Comparison of the Diagnostic Accuracies of Procalcitonin and C-Reactive Protein for Spontaneous Bacterial Peritonitis in Patients with Cirrhosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Quality Assessment
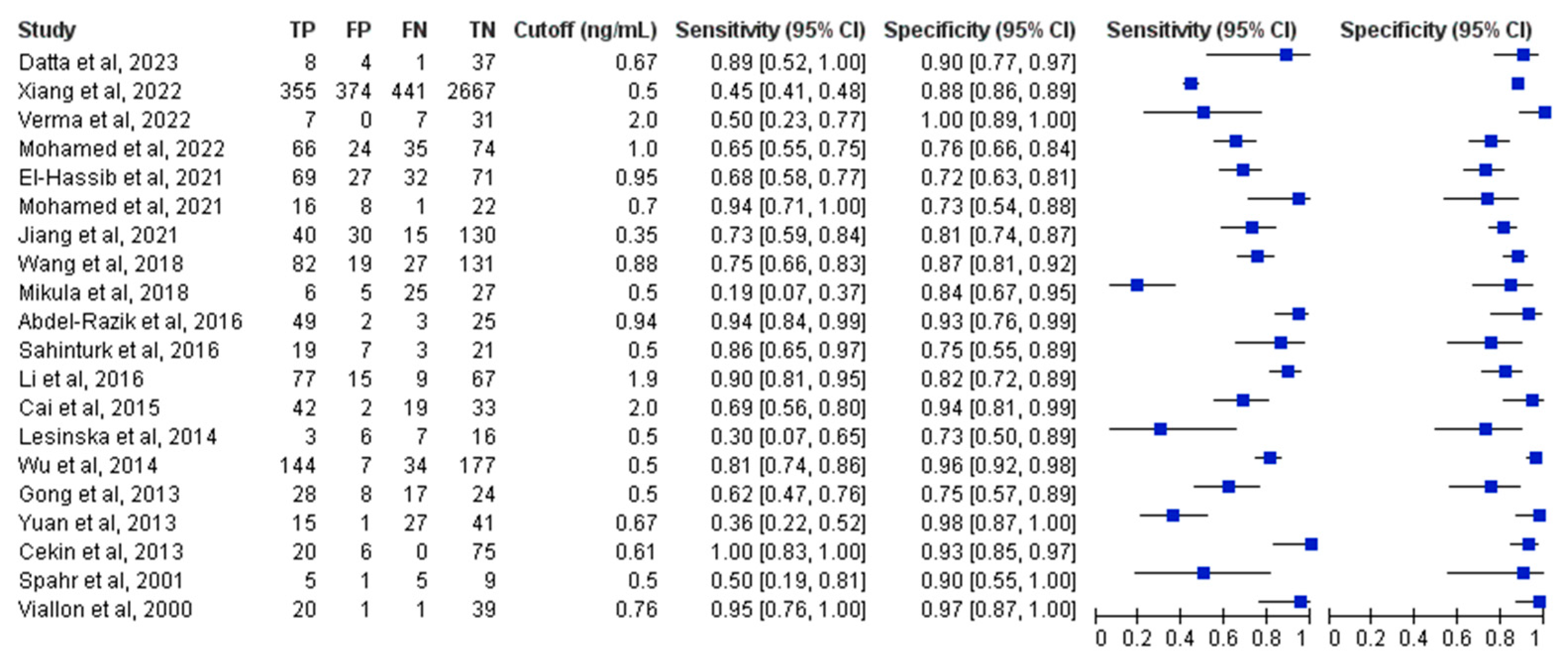
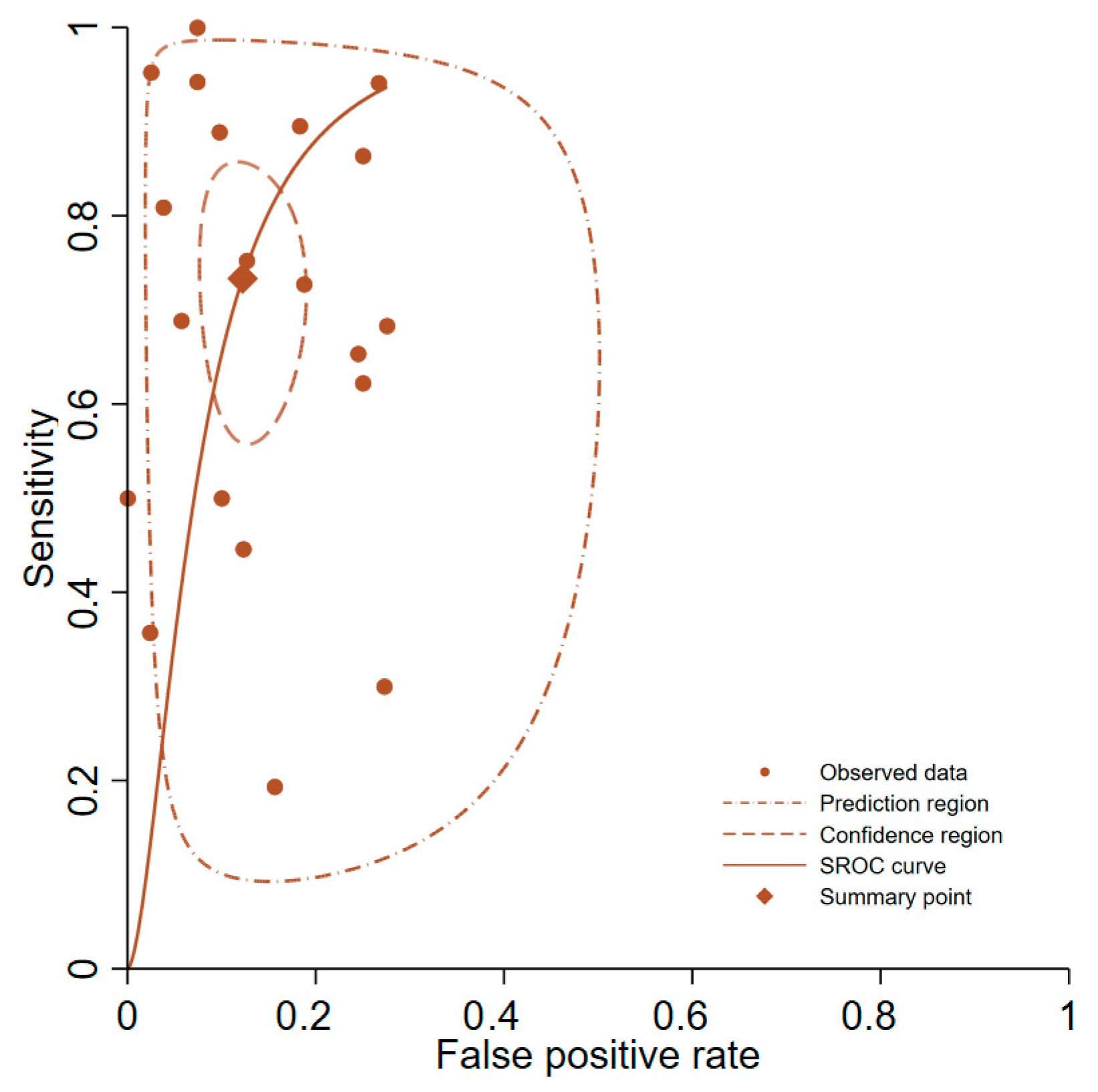
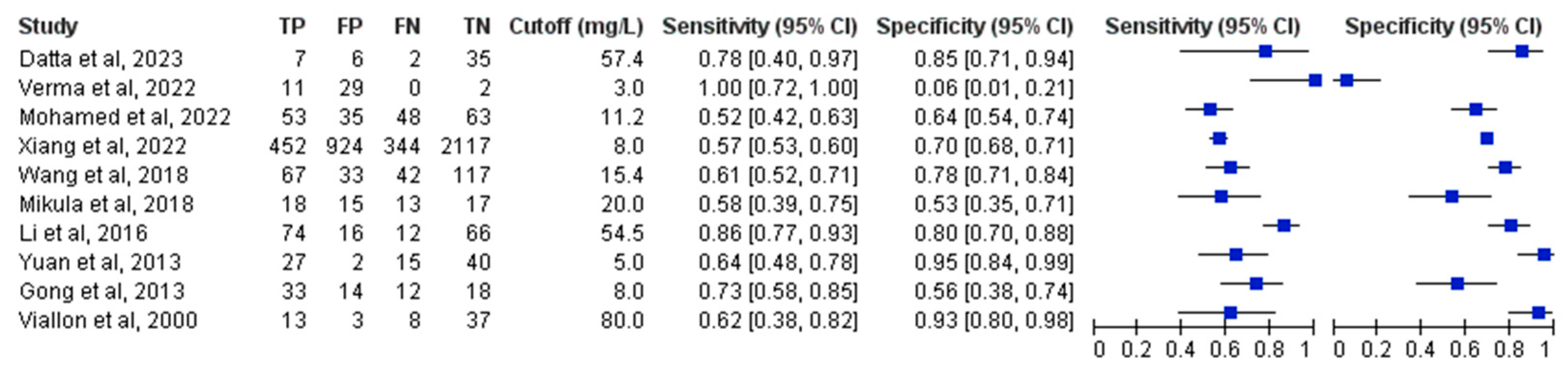
3.4. Primary Analysis of Overall Accuracy
3.5. Subgroup and Sensitivity Analyses
3.6. Head-to-Head Performance Analysis of PCT and CRP
3.7. Publication Bias
3.8. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCT | procalcitonin |
| SBP | spontaneous bacterial peritonitis |
| CRP | C-reactive protein |
| SROC | summary receiver operating characteristic |
| HSROC | hierarchical summary receiver operating characteristic |
References
- Bonnel, A.R.; Bunchorntavakul, C.; Reddy, K.R. Immune dysfunction and infections in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2011, 9, 727–738. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Chamroonkul, N.; Chavalitdhamrong, D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J. Hepatol. 2016, 8, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Borzio, M.; Salerno, F.; Piantoni, L.; Cazzaniga, M.; Angeli, P.; Bissoli, F.; Boccia, S.; Colloredo-Mels, G.; Corigliano, P.; Fornaciari, G.; et al. Bacterial infection in patients with advanced cirrhosis: A multicentre prospective study. Dig. Liver Dis. 2001, 33, 41–48. [Google Scholar] [CrossRef]
- Merli, M.; Lucidi, C.; Giannelli, V.; Giusto, M.; Riggio, O.; Falcone, M.; Ridola, L.; Attili, A.F.; Venditti, M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin. Gastroenterol. Hepatol. 2010, 8, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Caly, W.R.; Strauss, E. A prospective study of bacterial infections in patients with cirrhosis. J. Hepatol. 1993, 18, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; García-Tsao, G.; Navasa, M.; Piddock, L.J.V.; Planas, R.; Bernard, B.; Inadomi, J.M.; the International Ascites Club. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: A consensus document. J. Hepatol. 2000, 32, 142–153. [Google Scholar] [CrossRef] [PubMed]
- De Gottardi, A.; Thévenot, T.; Spahr, L.; Morard, I.; Bresson-Hadni, S.; Torres, F.; Giostra, E.; Hadengue, A. Risk of complications after abdominal paracentesis in cirrhotic patients: A prospective study. Clin. Gastroenterol. Hepatol. 2009, 7, 906–909. [Google Scholar] [CrossRef]
