Risk Factors and Biomarkers for Pulmonary Toxicities Associated with Immune Checkpoint Inhibitors
Abstract
1. Introduction
1.1. Existing Knowledge
1.2. Novel Findings
2. Materials and Methods
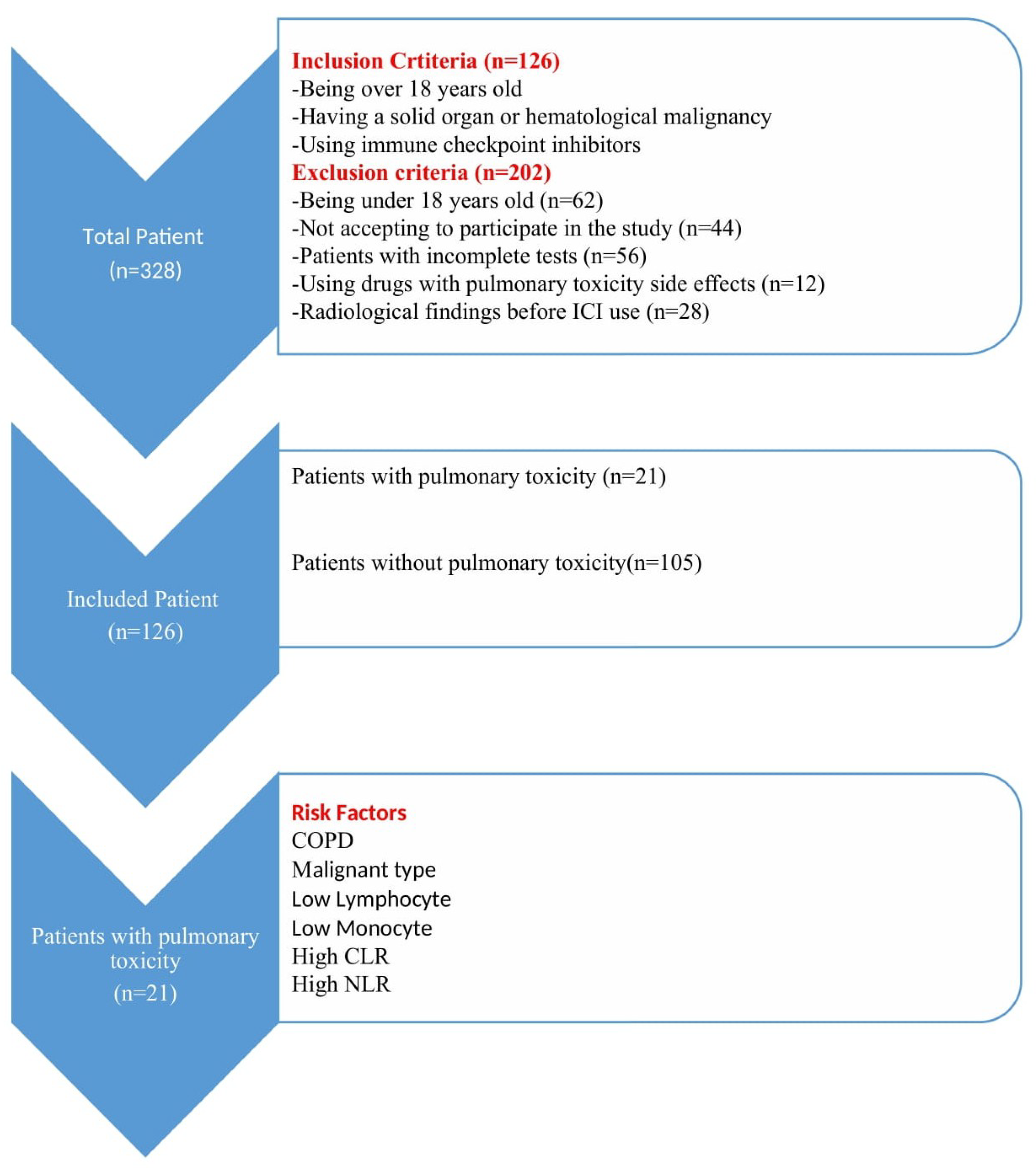
2.1. Study Design
2.2. Participants
2.3. Variables
2.4. Data Source/Measurement
2.5. Monitoring and Assessment
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [PubMed]
- Willsmore, Z.N.; Coumbe, B.G.T.; Crescioli, S.; Reci, S.; Gupta, A.; Harris, R.J.; Chenoweth, A.; Chauhan, J.; Bax, H.J.; McCraw, A.; et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: Treatment of melanoma and immune mechanisms of action. Eur. J. Immunol. 2021, 51, 544–556. [Google Scholar] [PubMed]
- Ma, W.; Xue, R.; Zhu, Z.; Farrukh, H.; Song, W.; Li, T.; Zheng, L.; Pan, C.X. Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp. Hematol. Oncol. 2023, 12, 10. [Google Scholar] [PubMed]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar]
- Valente, M.; Colucci, M.; Vegni, V.; Croce, V.; Bellan, C.; Rossi, G.; Gibilisco, G.; Frongia, F.; Guazzo, R.; Ghiribelli, C.; et al. A Multidisciplinary Approach to Improve the Management of Immune-Checkpoint Inhibitor-Related Pneumonitis. Onco Targets Ther. 2024, 17, 673–681. [Google Scholar]
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [PubMed]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounant, V.; Moro-Sibilot, D.; Michot, J.M.; Raimbourg, J.; Girard, N.; Guisier, F.; et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [PubMed]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated with Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Awad, M.M.; Sholl, L.M.; Maattala, J.A.; Taibi, M.; Hatabu, H.; Ott, P.A.; Armand, P.F.; Hodi, F.S. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin. Cancer Res. 2016, 22, 6051–6060. [Google Scholar]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016, 17, 1497–1508. [Google Scholar]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar]
- Toh, C.K.; Wong, E.H.; Lim, W.T.; Leong, S.S.; Fong, K.W.; Wee, J.; Tan, E.H. The impact of smoking status on the behavior and survival outcome of patients with advanced non-small cell lung cancer: A retrospective analysis. Chest 2004, 126, 1750–1756. [Google Scholar]
