Conduction System Pacing Versus Biventricular Cardiac Resynchronization Pacing: Meta-Analysis on Outcomes in Patients with Non-Left Bundle Branch Block
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Meta-Analysis
3. Results
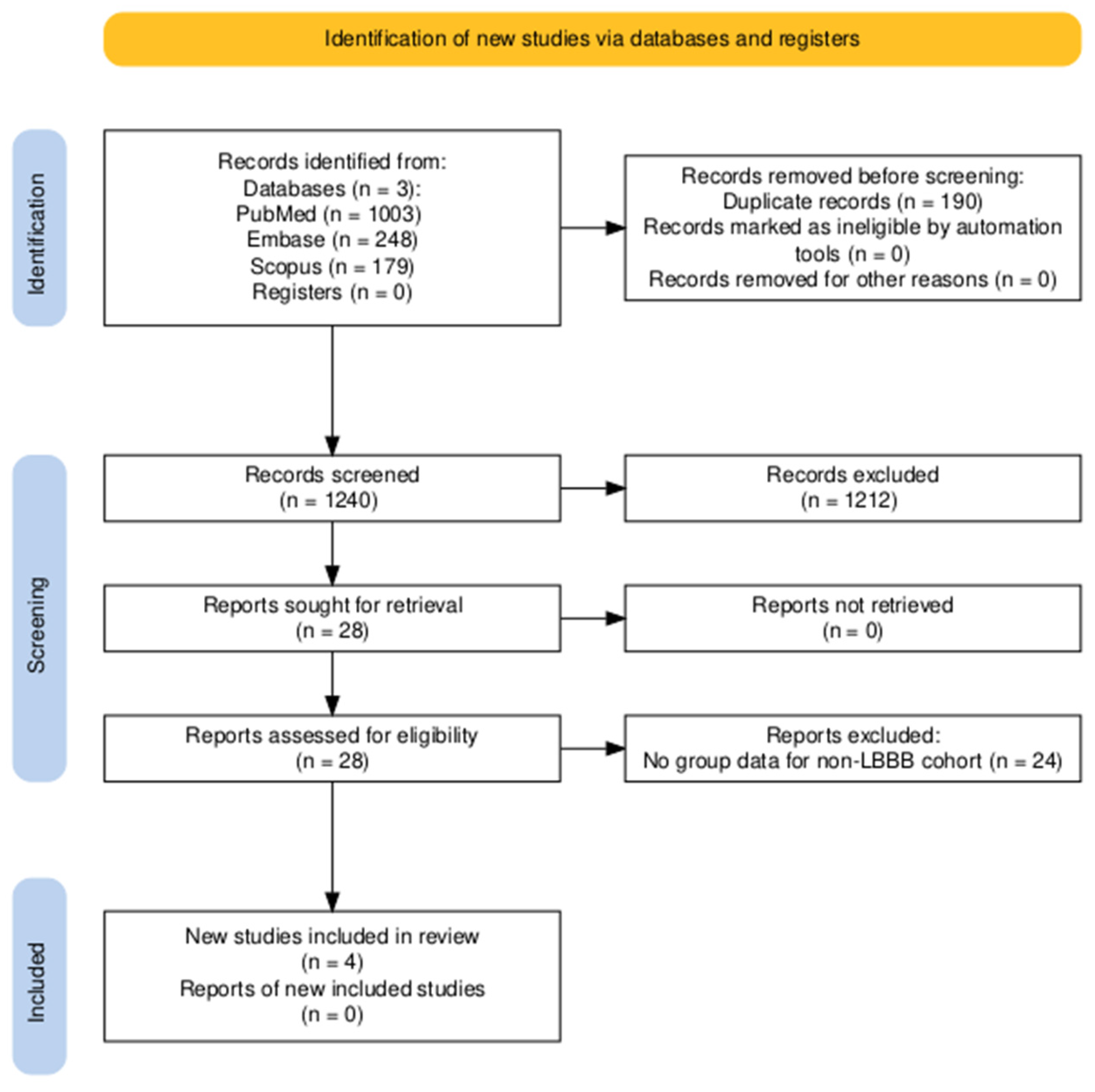
3.1. Study Selection
3.2. Study Characteristics
3.3. Statistical Analysis
4. Discussion
4.1. Difference in Outcomes Between CSP and BVP in Non-LBBB
4.2. Choosing Between CSP and BVP in Non-LBBB
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AV | Atrioventricular |
| BVP | Biventricular pacing |
| CI | Confidence interval |
| CRT | Cardiac resynchronization therapy |
| CSP | Conduction system pacing |
| HBP | His bundle pacing |
| HFrEF | Heart failure with reduced ejection fraction |
| HOT-CRT | His bundle optimized cardiac resynchronization therapy |
| IVCD | Intraventricular conduction delay |
| LBBB | Left bundle branch block |
| LBBP | Left bundle branch pacing |
| LOT-CRT | Left bundle branch optimized cardiac resynchronization therapy |
| LVEF | Left ventricular ejection fraction |
| MD | Mean difference |
| NYHA | New York Heart Association |
| RR | Relative risk |
| RBBB | Right bundle branch block |
References
- Chung, M.K.; Patton, K.K.; Lau, C.-P.; Dal Forno, A.R.J.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.-M.; Chung, E.H.; Cronin, E.M.; et al. 2023 HRS/APHRS/LAHRS Guideline on Cardiac Physiologic Pacing for the Avoidance and Mitigation of Heart Failure. Heart Rhythm 2023, 20, e17–e91. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, C.; Kwok, C.S.; Satchithananda, D.K.; Patwala, A.; Khan, M.A.; Zaidi, A.; Ahmed, F.Z.; Mamas, M.A. Cardiac Resynchronisation Therapy is not Associated with a Reduction in Mortality or Heart Failure Hospitalisation in Patients with Non-Left Bundle Branch Block QRS Morphology: Meta-Analysis of Randomised Controlled Trials. Heart 2015, 101, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Felix, I.; Collini, M.; Fonseca, R.; Guida, C.; Armaganijan, L.; Healey, J.S.; Carvalho, G. Conduction System Pacing versus Biventricular Pacing in Heart Failure with Reduced Ejection Fraction: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Heart Rhythm 2024, 21, 881–889. [Google Scholar] [CrossRef]