- Essmann, L.; Wirz, Y.; Gregoriano, C.; Schuetz, P. One biomarker does not fit all: Tailoring anti-infective therapy through utilization of procalcitonin and other specific biomarkers. Expert Rev. Mol. Diagn. 2023, 23, 739–752. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Singer, M.; Dal-Pizzol, F. Biomarkers for sepsis: More than just fever and leukocytosis—A narrative review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Bogossian, E.; Menozzi, M. The future of biomarkers. Crit. Care Clin. 2020, 36, 177–187. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Yeh, H.-T.; Lu, S.-W.; Tsai, Y.-C.; Tsai, Y.-C.; Yen, C.-C. Diagnostic accuracy of procalcitonin in adult non-neutropenic cancer patients with suspected infection: A systematic review and meta-analysis. BMC Infect. Dis. 2024, 24, 278. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Chen, C.-J.; Shao, S.-C.; Li, C.; Hsiao, C.-H.; Niu, K.-Y.; Yen, C.-C. Comparison of the Diagnostic Accuracies of Monocyte Distribution Width, Procalcitonin, and C-Reactive Protein for Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Med. 2023, 51, e106–e114. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-L.; Yeh, H.-T.; Niu, K.-Y.; Chen, C.-B.; Seak, C.-J.; Yen, C.-C. Diagnostic performances of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. Eur. J. Emerg. Med. 2025; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Li, J.-J.; Hsiao, C.-H.; Yen, C.-C. Clinical Characteristics and In-Hospital Outcomes in Patients with Iliopsoas Abscess: A Multicenter Study. J. Clin. Med. 2023, 12, 2760. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-C.; Yeh, H.; Ho, C.-F.; Hsiao, C.-H.; Niu, K.-Y.; Yeh, C.-C.; Lu, J.-X.; Wu, C.-C.; Chang, Y.-C.; Ng, C.-J. Risk factors for 30-day mortality in patients with head and neck cancer bleeding in the emergency department. Am. J. Emerg. Med. 2022, 58, 9–15. [Google Scholar] [CrossRef]
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C. Procalcitonin increase after endotoxin injection in normal subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar]
- Takata, S.; Wada, H.; Tamura, M.; Koide, T.; Higaki, M.; Mikura, S.-I.; Yasutake, T.; Hirao, S.; Nakamura, M.; Honda, K.; et al. Kinetics of c-reactive protein (CRP) and serum amyloid A protein (SAA) in patients with community-acquired pneumonia (CAP), as presented with biologic half-life times. Biomarkers 2011, 16, 530–535. [Google Scholar] [CrossRef]