- National Cancer Institute. Cancer Statistics. 2024. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 11 December 2024).
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- Watanabe, S.; Kimura, H.; Takato, H.; Waseda, Y.; Hara, J.; Sone, T.; Abo, M.; Maeda, S.; Matsushita, T.; Kasahara, K. Severe pneumonitis after nivolumab treatment in a patient with melanoma. Allergol. Int. 2016, 65, 487–489. [Google Scholar] [PubMed]
- Chow, L.Q. Exploring novel immune-related toxicities and endpoints with immune-checkpoint inhibitors in non-small cell lung cancer. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, e96–e102. [Google Scholar]
- Cui, P.F.; Ma, J.X.; Wang, F.X.; Zhang, J.; Tao, H.T.; Hu, Y. Pneumonitis and pneumonitis-related death in cancer patients treated with programmed cell death-1 inhibitors: A systematic review and meta-analysis. Ther. Clin. Risk Manag. 2017, 13, 1259–1271. [Google Scholar] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar]
- Chiang, C.L.; Chen, Y.W.; Wu, M.H.; Huang, H.C.; Tsai, C.M.; Chiu, C.H. Radiation recall pneumonitis induced by epidermal growth factor receptor–tyrosine kinase inhibitor in patients with advanced non-small-cell lung cancer. J. Chin. Med. Assoc. 2016, 79, 248–255. [Google Scholar]
- Egami, S.; Kawazoe, H.; Hashimoto, H.; Uozumi, R.; Arami, T.; Sakiyama, N.; Ohe, Y.; Nakada, H.; Aomori, T.; Ikemura, S.; et al. Absolute Lymphocyte Count Predicts Immune-Related Adverse Events in Patients with Non–Small-Cell Lung Cancer Treated With Nivolumab Monotherapy: A Multicenter Retrospective Study. Front. Oncol. 2021, 11, 618570. [Google Scholar]
- Matsukane, R.; Watanabe, H.; Minami, H.; Hata, K.; Suetsugu, K.; Tsuji, T.; Masuda, S.; Okamoto, I.; Nakagawa, T.; Ito, T.; et al. Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune-related adverse events. Sci. Rep. 2021, 11, 1324. [Google Scholar]
- Egami, S.; Kawazoe, H.; Hashimoto, H.; Uozumi, R.; Arami, T.; Sakiyama, N.; Ohe, Y.; Nakada, H.; Aomori, T.; Ikemura, S.; et al. Peripheral blood biomarkers predict immune-related adverse events in non-small cell lung cancer patients treated with pembrolizumab: A multicenter retrospective study. J. Cancer 2021, 12, 2105–2112. [Google Scholar]