- Herweg, B.; Mumtaz, M.; Vijayaraman, P. Conduction System Pacing for CRT: A Physiological Alternative. Arrhythmia Electrophysiol. Rev. 2025, 14, e04. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Pokharel, P.; Subzposh, F.A.; Oren, J.W.; Storm, R.H.; Batul, S.A.; Beer, D.A.; Hughes, G.; Leri, G.; Manganiello, M.; et al. His-Purkinje Conduction System Pacing Optimized Trial of Cardiac Resynchronization Therapy vs Biventricular Pacing. JACC Clin. Electrophysiol. 2023, 9, 2628–2638. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses 2021. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 6 July 2025).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Bonner, A.; Alexander, P.E.; Siemieniuk, R.A.; Furukawa, T.A.; Rochwerg, B.; Hazlewood, G.S.; Alhazzani, W.; Mustafa, R.A.; Murad, M.H.; et al. Advances in the GRADE Approach to Rate the Certainty in Estimates from a Network Meta-Analysis. J. Clin. Epidemiol. 2018, 93, 36–44. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Zalavadia, D.; Haseeb, A.; Dye, C.; Madan, N.; Skeete, J.R.; Vipparthy, S.C.; Young, W.; Ravi, V.; Rajakumar, C.; et al. Clinical Outcomes of Conduction System Pacing Compared to Biventricular Pacing in Patients Requiring Cardiac Resynchronization Therapy. Heart Rhythm 2022, 19, 1263–1271. [Google Scholar] [CrossRef]
- Pujol-López, M.; Jiménez Arjona, R.; Guasch, E.; Borràs, R.; Doltra, A.; Vázquez-Calvo, S.; Roca-Luque, I.; Garre, P.; Ferró, E.; Niebla, M.; et al. Conduction System Pacing vs. Biventricular Pacing in Patients with Ventricular Dysfunction and AV Block. Pacing Clin. Electrophysiol. PACE 2022, 45, 1115–1123. [Google Scholar] [CrossRef]
- Tan, E.S.J.; Soh, R.; Lee, J.-Y.; Boey, E.; de Leon, J.; Chan, S.P.; Yeo, W.T.; Lim, T.W.; Seow, S.-C.; Kojodjojo, P. Conduction System versus Biventricular Pacing in Heart Failure with Non-Left Bundle Branch Block. J. Cardiovasc. Electrophysiol. 2023, 34, 976–983. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Bai, Y.; Wang, J.; Qin, S.; Bai, J.; Wang, W.; Liang, Y.; Chen, H.; Su, Y.; et al. Electrical Resynchronization and Clinical Outcomes During Long-Term Follow-Up in Intraventricular Conduction Delay Patients Applied Left Bundle Branch Pacing-Optimized Cardiac Resynchronization Therapy. Circ. Arrhythm. Electrophysiol. 2023, 16, e011761. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing Prisma 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Strauss, D.G.; Selvester, R.H.; Wagner, G.S. Defining Left Bundle Branch Block in the Era of Cardiac Resynchronization Therapy. Am. J. Cardiol. 2011, 107, 927–934. [Google Scholar] [CrossRef]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M.; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef]