- Póvoa, P.; Salluh, J.I.F. Biomarker-guided antibiotic therapy in adult critically ill patients: A critical review. Ann. Intensive Care 2012, 2, 32. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Qu, C.; Zeng, B.; Liang, S.; Luo, Z.; Wang, X.; Zhong, C. Diagnostic Accuracy of Serum Procalcitonin for Spontaneous Bacterial Peritonitis Due to End-stage Liver Disease: A Meta-analysis. Medicine 2015, 94, e2077. [Google Scholar] [CrossRef]
- Deeks, J.J.; Bossuyt, P.M.; Leeflang, M.M.; Takwoingi, Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Salameh, J.-P.; Bossuyt, P.M.; A McGrath, T.; Thombs, B.D.; Hyde, C.J.; Macaskill, P.; Deeks, J.J.; Leeflang, M.; A Korevaar, D.; Whiting, P.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. [Google Scholar] [CrossRef]
- Leeflang, M.M.; Deeks, J.J.; Gatsonis, C.; Bossuyt, P.M. Systematic reviews of diagnostic test accuracy. Ann. Intern. Med. 2008, 149, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 2001, 20, 2865–2884. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Moses, L.E.; Shapiro, D.; Littenberg, B. Combining independent studies of a diagnostic test into a summary ROC curve: Data-analytic approaches and some additional considerations. Stat. Med. 1993, 12, 1293–1316. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Mustafa, R.A.; Brozek, J.; Steingart, K.R.; Leeflang, M.; Murad, M.H.; Bossuyt, P.; Glasziou, P.; Jaeschke, R.; Lange, S.; et al. GRADE guidelines: 21 part 2. Test accuracy: Inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2020, 122, 142–152. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Mustafa, R.A.; Brozek, J.; Steingart, K.R.; Leeflang, M.; Murad, M.H.; Bossuyt, P.; Glasziou, P.; Jaeschke, R.; Lange, S.; et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. J. Clin. Epidemiol. 2020, 122, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Datta, I.K.; Bhuiyan, T.M. Significance of Serum Procalcitonin and C-Reactive Protein in the Diagnosis and Prediction of Spontaneous Bacterial Peritonitis in Decompensated Chronic Liver Disease. Mymensingh Med. J. MMJ 2023, 32, 1163–1168. [Google Scholar]
