Short-Term Anticoagulation After Cardioversion in New-Onset Atrial Fibrillation and Low Thromboembolic Risk: A Real-World International Investigation
Abstract
1. Introduction
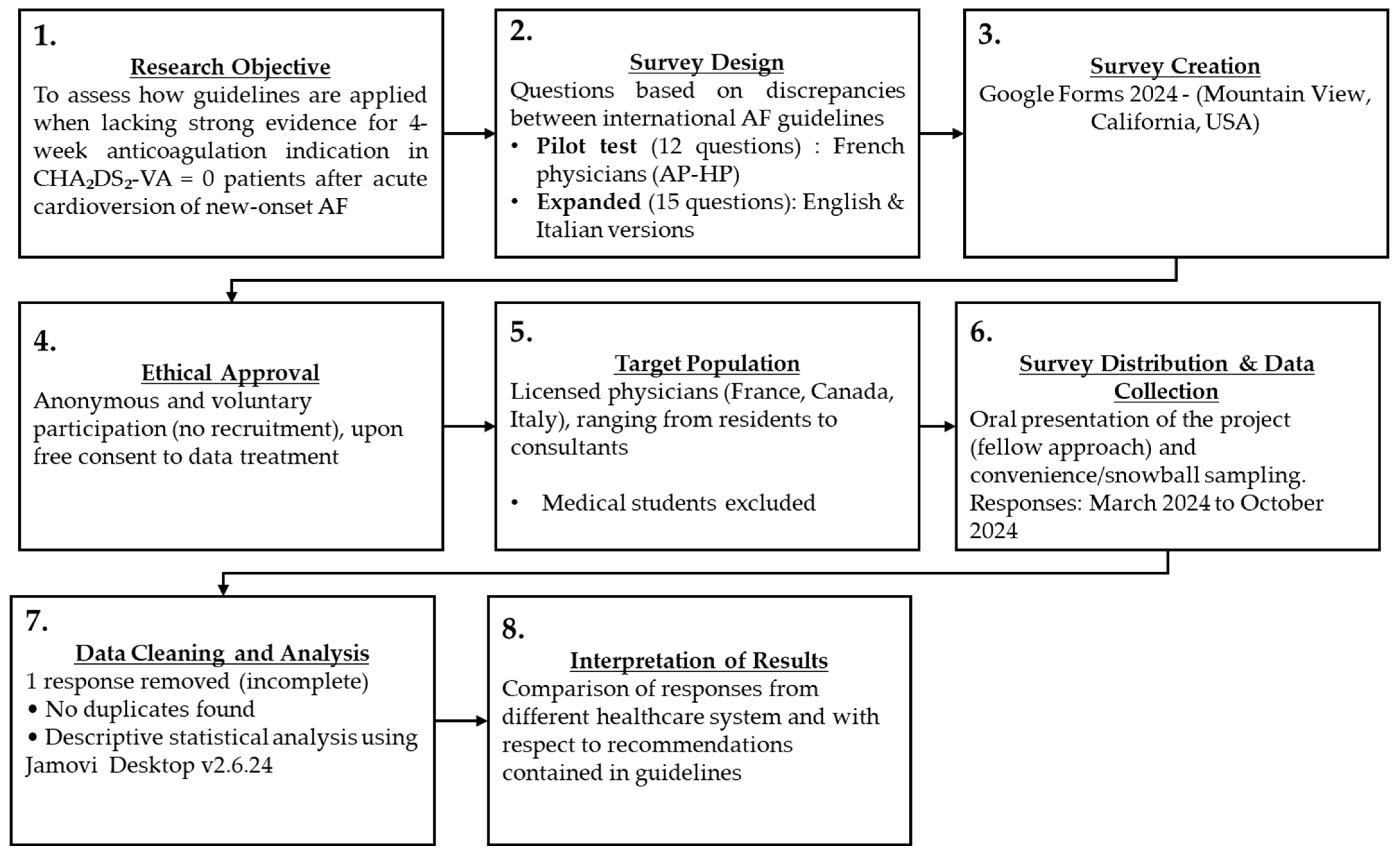
2. Materials and Methods
2.1. Survey Creation
2.2. Survey Administration
- I.