- Surawicz, B.; Childers, R.; Deal, B.J.; Gettes, L.S. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. J. Am. Coll. Cardiol. 2009, 53, 976–981. [Google Scholar] [CrossRef]
- Van Stipdonk, A.M.W.; Hoogland, R.; Ter Horst, I.; Kloosterman, M.; Vanbelle, S.; Crijns, H.J.G.M.; Prinzen, F.W.; Meine, M.; Maass, A.H.; Vernooy, K. Evaluating Electrocardiography-Based Identification of Cardiac Resynchronization Therapy Responders Beyond Current Left Bundle Branch Block Definitions. JACC Clin. Electrophysiol. 2020, 6, 193–203. [Google Scholar] [CrossRef]
- Pujol-López, M.; Graterol, F.R.; Borràs, R.; Garcia-Ribas, C.; Guichard, J.B.; Regany-Closa, M.; Jiménez-Arjona, R.; Niebla, M.; Poza, M.; Carro, E.; et al. Clinical Response to Resynchronization Therapy: Conduction System Pacing vs Biventricular Pacing. CONSYST-CRT trial. JACC Clin. Electrophysiol. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Glikson, M.; Jastrzebski, M.; Gold, M.R.; Ellenbogen, K.; Burri, H. Conventional Biventricular Pacing Is Still Preferred to Conduction System Pacing for Atrioventricular Block in Patients with Reduced Ejection Fraction and Narrow QRS. Europace 2023, 26, euad337. [Google Scholar] [CrossRef]
- Zweerink, A.; Zubarev, S.; Bakelants, E.; Potyagaylo, D.; Stettler, C.; Chmelevsky, M.; Lozeron, E.D.; Hachulla, A.-L.; Vallée, J.-P.; Burri, H. His-Optimized Cardiac Resynchronization Therapy With Ventricular Fusion Pacing for Electrical Resynchronization in Heart Failure. JACC Clin. Electrophysiol. 2021, 7, 881–892. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Moskal, P.; Huybrechts, W.; Curila, K.; Sreekumar, P.; Rademakers, L.M.; Ponnusamy, S.S.; Herweg, B.; Sharma, P.S.; Bednarek, A.; et al. Left Bundle Branch-Optimized Cardiac Resynchronization Therapy (LOT-CRT): Results from an International LBBAP Collaborative Study Group. Heart Rhythm 2022, 19, 13–21. [Google Scholar] [CrossRef]
- Sharma, P.S.; Naperkowski, A.; Bauch, T.D.; Chan, J.Y.S.; Arnold, A.D.; Whinnett, Z.I.; Ellenbogen, K.A.; Vijayaraman, P. Permanent His Bundle Pacing for Cardiac Resynchronization Therapy in Patients With Heart Failure and Right Bundle Branch Block. Circ. Arrhythm. Electrophysiol. 2018, 11, e006613. [Google Scholar] [CrossRef]
