dose-response pattern, highlighting how frequent exacerbations affect myocardial function.
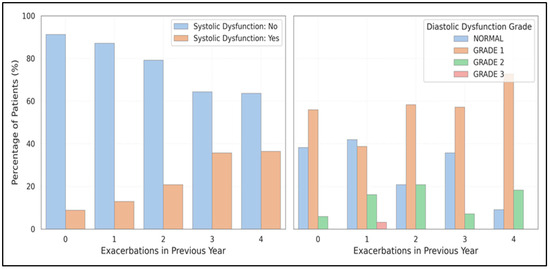
Figure 4.
Illustrates the relationship between exacerbation frequency and left ventricular dysfunction, showing the dose-response relationship between the number of exacerbations in the past year and the prevalence of systolic and diastolic dysfunction.
3.5.4. Pulmonary Function Correlation
Negative correlations were found between FEV1/FVC and the E/A ratio (r = −0.32, p < 0.001), as well as between FEV1% predicted and the E/e′ ratio (r = −0.31, p = 0.001). Additionally, LVEF showed a positive correlation with both FEV1/FVC and FEV1% predicted (r = 0.27–0.30, p ≤ 0.004), indicating that worsening airflow obstruction is linked to more severe left ventricular impairment.
3.6. Multivariate Analysis
Among the candidate predictors tested, only GOLD stage IV was independently associated with diastolic dysfunction (OR: 12.09, 95% CI: 3.49–41.87, p < 0.001). None of the predictors reached statistical significance for systolic dysfunction in the multivariate model, although GOLD stage IV (OR: 2.37, p = 0.164) and ≥3 exacerbations (OR: 2.02, p = 0.522) exhibited a consistent directional trend (Figure 5).
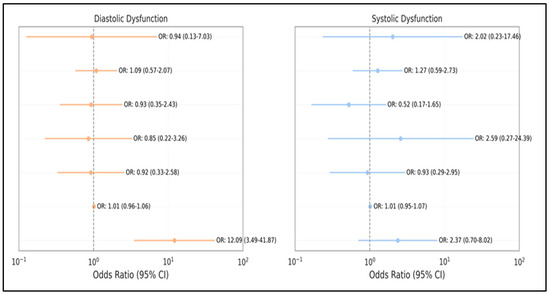
Figure 5.
Forest plot of independent predictors for left ventricular dysfunction. The plot displays odds ratios with 95% confidence intervals for significant predictors of both diastolic and systolic dysfunction identified in multivariate analysis.
4. Discussion
The current study revealed a significant prevalence of left ventricular dysfunction in COPD patients, with 67.5% exhibiting diastolic dysfunction and 18.4% demonstrating systolic dysfunction. Both types of dysfunctions showed strong, statistically significant links to increasing severity of COPD (GOLD staging), diminished pulmonary function, oxygen desaturation, and frequent exacerbations. GOLD stage IV, older age, and frequency of exacerbations independently predicted diastolic dysfunction. In contrast, systolic dysfunction was independently linked to GOLD stage IV, oxygen saturation below 88%, and three or more exacerbations in the past year.
The prevalence of systolic dysfunction observed in our study aligns with that of Kibbler et al. [5], who recently noted a pooled rate of 15.8% in their meta-analysis. The slightly higher rate in our cohort (18.4%) probably reflects the higher concentration of patients in GOLD III and IV stages. Freixa et al. [22] and Huang et al. [23] also reported an increase in LV dysfunction in later stages of COPD, especially among GOLD IV patients. Our findings enhance this understanding through a representative cohort significantly leaning towards advanced disease, with a 92.2% prevalence of diastolic dysfunction in GOLD IV patients. The group with normal function significantly decreases in GOLD IV, highlighting the impact of advanced COPD on left ventricular relaxation.
Previous studies by Sabit et al. [24] and Funk et al. [25] identified significant correlations between FEV1 and echocardiographic indicators of LV diastolic dysfunction. Our results corroborate with significant relationships between both FEV1% predicted and the E/e′ ratio. Furthermore, our data support the influence of oxygen desaturation on cardiac function, consistent with earlier research connecting chronic hypoxemia to myocardial dysfunction and pulmonary hypertension [26,27].
In earlier studies, MacDonald et al. [27] and Patel et al. [28] emphasized the connection between exacerbations and cardiac outcomes. The current study’s findings reinforce this connection, showing a clear dose-response relationship between exacerbation frequency and diastolic and systolic dysfunction. These results highlight the cardiovascular burden associated with exacerbation-prone COPD phenotypes.
Multiple overlapping mechanisms might clarify the observed relationships. Cigarette smoking leads to oxidative stress, systemic inflammation, and direct myocardial damage [28]. Despite no significant differences in overall cardiac dysfunction rates between smokers and non-smokers, smokers in our study displayed more severe (Grade 2) diastolic dysfunction.