- Michailidou, D.; Khaki, A.R.; Morelli, M.P.; Diamantopoulos, L.; Singh, N.; Grivas, P. Association of blood biomarkers and autoimmunity with immune-related adverse events in patients with cancer treated with immune checkpoint inhibitors. Sci. Rep. 2021, 11, 9029. [Google Scholar]
| Variables | n (%) or Mean ± SD | p | |||
|---|---|---|---|---|---|
| Total (n = 126) | Toxicity (+) (n = 21) | Toxicity (−) (n = 105) | |||
| Age | 62.93 ± 12.94 | 63.10 ± 14.24 | 62.90 ± 12.73 | 0.532 | |
| Sex | Male | 102 (81) | 20 (95.2) | 82 (78.1) | 0.068 |
| Female | 24 (19) | 1 (4.8) | 23 (21.9) | ||
| Smoke | Yes | 86 (68.3) | 18 (85.7) | 68 (64.8) | 0.060 |
| No | 40 (31.7) | 3 (14.3) | 37 (35.2) | ||
| Type of Malignancy | Lung Cancer | 52 (41.3) | 13 (61.9) | 39 (37.1) | 0.000 |
| Malignant Melanoma | 36 (28.6) | 1 (4.8) | 35 (33.3) | ||
| Kidney and Bladder | 11 (8.7) | 1 (4.8) | 10 (9.5) | ||
| Lymphoma | 17 (13.5) | 1 (4.8) | 16 (15.2) | ||
| Mesothelioma | 4 (3.2) | 3 (14.3) | 1 (1) | ||
| Nasopharynx | 2 (1.6) | 0 (0) | 2 (1.9) | ||
| Esophagus | 1 (0.8) | 1 (4.8) | 0 (0) | ||
| Testis | 1 (0.8) | 0 (0) | 1 (1) | ||
| Prostate | 1 (0.8) | 1 (4.8) | 0 (0) | ||
| Hepatocellular Carcinoma | 1 (0.8) | 0 (0) | 1 (1) | ||
| COPD | Yes | 41 (32.5) | 11 (52.4) | 30 (28.6) | 0.034 |
| No | 85 (67.5) | 10 (47.6) | 75 (71.4) | ||
| HT | Yes | 40 (31.7) | 6 (28.6) | 34 (32.4) | 0.732 |
| No | 86 (68.3) | 15 (71.4) | 71 (67.6) | ||
| DM | Yes | 34 (27) | 6 (28.6) | 28 (26.7) | 0.858 |
| No | 92 (73) | 15 (71.4) | 77 (73.3) | ||
| Heart Diseases | Yes | 32 (25.4) | 5 (23.8) | 27 (25.7) | 0.855 |
| No | 94 (74.6) | 16 (76.2) | 78 (74.3) | ||
| Chronic Liver Disease | Yes | 11 (8.7) | 1 (4.8) | 10 (9.5) | 0.398 |
| No | 115 (91.3) | 20 (95.2) | 95 (90.5) | ||
| Rheumatological Diseases | Yes | 7 (5.6) | 3 (14.3) | 4 (3.8) | 0.056 |
| No | 119 (94.4) | 18 (85.7) | 101 (96.2) | ||
| Chronic Kidney Disease | Yes | 3 (2.4) | 0 (0) | 3 (2.9) | 0.083 |
| No | 123 (97.6) | 21 (100) | 102 (97.1) | ||