- Graterol, F.R.; Pujol-López, M.; Borràs, R.; Ayala, B.; Uribe, L.; Guasch, E.; Regany-Closa, M.; Niebla, M.; Carro, E.; Guichard, J.-B.; et al. Predictors of Failed Left Bundle Branch Pacing Implant in Heart Failure with Reduced Ejection Fraction: Importance of Left Ventricular Diameter and QRS Morphology. Heart Rhythm 2024, 21, 2571–2578. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Cano, O.; Ponnusamy, S.S.; Molina-Lerma, M.; Chan, J.Y.S.; Padala, S.K.; Sharma, P.S.; Whinnett, Z.I.; Herweg, B.; Upadhyay, G.A.; et al. Left Bundle Branch Area Pacing in Patients with Heart Failure and Right Bundle Branch Block: Results from International LBBAP Collaborative-Study Group. Heart Rhythm O2 2022, 3, 358–367. [Google Scholar] [CrossRef]
- Upadhyay, G.A.; Cherian, T.; Shatz, D.Y.; Beaser, A.D.; Aziz, Z.; Ozcan, C.; Broman, M.T.; Nayak, H.M.; Tung, R. Intracardiac Delineation of Septal Conduction in Left Bundle-Branch Block Patterns: Mechanistic Evidence of Left Intrahisian Block Circumvented by His Bundle Pacing. Circulation 2019, 139, 1876–1888. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Ponnusamy, S.; Cano, Ó.; Sharma, P.S.; Naperkowski, A.; Subsposh, F.A.; Moskal, P.; Bednarek, A.; Dal Forno, A.R.; Young, W.; et al. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2021, 7, 135–147. [Google Scholar] [CrossRef]
- Cleland, J.G.; Abraham, W.T.; Linde, C.; Gold, M.R.; Young, J.B.; Claude Daubert, J.; Sherfesee, L.; Wells, G.A.; Tang, A.S.L. An Individual Patient Meta-Analysis of Five Randomized Trials Assessing the Effects of Cardiac Resynchronization Therapy on Morbidity and Mortality in Patients with Symptomatic Heart Failure. Eur. Heart J. 2013, 34, 3547–3556. [Google Scholar] [CrossRef]
- Ploux, S.; Whinnett, Z.; Lumens, J.; Denis, A.; Zemmoura, A.; De Guillebon, M.; Ramoul, K.; Ritter, P.; Jaïs, P.; Clementy, J.; et al. Acute Hemodynamic Response to Biventricular Pacing in Heart Failure Patients with Narrow, Moderately, and Severely Prolonged QRS Duration. Heart Rhythm 2012, 9, 1247–1250. [Google Scholar] [CrossRef]
- Whinnett, Z.I.; Shun-Shin, M.J.; Tanner, M.; Foley, P.; Chandrasekaran, B.; Moore, P.; Adhya, S.; Qureshi, N.; Muthumala, A.; Lane, R.; et al. Effects of Haemodynamically Atrio-ventricular Optimized His Bundle Pacing on Heart Failure Symptoms and Exercise Capacity: The His Optimized Pacing Evaluated for Heart Failure (HOPE-HF) Randomized, Double-Blind, Cross-over Trial. Eur. J. Heart Fail. 2023, 25, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Burri, H.; Abdin, A.; Cano, O.; Curila, K.; De Pooter, J.; Diaz, J.C.; Drossart, I.; Huang, W.; Israel, C.W.; et al. European Society of Cardiology (ESC) Clinical Consensus Statement on Indications for Conduction System Pacing, with Special Contribution of the European Heart Rhythm Association of the ESC and Endorsed by the Asia Pacific Heart Rhythm Society, the Canadian Heart Rhythm Society, the Heart Rhythm Society, and the Latin American Heart Rhythm Society. Europace 2025, 27, euaf050. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.S.; Murugan, M.; Ganesan, V.; Vijayaraman, P. Predictors of Procedural Failure of Left Bundle Branch Pacing in Scarred Left Ventricle. J. Cardiovasc. Electrophysiol. 2023, 34, 760–764. [Google Scholar] [CrossRef]
- Elliott, M.K.; Strocchi, M.; Sieniewicz, B.J.; Sidhu, B.; Mehta, V.; Wijesuriya, N.; Behar, J.M.; Thorpe, A.; Martic, D.; Wong, T.; et al. Biventricular Endocardial Pacing and Left Bundle Branch Area Pacing for Cardiac Resynchronization: Mechanistic Insights from Electrocardiographic Imaging, Acute Hemodynamic Response, and Magnetic Resonance Imaging. Heart Rhythm 2023, 20, 207–216. [Google Scholar] [CrossRef]
- Hu, Q.; You, H.; Chen, K.; Dai, Y.; Lu, W.; Li, Y.; Cheng, C.; Zhou, Y.; Wang, J.; Chen, R.; et al. Distance between the Lead-Implanted Site and Tricuspid Valve Annulus in Patients with Left Bundle Branch Pacing: Effects on Postoperative Tricuspid Regurgitation Deterioration. Heart Rhythm 2023, 20, 217–223. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Fan, X.; Wang, Q.; Wang, Z.; Li, H.; Tao, J.; Wang, H.; Liu, Z.; Yao, Y. Tricuspid Regurgitation Outcomes in Left Bundle Branch Area Pacing and Comparison with Right Ventricular Septal Pacing. Heart Rhythm 2022, 19, 1202–1203. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Nair, D.G.; Doshi, S.K.; Doshi, R.N.; Chovanec, M.; Ganz, L.; Sabet, L.; Jiang, C.; Neuzil, P. First-in-Human Study of a Leadless Pacemaker System for Left Bundle Branch Area Pacing. Heart Rhythm. 2025. online ahead of print. [Google Scholar] [CrossRef]
| Reference | Study Type | Non-LBBB Type | CSP Type | Arm | n | Age, y * | Male, % | HTN, % | DM, % | IHD, % | AF, % | CKD, % | NYHA III–IV, % | Follow Up, Days |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vijayaraman et al., 2022 [13] | Retrospective | RBBB (19%), IVCD (19%), AVB (40%), normal QRS (21%) | HBP, LBBP, LOT-CRT | CSP | 152 | 73 † | 70 | 69 | 37 | 54 | 66 | NR | NR | 810 |
| BVP | 78 | 74 † | 73 | 77 | 51 | 63 | 60 | NR | NR | |||||
| Pujol-Lopez et al., 2022 [14] | Retrospective | AVB | HPB (72%), LBBP (28%) | CSP | 25 | 72 (9) | 68 | NR | NR | NR | 16 | NR | 64 | 365 |
| BVP | 25 | 69 (8) | 76 | NR | NR | NR | 16 | NR | 72 | |||||