- Participants were not actively recruited. Instead, they voluntarily chose to take part in the survey, upon reviewing and consenting to Google Forms’ data usage disclaimer with respect to data collection, storage, and protection. A dedicated section at the beginning of the survey explicitly communicated this and included the following consent statement: “By participating in this survey, respondents acknowledge and agree to the Google Forms Terms of Service and consent to the use of their responses solely for research purposes, in compliance with the EU General Data Protection Regulation (GDPR).” (See Supplementary Materials, page 2). Additionally, although Google Forms may be used to collect and process personal data, no sensitive information (e.g., names, dates of birth, email addresses, or postal addresses) was requested for the survey completion.
- II.
- The authors developed and distributed the survey using Google Forms, thereby agreeing to its Terms of Service and Guidelines (see Supplementary Materials pages. 2–6; Google Forms Policies and Guidelines).
- III.
- All authors were fully committed to adhering to these policies throughout the research process, according to the latest version of the Declaration of Helsinki.
3. Results
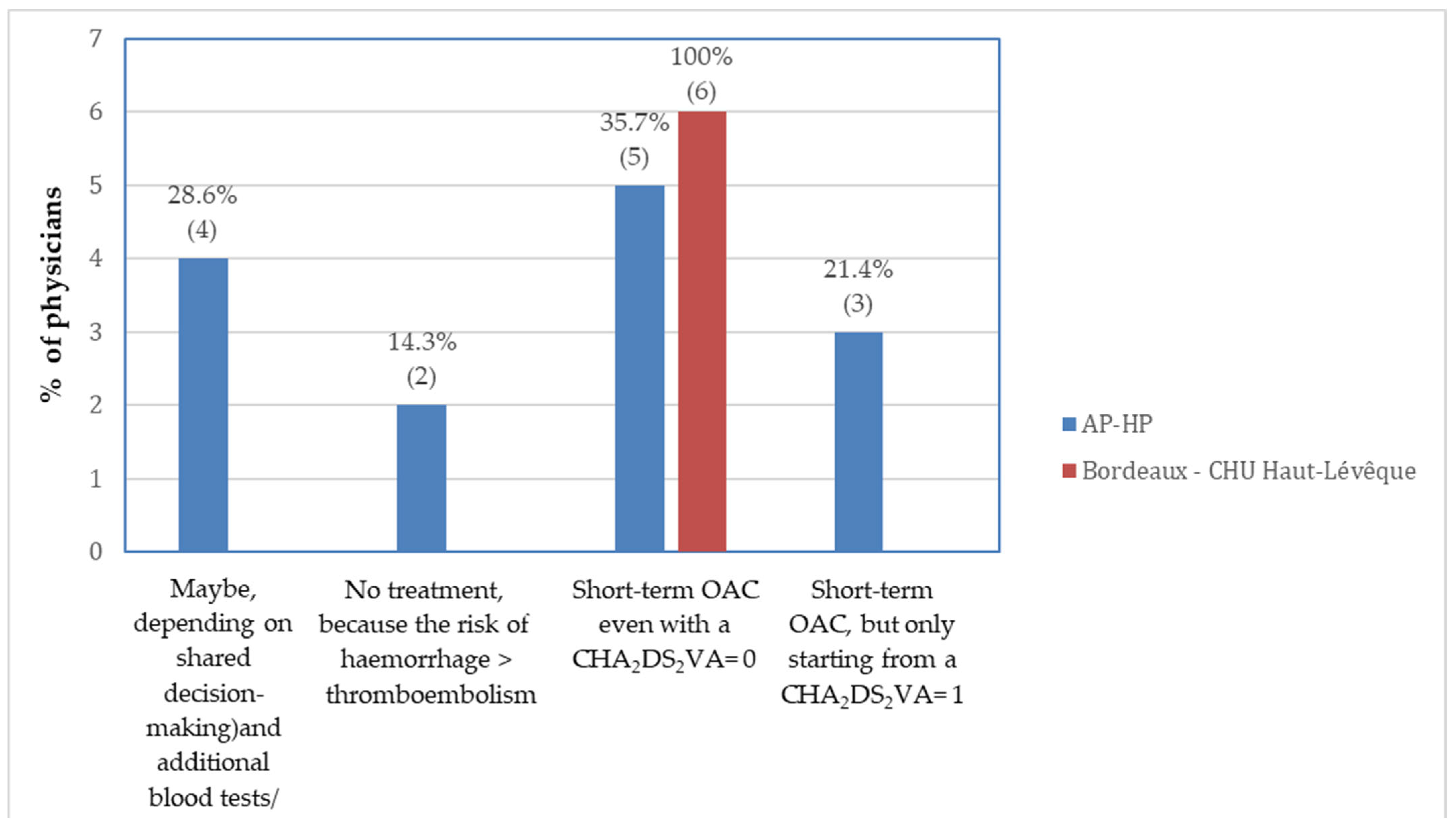
3.1. Stratification by Work Experience
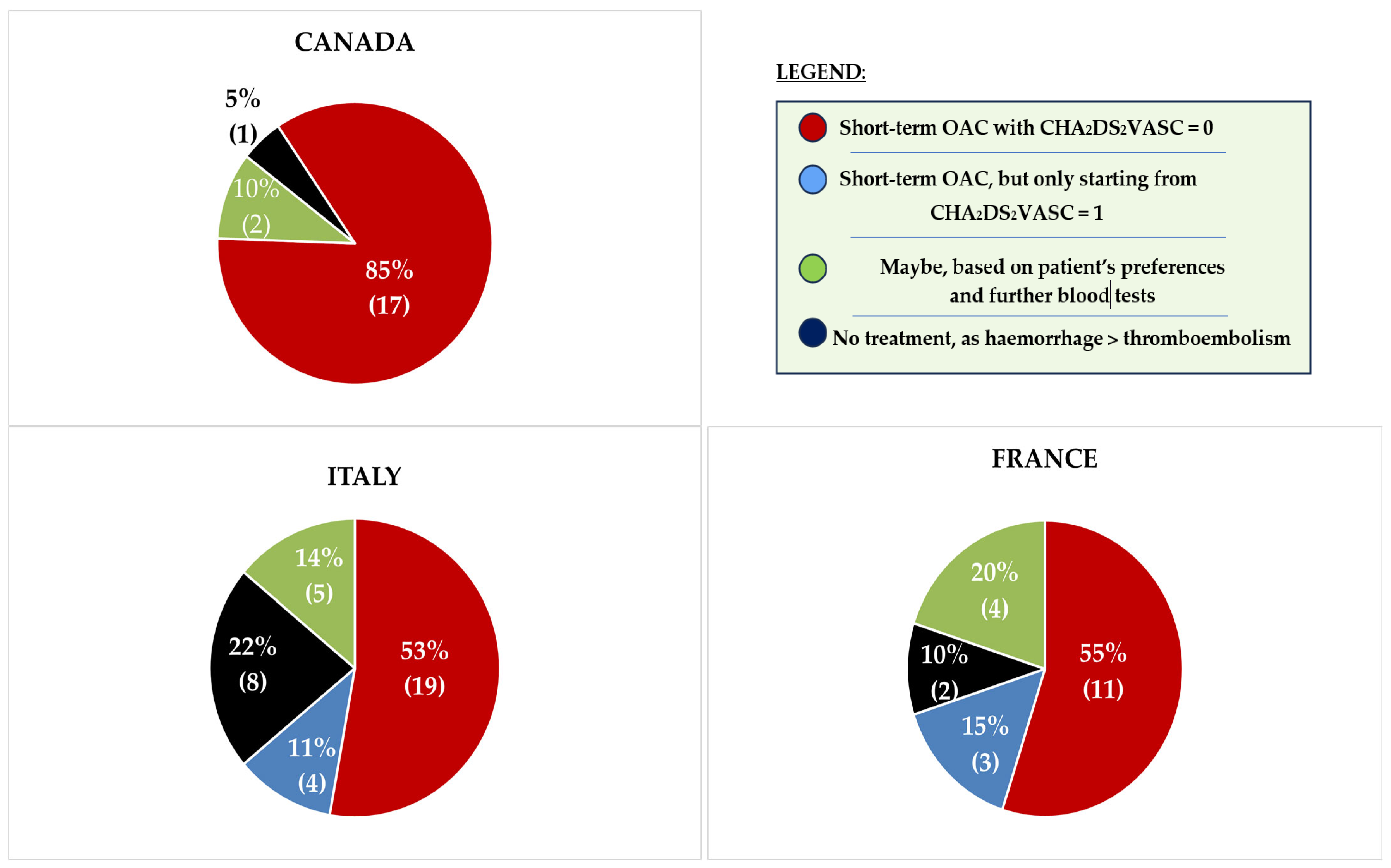
3.2. Stratification by Healthcare System
4. Discussion
4.1. Evidence Regarding Peri-Cardioversion OAC
- I.