| Neurological Diseases | Yes | 7 (5.6) | 1 (4.8) | 6 (5.7) | 0.862 |
| No | 119 (94.4) | 20 (95.2) | 99 (94.3) | ||
| CCI Risk Score | Low | 22 (17.5) | 2 (9.5) | 20 (19) | 0.589 |
| Medium | 15 (11.9) | 3 (14.3) | 12 (11.4) | ||
| High | 42 (33.3) | 6 (28.6) | 36 (34.3) | ||
| Very High | 47 (37.3) | 10 (47.6) | 37 (35.2) | ||
| RT | Yes | 16 (12.7) | 4 (19) | 12 (11.4) | 0.425 |
| No | 110 (87.3) | 17 (81) | 93 (88.6) | ||
| Indication for ICI | Early-Stage Malignancies | 43 (34.1) | 10 (47.6) | 33 (31.4) | 0.351 |
| Advanced-Stage Malignancies | 50 (39.7) | 7 (33.3) | 43 (41) | ||
| Relapse | 33 (26.2) | 4 (19) | 29 (27.6) | ||
| ICI usage time | <3 months | 2 (1.6) | 2 (9.5) | 0 (0) | 0.010 |
| 3 months–6 months | 22 (17.5) | 5 (23.8) | 17 (16.2) | ||
| 6 months–1 year | 56 (44.4) | 7 (33.3) | 49 (46.7) | ||
| >1 year | 46 (36.5) | 7 (33.3) | 39 (37.1) | ||
| Time to ICI-Related Radiological Abnormalities | No | 91 (72.2) | 0 (0) | 91 (86.7) | 0.000 |
| 0–6 months | 7 (5.6) | 2 (9.5) | 5 (4.8) | ||
| 6 months–1 year | 13 (10.3) | 7 (33.3) | 6 (5.7) | ||
| >1 year | 15 (11.9) | 12 (57.1) | 3 (2.9) | ||
| Second ICI (Durvalumab, İpilimumab) Use | Yes | 16 (12.7) | 1 (4.8) | 15 (14.3) | 0.231 |
| No | 110 (87.3) | 20 (95.2) | 90 (85.7) | ||
| Fever | Yes | 11 (8.7) | 2 (9.5) | 9 (8.6) | 0.888 |
| No | 115 (91.3) | 19 (90.5) | 96 (91.4) | ||
| Weakness–Fatigue | Yes | 74 (58.7) | 14 (66.7) | 60 (57.1) | 0.412 |
| No | 52 (41.3) | 7 (33.3) | 45 (42.9) | ||
| Headache–Dizziness | Yes | 49 (38.9) | 9 (42.9) | 40 (38.1) | 0.690 |
| No | 77 (61.1) | 12 (57.1) | 65 (61.9) | ||
| Dyspnea | Yes | 68 (54) | 16 (76.2) | 52 (49.5) | 0.025 |
| No | 58 (46) | 5 (23.8) | 53 (50.5) | ||
| Cough | Yes | 65 (51.6) | 17 (81) | 48 (45.7) | 0.003 |
| No | 61 (48.4) | 4 (19) | 57 (54.3) | ||
| Sputum | Yes | 39 (31) | 12 (57.1) | 27 (25.7) | 0.004 |
| No | 87 (69) | 9 (42.9) | 78 (74.3) | ||
| Chest Pain | Yes | 35 (27.8) | 5 (23.8) | 30 (28.6) | 0.657 |
| No | 91 (72.2) | 16 (76.2) | 75 (71.4) | ||
| Hemoptysis | Yes | 9 (7.1) | 3 (14.3) | 6 (5.7) | 0.164 |
| No | 117 (92.9) | 18 (85.7) | 99 (94.3) | ||
| Respiratory Function Tests | Normal | 94 (74.6) | 19 (90.5) | 75 (71.4) | 0.094 |