- Xiang, S.; Tan, J.; Tan, C.; Xu, Q.; Wen, Y.; Wang, T.; Yang, C.; Zhao, W. Establishment and Validation of a Non-Invasive Diagnostic Nomogram to Identify Spontaneous Bacterial Peritonitis in Patients with Decompensated Cirrhosis. Front. Med. 2022, 8, 797363. [Google Scholar] [CrossRef]
- Verma, R.; Satapathy, S.K.; Bilal, M. Procalcitonin and C-reactive protein in the diagnosis of spontaneous bacterial peritonitis. Transl. Gastroenterol. Hepatol. 2022, 7, 36. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Salah, W.; Hassan, M.B.; Eldeeb, H.H.; Adaroas, A.S.; Khattab, R.A.; Abostate, H.M.; Soliman, M.Y.; Habba, E.; Abd-Elsalam, S.; et al. MCP1, CRP and Procalcitonin as Novel Diagnostic Markers in Cirrhotic Patients with Spontaneous Bacterial Peritonitis. Open Biomark. J. 2022, 12. [Google Scholar] [CrossRef]
- Jiang, P.; Dou, R.; Cui, Z.; Tong, Y.; Li, C.; Jiang, S.; Bai, Y.; Zheng, J.; Wang, C.; Wang, Y. Value of albumin–bilirubin score combined with neutrophil count and procalcitonin in ascites in predicting spontaneous bacterial peritonitis in patients with cirrhotic ascites. J. Clin. Hepatol. 2021, 37, 2097–2101. [Google Scholar]
- El-Hassib, D.M.A.; Abo-Elmatty, D.M.; Mesbah, N.M.; Abd-Elsalam, S.; Bastawisy, S.A.; E, D.; Hassan, A.S.; Zahran, F.E.; Abdelghany, R.S.; Siddik, R.I.; et al. The role of micrornas (Mirna 155, mirna-146b) and procalcitonin as novel markers for the diagnosis of spontaneous bacterial peritonitis. Open Biomark. J. 2021, 11, 28–38. [Google Scholar] [CrossRef]
- Abu Rahma, M.Z.; Mahran, Z.G.; Shafik, E.A.; Mohareb, D.A.; El-Rady, N.M.A.; Mustafa, M.A.; Khalil, M.; Abo-Amer, Y.E.-E.; Abd-Elsalam, S. The role of serum procalcitonin level as an early marker of ascitic fluid infection in post hepatitic cirrhotic patients. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2021, 20, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Zhang, F.; Yang, N.; Xie, N.; Mao, Y.; Li, B. Combination of PCT, sNFI and dCHC for the diagnosis of ascites infection in cirrhotic patients. BMC Infect. Dis. 2018, 18, 389. [Google Scholar] [CrossRef]
- Mikuła, T.; Sapuła, M.; Jabłońska, J.; Kozłowska, J.; Stańczak, W.; Krankowska, D.; Wiercińska-Drapało, A. Significance of heparin-binding protein and D-dimers in the early diagnosis of spontaneous bacterial peritonitis. Mediat. Inflamm. 2018, 2018, 1969108. [Google Scholar] [CrossRef] [PubMed]