- Atrial stunning, a temporary depression in left atrial appendage (LAA) mechanical function, resulting in decreased LAA emptying velocities, potentially favouring intracardiac thrombus formation [21]. The intensity of this phenomenon depends on the duration of the preceding AF episode and it can occur after both electrical and pharmacological cardioversion [4].
- II.
- An inaccurate estimation of the real onset of AF. Up to 4–8% of patients whose AF lasts for just 6 to 48 h are completely asymptomatic [22], raising the risk of cardioversion being performed beyond the recommended 48-h window. This is clinically relevant, because longer AF episodes (even if asymptomatic) lead to more profound atrial stunning and a higher probability of post-cardioversion thrombus formation [22].
- III.
- Transient prothrombotic state associated with sinus rhythm restoration. Even if AF lasts < 48 h, increased atrial volume and pressure can lead to atrial stretching and endothelial dysfunction, further promoting blood stasis and thrombin synthesis [23], both of which are risk factors for intracardiac thrombus formation.
- IV.
- Pre-formed thrombus within the left atrial appendage (LAA) or left atrium (LA). Although it has been demonstrated that intra-atrial thrombi can form even within 48 h of AF onset, [24], Anselmino et al. [25] detected no intracardiac thrombi in patients in sinus rhythm, with CHA2DS2-VASc = 0–1 and with no past history of AF ablation undergoing TOE before AF pulmonary vein isolation (PVI), making this hypothesis unlikely in the study population described in this research. Moreover, the absence of echocardiographic evidence of LAA thrombus does not guarantee safe cardioversion without appropriate postprocedural OAC, nor does it prevent TECs from occurring once sinus rhythm is restored [5].
4.2. Study Results
4.3. Future Implications
- (1)
- Limiting the timing threshold for safe cardioversion without postprocedural OAC to <12 h.
- (2)
- Accounting for regional access, individual risk, and equity in prescribing OAC.
- (3)
- Implementing bleeding prevention strategies in systems with routine post-cardioversion OAC (e.g., Canada and China).