| Obstructive | 17 (13.5) | 2 (9.5) | 15 (14.3) | ||
| Restrictive | 15 (11.9) | 0 (0) | 15 (14.3) | ||
| Variables | n (%) or Mean ± SD | p | |||
|---|---|---|---|---|---|
| Total (n = 126) | Toxicity (+) (n = 21) | Toxicity (−) (n = 105) | |||
| Radiological Findings Before ICI Use | |||||
| Normal | Yes | 72 (57.1) | 17 (81) | 55 (52.4) | 0.016 |
| No | 54 (42.9) | 4 (19) | 50 (47.6) | ||
| Pleural Effusion | Yes | 7 (5.6) | 2 (9.5) | 5 (4.8) | 0.496 |
| No | 119 (94.4) | 19 (91.5) | 100 (95.2) | ||
| Nodule Mass | Yes | 74 (58.7) | 8 (38.1) | 66 (62.9) | 0.035 |
| No | 52 (41.3) | 13 (61.9) | 39 (37.1) | ||
| Cyst Cavity | Yes | 9 (7.1) | 3 (14.3) | 6 (5.7) | 0.164 |
| No | 117 (92.9) | 18 (85.7) | 99 (94.3) | ||
| Radiological findings after ICI use | |||||
| OP | Yes | 12 (9.5) | 12 (57.1) | 0 (0) | 0.000 |
| No | 114 (90.5) | 9 (42.9) | 105 (100) | ||
| IIP/NSIP | Yes | 5 (4) | 5 (23.8) | 0 (0) | 0.000 |
| No | 121 (96) | 16 (76.2) | 105 (100) | ||
| HP | Yes | 3 (2.4) | 3 (14.3) | 0 (0) | 0.000 |
| No | 123 (97.6) | 18 (85.7) | 105 (100) | ||
| Wbc (×103/mm3) | 10.32 ± 7.84 | 8.42 ± 6.99 | 10.69 ± 7.97 | 0.229 | |
| Hemoglobin | 11.61 ± 4.09 | 11.25 ± 2.82 | 11.67 ± 4.30 | 0.183 | |
| Hematocrit | 34.30 ± 7.43 | 33.88 ± 8.51 | 34.38 ± 7.24 | 0.416 | |
| Platelet (×103/mm3) | 254.30 ± 144.25 | 220.57 ± 137.79 | 261.05 ± 145.20 | 0.361 | |
| Neutrophil | 8090.40 ± 7448.90 | 6849.52 ± 6381.49 | 8338.57 ± 7647.58 | 0.176 | |
| Lymphocyte | 1272.34 ± 973.93 | 850.71 ± 651.02 | 1356.67 ± 1007.72 | 0.023 | |
| Monocyte | 721.94 ±551.64 | 554.05 ± 536.27 | 755.52 ± 551.01 | 0.024 | |
| Eosinophil | 162.62 ± 234.35 | 119.52 ±124.27 | 171.24 ±250.18 | 0.418 | |
| LMR | 2.30 ±1.99 | 2.95 ± 3.36 | 2.17 ± 1.57 | 0.503 | |
| NLR | 11.67 ± 15.29 | 13.56 ± 18.01 | 11.29 ± 14.75 | 0.369 | |
| CLR | 294.63 ±1815.92 | 1273.81 ± 4380.35 | 98.79 ±202.93 | 0.011 | |
| CRP | 70.55 ±97.35 | 110.63 ±121.72 | 62.53 ±90.28 | 0.036 | |
| Ferritin | 646.33 ± 1671.64 | 795.10 ±1758.66 | 616.58 ± 1660.86 | 0.487 | |
| R2 = 0.353 | B | p | O.R. | 95% C.I. for OR. | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| NLR | −0.063 | 0.031 | 0.939 | 0.887 | 0.994 |