- Şahintürk, Y.; Çekiç, B.; Görgülügil, G.Z.; Harmandar, F.A.; Uyar, S.; Çekin, Y.; Çekin, A.H. Cirrhotic Ascites management via procalcitonin level and a new approach B-mode gray-scale histogram. Turk. J. Gastroenterol. 2016, 27, 47–54. [Google Scholar] [CrossRef]
- Li, Y.-C.; Hu, W.-Y.; Li, J.; Luo, H.-X.; Zhou, J.; Chen, C.-J. Diagnostic value of serum PCT and CRP for spontaneous bacterial peritonitis in patients with liver cirrhosis. World Chin. J. Dig. 2016, 24, 3299–3303. [Google Scholar] [CrossRef]
- Abdel-Razik, A.; Mousa, N.; Elhammady, D.; Elhelaly, R.; Elzehery, R.; Elbaz, S.; Eissa, M.; El-Wakeel, N.; Eldars, W. Ascitic fluid calprotectin and serum procalcitonin as accurate diagnostic markers for spontaneous bacterial peritonitis. Gut Liver 2016, 10, 624–631. [Google Scholar] [CrossRef]
- Cai, Z.-H.; Fan, C.-L.; Zheng, J.-F.; Zhang, X.; Zhao, W.-M.; Li, B.; Li, L.; Dong, P.-L.; Ding, H.-G. Measurement of serum procalcitonin levels for the early diagnosis of spontaneous bacterial peritonitis in patients with decompensated liver cirrhosis. BMC Infect. Dis. 2015, 15, 55. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, F.; Zeng, T.; Xu, H.; Lei, Y.; Zhong, S.; Zhou, Z.; Ren, H. Role of serum procalcitonin assay for diagnosis of spontaneous bacterial peritonitis in end-stage liver diseases. Acta Acad. Med. Sin. 2014, 36, 37–41. [Google Scholar]
- Lesińska, M.; Hartleb, M.; Gutkowski, K.; Nowakowska-Duława, E. Procalcitonin and macrophage inflammatory protein-1 beta (MIP-1β) in serum and peritoneal fluid of patients with decompensated cirrhosis and spontaneous bacterial peritonitis. Adv. Med. Sci. 2014, 59, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.-Y.; Ke, Z.-Q.; Wang, M.; Li, Y. Procalcitonin and C-reactive protein in the diagnosis and prediction of spontaneous bacterial peritonitis associated with chronic severe hepatitis B. Ann. Lab. Med. 2013, 33, 449–454. [Google Scholar] [CrossRef]
- Gong, Y.X.; Cui, S.N.; Li, L.; Wang, M.M.; Guo, N. Diagnostic value of human neutrophil peptide in spontaneous bacterial peritonitis. Chin. J. Hepatol. 2013, 21, 944–948. [Google Scholar]