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| CV | Cardioversion |
| DOAC(s) | Direct oral anticoagulant(s) |
| OAC | Oral anticoagulation |
| TECs | Thromboembolic complications |
References
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial fibrillation: Epidemiology, screening and digital health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Annovi, A.; Sanchis-Gomar, F.; Saccenti, C.; Meschi, T.; Ticinesi, A.; Cervellin, G. Effectiveness and safety of electrical cardioversion for acute-onset atrial fibrillation in the emergency department: A real-world 10-year single center experience. Clin. Exp. Emerg. Med. 2019, 6, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Pancholy, S.B.; Sharma, P.S.; Pancholy, D.S.; Patel, T.M.; Callans, D.J.; Marchlinski, F.E. Meta-Analysis of Gender Differences in Residual Stroke Risk and Major Bleeding in Patients With Nonvalvular Atrial Fibrillation Treated With Oral Anticoagulants. Am. J. Cardiol. 2014, 113, 485–490. [Google Scholar] [CrossRef]
- Khan, I.A. Atrial stunning: Basics and clinical considerations. Int. J. Cardiol. 2003, 92, 113–128. [Google Scholar] [CrossRef]
- Airaksinen, K.E.J.; Grönberg, T.; Nuotio, I.; Nikkinen, M.; Ylitalo, A.; Biancari, F.; Hartikainen, J.E.K. Thromboembolic Complications After Cardioversion of Acute Atrial Fibrillation. J. Am. Coll. Cardiol. 2013, 62, 1187–1192. [Google Scholar] [CrossRef]
- Scheuermeyer, F.X.; Grafstein, E.; Stenstrom, R.; Innes, G.; Poureslami, I.; Sighary, M. Thirty-day Outcomes of Emergency Department Patients Undergoing Electrical Cardioversion for Atrial Fibrillation or Flutter. Acad. Emerg. Med. 2010, 17, 408–415. [Google Scholar] [CrossRef]
- Palomäki, A.; Mustonen, P.; Hartikainen, J.E.K.; Nuotio, I.; Kiviniemi, T.; Ylitalo, A.; Hartikainen, P.; Lehtola, H.; Luite, R.; Airaksinen, K.E.J. Strokes after cardioversion of atrial fibrillation—The FibStroke study. Int. J. Cardiol. 2016, 203, 269–273. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Andrade, J.G.; Aguilar, M.; Atzema, C.; Bell, A.; Cairns, J.A.; Cheung, C.C.; Cox, J.L.; Dorian, P.; Gladstone, D.J.; Healey, J.S.; et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can. J. Cardiol. 2020, 36, 1847–1948. [Google Scholar] [CrossRef]
- Ma, C.-S.; Wu, S.-L.; Liu, S.-W.; Han, Y.-L. Chinese Guidelines for the Diagnosis and Management of Atrial Fibrillation. J. Geriatr. Cardiol. 2024, 21, 251–314. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Atrial Fibrillation: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng196/chapter/Recommendations (accessed on 31 December 2024).
- Brieger, D.; Amerena, J.; Attia, J.; Bajorek, B.; Chan, K.H.; Connell, C.; Freedman, B.; Ferguson, C.; Hall, T.; Haqqani, H.; et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ. 2018, 27, 1209–1266. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Nuotio, I.; Hartikainen, J.E.K.; Grönberg, T.; Biancari, F.; Airaksinen, K.E.J. Time to Cardioversion for Acute Atrial Fibrillation and Thromboembolic Complications. JAMA 2014, 312, 647. [Google Scholar] [CrossRef]
- Grönberg, T.; Hartikainen, J.E.K.; Nuotio, I.; Biancari, F.; Ylitalo, A.; Airaksinen, K.E.J. Anticoagulation, CHA2DS2VASc Score, and Thromboembolic Risk of Cardioversion of Acute Atrial Fibrillation (from the FinCV Study). Am. J. Cardiol. 2016, 117, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Khunger, M.; Seicean, S.; Chung, M.K.; Tchou, P.J. Incidence of Thromboembolic Complications Within 30 Days of Electrical Cardioversion Performed Within 48 Hours of Atrial Fibrillation Onset. JACC Clin. Electrophysiol. 2016, 2, 487–494. [Google Scholar] [CrossRef]
- Hansen, M.L.; Jepsen, R.M.H.G.; Olesen, J.B.; Ruwald, M.H.; Karasoy, D.; Gislason, G.H.; Hansen, J.; Køber, L.; Husted, S.; Torp-Pedersen, C. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. EP Eur. 2015, 17, 18–23. [Google Scholar] [CrossRef]