Hypoxemia hinders myocardial oxygen delivery and exacerbates systolic and diastolic dysfunction by directly impacting myocardial relaxation, increased afterload, and remodeling of pulmonary vasculature [26,27]. In our group, lower SpO2 was linked to both types of dysfunctions, with patients having saturations above 92% showing significantly reduced rates of cardiac impairment.
Systemic inflammation—long seen as a hallmark of COPD—can drive myocardial fibrosis and endothelial dysfunction, mechanisms that may contribute to diastolic stiffness and subclinical systolic failure [29,30]. In advanced COPD, pulmonary hyperinflation further limits cardiac filling by decreasing venous return and modifying ventricular interdependence [31,32].
Moreover, autonomic dysfunction in COPD, characterized by altered heart rate variability and increased sympathetic tone, may facilitate myocardial remodeling and be a factor in LV dysfunction [33]. Another previous study suggests that frequent exacerbations function as acute “cardiac stress tests”, emphasizing the need for more research into the cardiovascular impacts of these events [34].
Given the common occurrence of LV dysfunction and its link to disease severity, exacerbation, and hypoxemia, it may be an important complementary evaluation of cardiac health in COPD patients, especially those classified as GOLD stages III and IV. Transthoracic echocardiography is a cost-effective and readily available method for detecting subclinical cardiac issues. Identifying diastolic and systolic dysfunction early may inform pulmonary and cardiovascular treatment strategies.
Integrating complementary cardiopulmonary assessments into COPD management can impact treatment choices. Optimizing bronchodilator therapy can alleviate hyperinflation and enhance venous return, which may positively affect cardiac function. Clinicians should also be mindful of the cardiovascular safety profiles of medications prescribed for COPD.
Additionally, preventing exacerbations—through the guideline-based application of inhaled therapies, pulmonary rehabilitation, and vaccination—could help limit cardiovascular complications [35]. Long-term oxygen therapy has been demonstrated to enhance survival rates and cardiac function for patients with hypoxemia [36]. Integrative care models that include pulmonologists and cardiologists may be especially beneficial for this high-risk group.
This study possesses several significant strengths, including a strong representation of GOLD III–IV patients and standardized evaluations of pulmonary and cardiac function. Blinding the echocardiography operator to the clinical status helps minimize the risk of detection bias. Our research showed that 67.5% of COPD patients exhibit diastolic dysfunction, with 18.4% also experiencing systolic dysfunction. This underscores the importance of early detection and proactive management of cardiovascular issues in this group. Using machine learning can improve early diagnosis by revealing complex, non-linear connections among variables such as spirometric indices, echocardiographic data, biomarkers, and comorbidities—patterns that traditional statistical methods might overlook [16,37].
While the study offers valuable insights, it has some limitations. For instance, it did not fully account for conditions such as diabetes, high blood pressure, and kidney issues, which may also affect heart performance outside of COPD. The analysis of pulmonary hypertension and hypoxemia was constrained because PASP measurements were not always available. Furthermore, without a healthy control group matched for age and sex, it is challenging to determine precisely how COPD affects the heart. As a cross-sectional observational study, it cannot establish causality either. The findings may also vary since they are based on a real-world clinical population. Future studies should include well-matched control groups to clarify the specific impact of COPD on the heart. Long-term research is necessary to explore causal relationships and track the progression of heart dysfunction. Gathering detailed data on other health conditions and ensuring accurate PASP measurement, particularly in relation to hypoxemia and pulmonary hypertension, will enhance our understanding of the heart–lung connection. Ultimately, early detection of heart involvement in COPD could improve risk assessment and enable more timely treatment.
5. Conclusions
This study provides strong evidence of a significant burden of left ventricular (LV) dysfunction in COPD patients, especially in advanced stages. Diastolic dysfunction was observed in roughly two-thirds of participants, while nearly 20% showed systolic dysfunction; both were linked to worse pulmonary function, hypoxemia, and frequent exacerbations. These findings underscore the importance of proactive cardiovascular screening in COPD patients, particularly those with severe disease, oxygen desaturation, or exacerbation-prone features. Early detection of cardiac issues—even without traditional symptoms—may enable more personalized and integrated treatment strategies. The connections found support a multifactorial pathophysiology involving systemic inflammation, hypoxemia, autonomic imbalance, and direct pulmonary–cardiac interactions. Future research should focus on prospective, longitudinal studies to identify causative factors and assess as to whether early cardiac screening and targeted therapies can improve outcomes for such a high-risk group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina61071260/s1, Figure S1: Cardiac Dysfunction by Smoking Status.