- Cekin, Y.; Cekin, A.H.; Duman, A.; Yilmaz, U.; Yesil, B.; Yolcular, B.O. The role of serum procalcitonin levels in predicting ascitic fluid infection in hospitalized cirrhotic and non-cirrhotic patients. Int. J. Med. Sci. 2013, 10, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Spahr, L.; Morard, I.; Hadengue, A.; Vadas, L.; Pugin, J. Procalcitonin is not an accurate marker of spontaneous bacterial peritonitis in patients with cirrhosis. Hepato-Gastroenterology 2001, 48, 502–505. [Google Scholar]
- Viallon, A.; Zeni, F.; Pouzet, V.; Lambert, C.; Quenet, S.; Aubert, G.; Guyomarch, S.; Tardy, B.; Bertrand, J.-C. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: Diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000, 26, 1082–1088. [Google Scholar] [CrossRef]
- Oruc, N.; Ozutemiz, O.; Yuce, G.; Akarca, U.S.; Ersoz, G.; Gunsar, F.; Batur, Y. Serum procalcitonin and CRP levels in non-alcoholic fatty liver disease: A case control study. BMC Gastroenterol. 2009, 9, 16. [Google Scholar] [CrossRef]
- Elefsiniotis, I.S.; Skounakis, M.; Vezali, E.; Pantazis, K.D.; Petrocheilou, A.; Pirounaki, M.; Papatsibas, G.; Kontou-Kastellanou, C.; Moulakakis, A. Clinical significance of serum procalcitonin levels in patients with acute or chronic liver disease. Eur. J. Gastroenterol. Hepatol. 2006, 18, 525–530. [Google Scholar] [CrossRef]
- Rule, J.A.; Hynan, L.S.; Attar, N.; Sanders, C.; Korzun, W.J.; Lee, W.M.; Acute Liver Failure Study Group; Bruns, H. Procalcitonin identifies cell injury, not bacterial infection, in acute liver failure. PLoS ONE 2015, 10, e0138566. [Google Scholar] [CrossRef]
- Sugihara, T.; Koda, M.; Okamoto, T.; Miyoshi, K.; Matono, T.; Oyama, K.; Hosho, K.; Okano, J.-I.; Isomoto, H. Serum procalcitonin in patients with acute liver failure. Yonago Acta Med. 2017, 60, 40–46. [Google Scholar] [PubMed]
- Mallet, M.; Tripon, S.; Thabut, D.; Rudler, M. THU-405—Elevated Levels of Procalcitonin during Acute Liver Failure are not Associated with Sepsis or Worth Outcome. J. Hepatol. 2016, 64 (Suppl. S2), S308–S309. [Google Scholar] [CrossRef]
- Whang, K.T.; Vath, S.D.; Becker, K.L.; Snider, R.H.; Nylen, E.S.; Muller, B.; Li, Q.; Tamarkin, L.; White, J.C. Procalcitonin and proinflammatory cytokine interactions in sepsis. Shock 2000, 14, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-W.; Niu, K.-Y.; Pai, C.-P.; Lin, S.-H.; Chen, C.-B.; Lo, Y.-T.; Lee, Y.-C.; Seak, C.-J.; Yen, C.-C. Novel Prediction Score for Arterial–Esophageal Fistula in Patients with Esophageal Cancer Bleeding: A Multicenter Study. Cancers 2024, 16, 804. [Google Scholar] [CrossRef]