- Tampieri, A.; Cipriano, V.; Mucci, F.; Rusconi, A.M.; Lenzi, T.; Cenni, P. Safety of cardioversion in atrial fibrillation lasting less than 48 h without post-procedural anticoagulation in patients at low cardioembolic risk. Intern. Emerg. Med. 2018, 13, 87–93. [Google Scholar] [CrossRef]
- Dagres, N.; Kornej, J.; Hindricks, G. Prevention of Thromboembolism After Cardioversion of Recent-Onset Atrial Fibrillation. J. Am. Coll. Cardiol. 2013, 62, 1193–1194. [Google Scholar] [CrossRef]
- Grimm, R.A.; Stewart, W.J.; Maloney, J.D.; Cohen, G.I.; Pearce, G.L.; Salcedo, E.E.; Klein, A.L. Impact of electrical cardioversion for atrial fibrillation on left atrial appendage function and spontaneous echo contrast: Characterization by simultaneous transesophageal echocardiography. J. Am. Coll. Cardiol. 1993, 22, 1359–1366. [Google Scholar] [CrossRef]
- Flaker, G.C.; Belew, K.; Beckman, K.; Vidaillet, H.; Kron, J.; Safford, R.; Mickel, M.; Barrell, P. AFFIRM Investigators Asymptomatic atrial fibrillation: Demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am. Heart J. 2005, 149, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Prothrombotic markers in atrial fibrillation: What is new? Available online: https://pubmed.ncbi.nlm.nih.gov/11511115/ (accessed on 8 January 2025).
- Stoddard, M.F.; Dawkins, P.R.; Prince, C.R.; Ammash, N.M. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: A transesophageal echocardiographic study. J. Am. Coll. Cardiol. 1995, 25, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Anselmino, M.; Garberoglio, L.; Gili, S.; Bertaglia, E.; Stabile, G.; Marazzi, R.; Themistoclakis, S.; Solimene, F.; Frea, S.; Marra, W.G.; et al. Left atrial appendage thrombi relate to easily accessible clinical parameters in patients undergoing atrial fibrillation transcatheter ablation: A multicenter study. Int. J. Cardiol. 2017, 241, 218–222. [Google Scholar] [CrossRef]
- Andrade, J.G.; Macle, L.; Verma, A.; Mitchell, L.B. Comment on “The Canadian Cardiovascular Society 2018 guideline update for atrial fibrillation—A different perspective”. CJEM 2020, 22, E3. [Google Scholar] [CrossRef]
- Lamberts, M.; Staerk, L.; Olesen, J.B.; Fosbøl, E.L.; Hansen, M.L.; Harboe, L.; Lefevre, C.; Evans, D.; Gislason, G.H. Major Bleeding Complications and Persistence with Oral Anticoagulation in Non-Valvular Atrial Fibrillation: Contemporary Findings in Real-Life Danish Patients. J. Am. Heart Assoc. 2017, 6, e004517. [Google Scholar] [CrossRef]
- Saglietto, A.; De Ferrari, G.M.; Gaita, F.; Anselmino, M. Short-term anticoagulation after acute cardioversion of early-onset atrial fibrillation. Eur. J. Clin. Investig. 2020, 50, e13316. [Google Scholar] [CrossRef]
- Migliore, F.; Providencia, R.; Farkowski, M.M.; Dan, G.A.; Daniel, S.; Potpara, T.S.; Jubele, K.; Chun, J.K.R.; de Asmundis, C.; Zorzi, A.; et al. Antithrombotic treatment management in low stroke risk patients un-dergoing cardioversion of atrial fibrillation. EP Eur. 2021, 23, 1502–1507. [Google Scholar] [CrossRef]
- Choudhry, N.K.; Fletcher, R.H.; Soumerai, S.B. Systematic Review: The Relationship between Clinical Experience and Quality of Health Care. Ann. Intern. Med. 2005, 142, 260–273. [Google Scholar] [CrossRef]
- Sohara, H.; Amitani, S.; Kurose, M.; Miyahara, K. Atrial fibrillation activates platelets and coagulation in a time-dependent manner: A study in patients with paroxysmal atrial fibrillation. J. Am. Coll. Cardiol. 1997, 29, 106–112. [Google Scholar] [CrossRef]
- Tamemoto, Y.; Shibata, Y.; Hashimoto, N.; Sato, H.; Hisaka, A. Involvement of multiple cytochrome P450 isoenzymes in drug interactions between ritonavir and direct oral anticoagulants. Drug Metab. Pharmacokinet. 2023, 53, 100498. [Google Scholar] [CrossRef]
- American College of Cardiology. Athletes and Anticoagulation: Return to Play After DVT/PE. Available online: https://www.acc.org/latest-in-cardiology/articles/2016/10/19/15/13/http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2016%2f10%2f19%2f15%2f13%2fathletes-and-anticoagulation (accessed on 1 February 2025).