Author Contributions
Conceptualization, R.B., T.D., S.R.; methodology, P.R. and P.B.; investigation, R.B. and T.D.; writing—original draft preparation, R.B. and T.D.; writing—review and editing, I.M.R. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Institutional Human Ethics Committee (IHEC) of Silchar Medical College and Hospital approved the study protocol (No. SME/ETHICS/17th Feb/2023/23, date: 15 July 2024).
Informed Consent Statement
Prior to enrolment, all participants provided written informed consent. The procedures adhered to the ethical standards of the committee responsible for human experimentation and the principles outlined in the Helsinki Declaration (1975, revised 2013). Patient identities and institutional identifiers were anonymized in all data handling and reporting.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors have no conflicts of interest.
Abbreviations
| IHEC | The Institutional Human Ethics Committee |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| COPD | Chronic obstructive pulmonary disease |
| FEV1 | Forced expiratory volume in 1 s |
| FVC | Forced vital capacity |
| ESR | Erythrocyte sedimentation rate |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| ASE | American Society of Echocardiography |
| LVSD | Left ventricular systolic dysfunction |
| LVEF | Left ventricular ejection fraction |
| SpO2 | Peripheral oxygen saturation |
| BMI | Body mass index |
| LA | Left atrium |
| TRV | Tricuspid regurgitation velocity |
| LVMi | Left ventricular mass index |
| RV | Right ventricular |
| LV | Left ventricular |
References
- World Health Organization (WHO). The Top 10 Causes of Death; WHO: Geneva, Switzerland, 2025. [Google Scholar]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef] [PubMed]
- Waschki, B.; Watz, H.; Holz, O.; Magnussen, H.; Olejnicka, B.; Welte, T.; Rabe, K.F.; Janciauskiene, S. Plasminogen Activator Inhibitor-1 Is Elevated in Patients with COPD Independent of Metabolic and Cardiovascular Function; Taylor & Francis: Abingdon, UK, 2017; pp. 981–987. [Google Scholar]
- Barnes, P.J.; Celli, B.R. Systemic Manifestations and Comorbidities of COPD; European Respiratory Society: Lausanne, Switzerland, 2009; Volume 33, pp. 1165–1185. [Google Scholar]
- Kibbler, J.; Wade, C.; Mussell, G.; Ripley, D.P.; Bourke, S.C.; Steer, J. Systematic Review and Meta-Analysis of Prevalence of Undiagnosed Major Cardiac Comorbidities in COPD. ERJ Open Res. 2023, 9, 548–2023. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W.; Maclay, J.; McAllister, D. Cardiovascular Injury and Repair in Chronic Obstructive Pulmonary Disease. Proc. Am. Thorac. Soc. 2008, 5, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Goudis, C.A. Chronic Obstructive Pulmonary Disease and Atrial Fibrillation: An Unknown Relationship. J. Cardiol. 2017, 69, 699–705. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Dransfield, M.T. Chronic Obstructive Pulmonary Disease and Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2013; Volume 162, pp. 237–251. [Google Scholar]
- Fabbri, L.M.; Luppi, F.; Beghé, B.; Rabe, K.F. Complex Chronic Comorbidities of COPD; European Respiratory Society: Lausanne, Switzerland, 2007; Volume 31, pp. 204–212. [Google Scholar]
- Sin, D.D.; Man, S.P. Why Are Patients with Chronic Obstructive Pulmonary Disease at Increased Risk of Cardiovascular Diseases? The Potential Role of Systemic Inflammation in Chronic Obstructive Pulmonary Disease; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; Volume 107, pp. 1514–1519. [Google Scholar]
- Hawkins, N.M.; Petrie, M.C.; Jhund, P.S.; Chalmers, G.W.; Dunn, F.G.; McMurray, J.J. Heart Failure and Chronic Obstructive Pulmonary Disease: Diagnostic Pitfalls and Epidemiology. Eur. J. Heart Fail. 2009, 11, 130–139. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Celli, B.R.; Decramer, M.; Wedzicha, J.A.; Wilson, K.C.; Agustí, A.; Criner, G.J.; MacNee, W.; Make, B.J.; Rennard, S.I.; Stockley, R.A.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Research Questions in COPD. Eur. Respir. J. 2015, 45, 879–905. [Google Scholar] [CrossRef]
- Houben-Wilke, S.; Jörres, R.A.; Bals, R.; Franssen, F.M.E.; Gläser, S.; Holle, R.; Karch, A.; Koch, A.; Magnussen, H.; Obst, A.; et al. Peripheral Artery Disease and Its Clinical Relevance in Patients with Chronic Obstructive Pulmonary Disease in the COPD and Systemic Consequences-Comorbidities Network Study. Am. J. Respir. Crit. Care Med. 2017, 195, 189–197. [Google Scholar] [CrossRef]