- Lu, S.-W.; Pai, C.-P.; Yang, T.-H.; Lu, J.-X.; Hsiao, C.-H.; Yen, C.-C. Clinical characteristics and risk factors for 30-day mortality in esophageal cancer patients with upper gastrointestinal bleeding: A multicenter study. Front. Oncol. 2023, 13, 1184710. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-K.; Yen, C.-C.; Chen, S.-Y.; Lo, H.-Y.; Ng, C.-J.; Chaou, C.-H. Prognostic value of cardiac troponin in dialysis patients with paroxysmal supraventricular tachycardia. Medicine 2022, 101, e30513. [Google Scholar] [CrossRef]
- Naaktgeboren, C.A.; Ochodo, E.A.; Van Enst, W.A.; de Groot, J.A.H.; Hooft, L.; Leeflang, M.M.G.; Bossuyt, P.M.; Moons, K.G.M.; Reitsma, J.B. Assessing variability in results in systematic reviews of diagnostic studies. BMC Med. Res. Methodol. 2016, 16, 6. [Google Scholar] [CrossRef]
- Yeh, H.-T.; Lu, S.-W.; Cheng, T.-H.; Lu, J.-X.; Hsiao, C.-H.; Yen, C.-C. Diagnostic accuracy of transthoracic echocardiography for acute type A aortic syndrome: A systematic review and meta-analysis. Biomed. J. 2025, 48, 100747. [Google Scholar] [CrossRef]
- Jian, S.-J.Z.; Cheng, T.-H.; Yen, C.-C. Prognostic accuracy of point-of-care ultrasound in patients with pulseless electrical activity: A systematic review and meta-analysis. Scand. J. Trauma Resusc. Emerg. Med. 2025, 33, 27. [Google Scholar] [CrossRef]
- Irwig, L.; Macaskill, P.; Glasziou, P.; Fahey, M. Meta-analytic methods for diagnostic test accuracy. J. Clin. Epidemiol. 1995, 48, 119–130; discussion 131–132. [Google Scholar] [CrossRef]
- Yang, T.-H.; Shao, S.-C.; Lee, Y.-C.; Hsiao, C.-H.; Yen, C.-C. Risk factors for peri-intubation cardiac arrest: A systematic review and meta-analysis. Biomed. J. 2023, 47, 100656. [Google Scholar] [CrossRef] [PubMed]


| Pretest Probability | Post-test Probability After a Positive Result | Post-test Probability After a Negative Result | False Positive * | False Negative * |
|---|---|---|---|---|
| 0.1 | 0.44 | 0.03 | 108 | 27 |
| 0.2 | 0.64 | 0.07 | 96 | 54 |
| 0.3 | 0.75 | 0.11 | 84 | 81 |
| 0.4 | 0.82 | 0.16 | 72 | 108 |
| 0.5 | 0.88 | 0.22 | 60 | 135 |
| 0.6 | 0.91 | 0.30 | 48 | 162 |
| 0.7 | 0.94 | 0.40 | 36 | 189 |
| Subgroup | Number of Studies | Pooled Sensitivity (95% CI) | SUBGROUP p Value in Sensitivity | Pooled Specificity (95% CI) | SUBGROUP p Value in Specificity | Positive Likelihood Ratio | Negative Likelihood Ratio | Pooled AUC (95% CI) | Diagnostic Odds Ratio |