- Weiske, N.; Baumbach, H.; Bürger, T. Treatment of venous thoracic inlet syndrome—Specialties in athletes. Phlebologie 2020, 49, 10–15. [Google Scholar] [CrossRef]
| Would You Start This Patient on Short-Term (4 Weeks) DOAC Treatment? | ||||||
|---|---|---|---|---|---|---|
| Years of Experience | Maybe, Based on Patient’s Preferences and Further Blood Tests | No Treatment Since the Haemorrhagic Risk > Thromboembolic Risk | Yes, for 4 Weeks but Only Starting from CHA2DS2-VA = 1 | Yes, for 4 Weeks with CHA2DS2-VA = 0 | Tot | |
| <5 | Observed | 6 | 2 | 4 | 29 | 41 |
| % of total | 8.7% | 2.9% | 5.8% | 42.0% | 59.4% | |
| >10 | 3 | 8 | 1 | 16 | 28 | |
| 4.3% | 11.6% | 1.4% | 23.2% | 40.6% | ||
| Total | 9 | 10 | 5 | 45 | 69 | |
| 13.0% | 14.5% | 7.2% | 65.2% | 100.0% | ||
| Association Testing | ||||||
| Value | Df | p-Value | ||||
| χ2 Test | 7.99 | 3 | 0.046 | |||
| Fisher’s Exact Test | 0.049 | |||||
| N | 69 | |||||
| Centre | |||
|---|---|---|---|
| Would You Start This Patient on Short-Term (4 Weeks) DOAC Treatment? | Canada | Europe | Tot |
| Maybe. Additional info and specific blood tests are required | 2 | 9 | 11 |
| No treatment since the haemorrhagic risk > thromboembolic risk | 1 | 10 | 11 |
| Yes, for 4 weeks but only starting from CHA2DS2VASc = 1 | 0 | 6 | 6 |
| Yes, for 4 weeks with CHA2DS2VASc = 0 | 17 | 30 | 47 |
| Total | 20 | 55 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poggio, A.; Sullivan, A.P.; Rampa, L.; Andrade, J.G.; Anselmino, M. Short-Term Anticoagulation After Cardioversion in New-Onset Atrial Fibrillation and Low Thromboembolic Risk: A Real-World International Investigation. Medicina 2025, 61, 1200. https://doi.org/10.3390/medicina61071200
Poggio A, Sullivan AP, Rampa L, Andrade JG, Anselmino M. Short-Term Anticoagulation After Cardioversion in New-Onset Atrial Fibrillation and Low Thromboembolic Risk: A Real-World International Investigation. Medicina. 2025; 61(7):1200. https://doi.org/10.3390/medicina61071200
Chicago/Turabian StylePoggio, Alan, Andrew P. Sullivan, Lorenzo Rampa, Jason G. Andrade, and Matteo Anselmino. 2025. "Short-Term Anticoagulation After Cardioversion in New-Onset Atrial Fibrillation and Low Thromboembolic Risk: A Real-World International Investigation" Medicina 61, no. 7: 1200. https://doi.org/10.3390/medicina61071200
APA StylePoggio, A., Sullivan, A. P., Rampa, L., Andrade, J. G., & Anselmino, M. (2025). Short-Term Anticoagulation After Cardioversion in New-Onset Atrial Fibrillation and Low Thromboembolic Risk: A Real-World International Investigation. Medicina, 61(7), 1200. https://doi.org/10.3390/medicina61071200