- Müllerova, H.; Agusti, A.; Erqou, S.; Mapel, D.W. Cardiovascular Comorbidity in COPD: Systematic Literature Review. Chest 2013, 144, 1163–1178. [Google Scholar] [CrossRef]
- Zaidi, S.A.J.; Ghafoor, A.; Kim, J.; Abbas, Z.; Lee, S.W. HeartEnsembleNet: An Innovative Hybrid Ensemble Learning Approach for Cardiovascular Risk Prediction. Healthcare 2025, 13, 507. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of Spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall/CRC: Boca Raton, FL, USA, 1994; ISBN 978-0-429-24659-3. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Freixa, X.; Portillo, K.; Paré, C.; Garcia-Aymerich, J.; Gomez, F.P.; Benet, M.; Roca, J.; Farrero, E.; Ferrer, J.; Fernandez-Palomeque, C. Echocardiographic Abnormalities in Patients with COPD at Their First Hospital Admission. Eur. Respir. J. 2013, 41, 784–791. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Feng, Y.; Zhang, J.; Bai, L.; Huang, W.; Li, M. Impact of Chronic Obstructive Pulmonary Diseases on Left Ventricular Diastolic Function in Hospitalized Elderly Patients. CIA 2014, 10, 81–87. [Google Scholar] [CrossRef][Green Version]
- Sabit, R.; Bolton, C.E.; Fraser, A.G.; Edwards, J.M.; Edwards, P.H.; Ionescu, A.A.; Cockcroft, J.R.; Shale, D.J. Sub-Clinical Left and Right Ventricular Dysfunction in Patients with COPD. Respir. Med. 2010, 104, 1171–1178. [Google Scholar] [CrossRef]
- Funk, G.-C.; Lang, I.; Schenk, P.; Valipour, A.; Hartl, S.; Burghuber, O.C. Left Ventricular Diastolic Dysfunction in Patients with COPD in the Presence and Absence of Elevated Pulmonary Arterial Pressure. Chest 2008, 133, 1354–1359. [Google Scholar] [CrossRef]
- Vonk-Noordegraaf, A.; Marcus, J.T.; Holverda, S.; Roseboom, B.; Postmus, P.E. Early Changes of Cardiac Structure and Function in COPD Patients with Mild Hypoxemia. Chest 2005, 127, 1898–1903. [Google Scholar] [CrossRef]
- Elwing, J.; Panos, R.J. Pulmonary Hypertension Associated with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2008, 3, 55–70. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Barua, R.S. The Pathophysiology of Cigarette Smoking and Cardiovascular Disease: An Update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D.D. Association between Chronic Obstructive Pulmonary Disease and Systemic Inflammation: A Systematic Review and a Meta-Analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling through Coronary Microvascular Endothelial Inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Watz, H.; Waschki, B.; Meyer, T.; Kretschmar, G.; Kirsten, A.; Claussen, M.; Magnussen, H. Decreasing Cardiac Chamber Sizes and Associated Heart Dysfunction in COPD. Chest 2010, 138, 32–38. [Google Scholar] [CrossRef]
- Jörgensen, K.; Müller, M.F.; Nel, J.; Upton, R.N.; Houltz, E.; Ricksten, S.-E. Reduced Intrathoracic Blood Volume and Left and Right Ventricular Dimensions in Patients with Severe Emphysema. Chest 2007, 131, 1050–1057. [Google Scholar] [CrossRef]
- Goudis, C.A.; Konstantinidis, A.K.; Ntalas, I.V.; Korantzopoulos, P. Electrocardiographic Abnormalities and Cardiac Arrhythmias in Chronic Obstructive Pulmonary Disease. Int. J. Cardiol. 2015, 199, 264–273. [Google Scholar] [CrossRef]
- Chang, C.L.; Robinson, S.C.; Mills, G.D.; Sullivan, G.D.; Karalus, N.C.; McLachlan, J.D.; Hancox, R.J. Biochemical Markers of Cardiac Dysfunction Predict Mortality in Acute Exacerbations of COPD. Thorax 2011, 66, 764–768. [Google Scholar] [CrossRef]
- Kunisaki, K.M.; Dransfield, M.T.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Crim, C.; Hartley, B.F.; Martinez, F.J.; Newby, D.E.; et al. Exacerbations of Chronic Obstructive Pulmonary Disease and Cardiac Events. A Post Hoc Cohort Analysis from the SUMMIT Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 51–57. [Google Scholar] [CrossRef]
- Ergan, B.; Nava, S. Long-Term Oxygen Therapy in COPD Patients Who Do Not Meet the Actual Recommendations. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 351–366. [Google Scholar] [CrossRef]
- Robertson, N.M.; Centner, C.S.; Siddharthan, T. Integrating Artificial Intelligence in the Diagnosis of COPD Globally: A Way Forward. Chronic Obstr. Pulm. Dis. J. COPD Found. 2023, 11, 114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).