|---|---|---|---|---|---|---|---|---|---|
| Overall group | 20 | 0.73 (0.61–0.83) | - | 0.88 (0.83–0.91) | - | 6.0 (4.1–8.8) | 0.30 (0.20–0.47) | 0.90 (0.87–0.92) | 19.7 (9.6–40.5) |
| PCT cutoff 0.5–1 (ng/mL) | 16 | 0.74 (0.58–0.85) | 0.87 | 0.87 (0.82–0.91) | 0.71 | 5.8 (3.7–9.0) | 0.30 (0.17–0.52) | 0.89 (0.86–0.92) | 19.2 (7.7–47.9) |
| 100% cirrhotic patients | 16 | 0.71 (0.58–0.81) | 0.35 | 0.85 (0.80–0.88) | 0.01 * | 4.6 (3.3–6.5) | 0.34 (0.23–0.52) | 0.87 (0.84–0.89) | 13.4 (6.7–26.8) |
| Excluding case–control study | 19 | 0.74 (0.60–0.84) | 0.83 | 0.87 (0.82–0.91) | 0.42 | 5.8 (3.9–8.6) | 0.30 (0.19–0.48) | 0.90 (0.87–0.93) | 19.4 (9.1–41.5) |
| Excluding studies using optimal cutoffs | 8 | 0.52 (0.37–0.67) | 0.01 * | 0.86 (0.79–0.91) | 0.57 | 3.8 (2.3–6.5) | 0.55 (0.40–0.77) | 0.82 (0.78–0.85) | 6.9 (3.1–15.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.-H.; Lin, C.-M.; Niu, K.-Y.; Lin, S.-H.; Chen, C.-B.; Chuang, C.-L.; Yen, C.-C. Comparison of the Diagnostic Accuracies of Procalcitonin and C-Reactive Protein for Spontaneous Bacterial Peritonitis in Patients with Cirrhosis: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1134. https://doi.org/10.3390/medicina61071134
Tang T-H, Lin C-M, Niu K-Y, Lin S-H, Chen C-B, Chuang C-L, Yen C-C. Comparison of the Diagnostic Accuracies of Procalcitonin and C-Reactive Protein for Spontaneous Bacterial Peritonitis in Patients with Cirrhosis: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(7):1134. https://doi.org/10.3390/medicina61071134
Chicago/Turabian StyleTang, Tzu-Hsuan, Ching-Min Lin, Kuang-Yu Niu, Shih-Hua Lin, Chen-Bin Chen, Chiao-Li Chuang, and Chieh-Ching Yen. 2025. "Comparison of the Diagnostic Accuracies of Procalcitonin and C-Reactive Protein for Spontaneous Bacterial Peritonitis in Patients with Cirrhosis: A Systematic Review and Meta-Analysis" Medicina 61, no. 7: 1134. https://doi.org/10.3390/medicina61071134
APA StyleTang, T.-H., Lin, C.-M., Niu, K.-Y., Lin, S.-H., Chen, C.-B., Chuang, C.-L., & Yen, C.-C. (2025). Comparison of the Diagnostic Accuracies of Procalcitonin and C-Reactive Protein for Spontaneous Bacterial Peritonitis in Patients with Cirrhosis: A Systematic Review and Meta-Analysis. Medicina, 61(7), 1134. https://doi.org/10.3390/medicina61071134