| CLR | 0.002 | 0.007 | 1.002 | 1.001 | 1.004 |
| Cough (ref:no) | 2.042 | 0.004 | 7.705 | 1.897 | 31.288 |
| COPD (ref:no) | 1.520 | 0.010 | 4.571 | 1.436 | 14.552 |
| Constant | −1.657 | 0.000 | 0.191 | ||
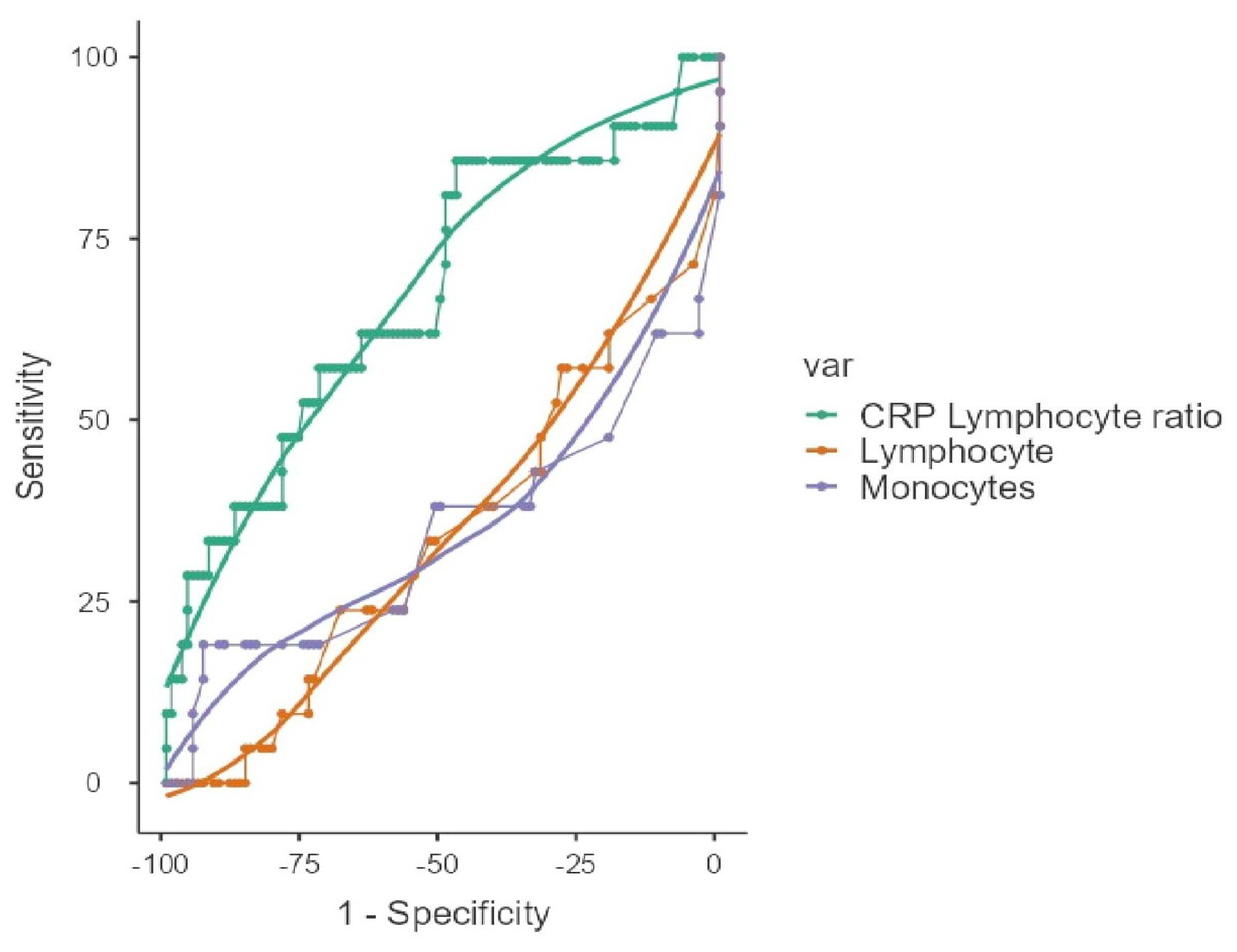
| ROC Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Scale | Cut-Off Point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden’s Index | AUC | |
| Lymphocyte | <950 | 57.14 | 67.62 | 26.09 | 88.75 | 0.248 | 0.657 | |
| Monocyte | <130 | 38.1 | 96.19 | 66.67 | 88.6 | 0.343 | 0.655 | |
| CLR | >15.2 | 85.71 | 47.62 | 24.66 | 94.34 | 0.333 | 0.677 | |
| Multivariate Logistic Regression Analysis | ||||||||
| 95% Confidence Interval | ||||||||
| Predictor | B | SE | p | Odds ratio | Lower | Upper | VIF | Tolerance |
| Intercept | −2.488 | 0.522 | <0.001 | 0.0830 | 0.0299 | 0.231 | ||
| Lymphocyte | −0.488 | 0.675 | 0.470 | 0.6142 | 0.1637 | 2.304 | 1.61 | 0.622 |
| Monocytes | 2.464 | 0.825 | 0.003 | 11.7569 | 2.3344 | 59.211 | 1.43 | 0.700 |
| CLR | 1.071 | 0.664 | 0.107 | 2.9183 | 0.7942 | 10.724 | 1.22 | 0.822 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzel, E.; Hanta, I.; Baydar Toprak, O.; Gurbuz, O.; Mete, B.; Bayram, E. Risk Factors and Biomarkers for Pulmonary Toxicities Associated with Immune Checkpoint Inhibitors. Medicina 2025, 61, 1258. https://doi.org/10.3390/medicina61071258
Guzel E, Hanta I, Baydar Toprak O, Gurbuz O, Mete B, Bayram E. Risk Factors and Biomarkers for Pulmonary Toxicities Associated with Immune Checkpoint Inhibitors. Medicina. 2025; 61(7):1258. https://doi.org/10.3390/medicina61071258
Chicago/Turabian StyleGuzel, Efraim, Ismail Hanta, Oya Baydar Toprak, Okan Gurbuz, Burak Mete, and Ertugrul Bayram. 2025. "Risk Factors and Biomarkers for Pulmonary Toxicities Associated with Immune Checkpoint Inhibitors" Medicina 61, no. 7: 1258. https://doi.org/10.3390/medicina61071258
APA StyleGuzel, E., Hanta, I., Baydar Toprak, O., Gurbuz, O., Mete, B., & Bayram, E. (2025). Risk Factors and Biomarkers for Pulmonary Toxicities Associated with Immune Checkpoint Inhibitors. Medicina, 61(7), 1258. https://doi.org/10.3390/medicina61071258