| Tan et al., 2023 [15] | Retrospective | RBBB (51%), IVCD (13%), AVB (36%) | HBP, LBBP | CSP | 48 | 70 (10) | 77 | 73 | 50 | 63 | 52 | 48 | NR | 578 |
| BVP | 48 | 70 (12) | 80 | 73 | 60 | 73 | 46 | 52 | NR | 728 | ||||
| Chen et al., 2023 [16] | Prospective | IVCD | LOT-CRT | CSP | 30 | 64 (13) | 77 | 30 | 20 | NR | 37 | NR | 63 | 730 |
| BVP | 55 | 64 (11) | 87 | 27 | 35 | NR | 26 | NR | 71 |
| Outcome | Relative Effect (95% CI) | Number of Participants (Studies) | Heterogeneity, % | Certainty of Evidence (GRADE) |
|---|---|---|---|---|
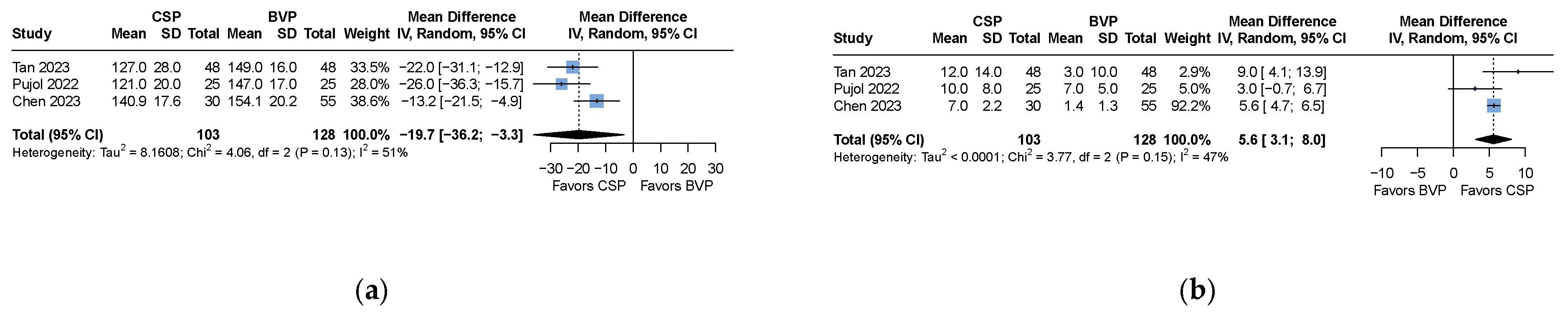
| QRS duration | MD −19.7 (−36.2 to −3.3) | 231 (3 studies) | 51 | ⊕⊖⊖⊖ Very low * |
| LVEF improvement | MD 5.6 (3.1 to 8.0) | 231 (3 studies) | 47 | ⊕⊖⊖⊖ Very low * |
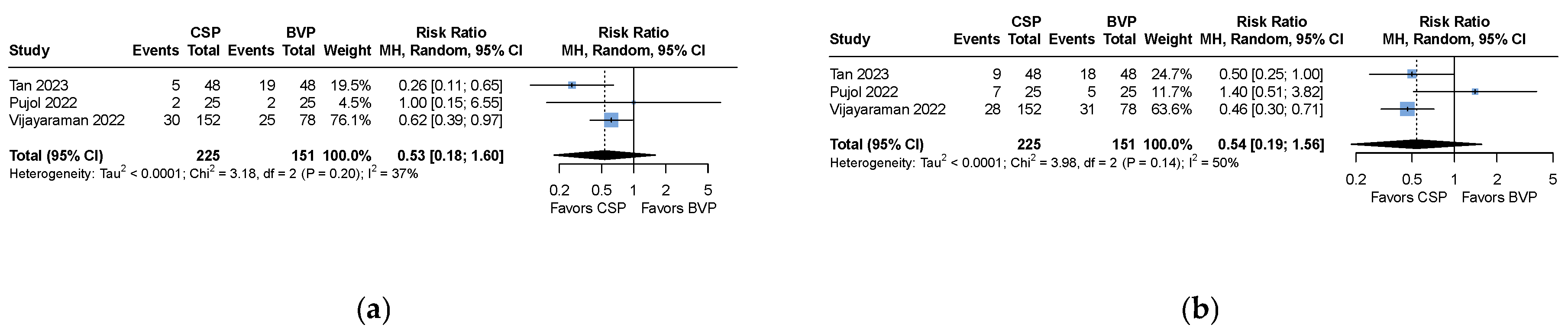
| All-cause mortality | RR 0.53 (CI 0.18 to 1.60) | 376 (3 studies) | 37 | ⊕⊕⊖⊖ Low |
| Heart failure hospitalization | RR 0.54 (0.19 to 1.56) | 376 (3 studies) | 50 | ⊕⊖⊖⊖ Very low * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pung, X.; Chua, J.J.L.; Fong, K.Y.; Chua, Y.Y.; Loo, G.J.M.; Ong, J.W.S.; Tay, J.C.K.; Teo, H.K.; Wang, Y.; Yeo, C.; et al. Conduction System Pacing Versus Biventricular Cardiac Resynchronization Pacing: Meta-Analysis on Outcomes in Patients with Non-Left Bundle Branch Block. Medicina 2025, 61, 1240. https://doi.org/10.3390/medicina61071240
Pung X, Chua JJL, Fong KY, Chua YY, Loo GJM, Ong JWS, Tay JCK, Teo HK, Wang Y, Yeo C, et al. Conduction System Pacing Versus Biventricular Cardiac Resynchronization Pacing: Meta-Analysis on Outcomes in Patients with Non-Left Bundle Branch Block. Medicina. 2025; 61(7):1240. https://doi.org/10.3390/medicina61071240
Chicago/Turabian StylePung, Xuanming, Joe J. L. Chua, Khi Yung Fong, Yi Yi Chua, Germaine J. M. Loo, Jonathan W. S. Ong, Julian C. K. Tay, Hooi Khee Teo, Yue Wang, Colin Yeo, and et al. 2025. "Conduction System Pacing Versus Biventricular Cardiac Resynchronization Pacing: Meta-Analysis on Outcomes in Patients with Non-Left Bundle Branch Block" Medicina 61, no. 7: 1240. https://doi.org/10.3390/medicina61071240
APA StylePung, X., Chua, J. J. L., Fong, K. Y., Chua, Y. Y., Loo, G. J. M., Ong, J. W. S., Tay, J. C. K., Teo, H. K., Wang, Y., Yeo, C., Lim, E. T. S., Ho, K. L., Chong, D. T. T., Ching, C. K., & Tan, V. H. (2025). Conduction System Pacing Versus Biventricular Cardiac Resynchronization Pacing: Meta-Analysis on Outcomes in Patients with Non-Left Bundle Branch Block. Medicina, 61(7), 1240. https://doi.org/10.3390/medicina61071240