Abstract
Background and Objectives: Percutaneous liver radiofrequency ablation (RFA) under monitored anesthesia care (MAC) carries a risk of hypoxia due to respiratory depression. Ensuring adequate oxygenation during such procedures is essential for patient safety. This study aimed to evaluate whether a high-flow nasal cannula (HFNC) improves oxygenation compared to a simple facemask during RFA. Materials and Methods: In this prospective, randomized controlled trial, 51 patients undergoing ultrasound-guided RFA under MAC were allocated to receive oxygen via an HFNC (30 L/min) or a facemask (6 L/min). Arterial blood gases were collected at the baseline and 5 min after oxygenation. The primary outcome was the arterial partial pressure of oxygen (PaO2). Secondary outcomes included hypoxia incidence (SpO2 < 95%), the difference in the ratio of the arterial partial pressure of oxygen to the fraction of inspired oxygen concentration (ΔP/F ratio), the difference in the arterial partial pressure of carbon dioxide (ΔPaCO2), respiratory rate (RR) changes, and patient satisfaction. Results: After adjustment for the baseline PaO2, the HFNC group showed significantly higher intra-procedural PaO2 compared to the facemask group (299 ± 18.6 vs. 194 ± 19.0 mmHg, p < 0.001). No significant differences were found in the ΔP/F ratio, ΔPaCO2, or patient satisfaction. Among the secondary outcomes, RR was more stable in the HFNC group throughout the procedure (Group × Time interaction, p = 0.003). Conclusions: The HFNC significantly improved intra-procedural PaO2 during RFA under MAC but did not reduce hypoxia incidence or improve other clinical outcomes compared to facemask oxygenation. The stability of RR observed with the HFNC may reflect a physiological advantage, though further studies are needed to determine its clinical relevance.
1. Introduction
Percutaneous liver radiofrequency ablation (RFA) is an effective, minimally invasive treatment for hepatocellular carcinoma and metastatic liver tumors and offers the benefits of reduced tissue trauma, no radiation exposure, and faster recovery compared to surgery [1,2,3]. However, RFA is frequently painful and requires deep sedation under monitored anesthesia care (MAC), which combines local anesthesia, sedatives, and opioids [4,5]. Sedatives and opioids administered during MAC reduce central respiratory drive and impair protective airway reflexes, which leads to hypoventilation, hypercapnia, and hypoxemia; moreover, pharyngeal muscle relaxation may result in upper airway obstruction, and thereby exacerbate desaturation [6,7] A leading cause of morbidity and mortality during procedural sedation, desaturation-related cardiopulmonary complications have incidences of up to 21.6 per 1000 cases in therapeutic procedures, such as endoscopic retrograde cholangiopancreatography, and mortality rates of up to 0.3 per 1000 cases in gastrointestinal endoscopy [8,9]. Therefore, to maintain patient safety and procedural success, advanced oxygen delivery systems are crucial to minimize the sedation-associated risks of hypoventilation and desaturation.
Conventional oxygen delivery modalities, such as nasal cannulas and simple facemasks, have a limited ability to provide consistent oxygenation. In contrast, the high-flow nasal cannula (HFNC) delivers heated, humidified oxygen at high flow rates, offers high inspired oxygen fractions (FiO2), and produces a mild positive end-expiratory pressure (PEEP)-like effect [10,11]. The HFNC has demonstrated benefits in reducing hypoxic events during procedural sedation in various interventions, including gastrointestinal endoscopy, bronchoscopy, and interventional radiology [12,13,14]. The physiological advantages of the HFNC may be particularly valuable during painful procedures such as liver RFA, where the depth of sedation may unpredictably deepen and increase the risk of respiratory compromise. However, the HFNC’s efficacy in liver RFA under MAC remains insufficiently elucidated.
This study aimed to evaluate whether, compared to a conventional facemask, an HFNC improves arterial oxygenation, as assessed by the intra-procedural partial pressure of arterial oxygen (PaO2), during liver RFA under MAC. We hypothesized that the HFNC, compared to the conventional facemask, would improve arterial oxygenation during liver RFA under MAC. Secondary outcomes included the incidence of hypoxia, changes in the ratio of the arterial partial pressure of oxygen to the fraction of inspired oxygen concentration (∆P/F ratio), changes in the arterial partial pressure of carbon dioxide (∆PaCO2), respiratory rate (RR), and patient satisfaction.
2. Materials and Methods
2.1. Participants
This prospective, randomized, controlled study was approved by the Institutional Review Board of Asan Medical Center (approval number: 2021-0714, 13 May 2021) and registered with the Clinical Research Information Service (registration number: KCT0006221, 17 May 2021). Written informed consent was obtained from all patients prior to enrollment. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Patients aged 20 to 80 years with an American Society of Anesthesiologists (ASA) Physical Status of I or II who were scheduled for ultrasound-guided percutaneous RFA under MAC between July and November 2021 were enrolled. The exclusion criteria included severe chronic pulmonary, cardiovascular, or cerebrovascular disease; a negative modified Allen test; and contraindications to remifentanil or propofol.
Eligible participants were randomized 1:1 to either the HFNC or facemask group using computer-generated block randomization (block size = 2) and sealed opaque envelopes. On the day of the procedure, an investigator (A) who was blinded to outcomes assigned the intervention. A separate investigator (B), who was also blinded, conducted all post-procedural assessments.
2.2. Anesthetic Management
All patients underwent continuous monitoring, including non-invasive blood pressure, electrocardiography, peripheral oxygen saturation (SpO2), bispectral index (BIS), and RR. RR data were automatically captured by the monitoring system using impedance pneumography and analyzed without further calculation. The monitored hemodynamic parameters were recorded at predefined timepoints (T0, T1, and T2): T0 was defined as the pre-procedural baseline before oxygen delivery; T1 was defined as the timepoint 5 min after the initiation of oxygen administration and sedation during spontaneous breathing; and T2 was defined as the timepoint at the end of the procedure. Following confirmation of collateral circulation with a modified Allen’s test, a 20-G radial arterial catheter was inserted for arterial blood sampling.
Baseline arterial blood gas analysis (ABGA; T0) was performed before oxygen delivery. In the facemask group, 100% oxygen was delivered at 6 L/min via a simple facemask, which approximates an FiO2 of 0.5. In the HFNC group, 100% heated and humidified oxygen was delivered at a flow rate of 30 L/min using an OptiFlow system (Fisher & Paykel®, Auckland, New Zealand).
Sedation was maintained with a target-controlled infusion of propofol (Marsh model, effect site concentration 0.8–1.5 μg/mL) and remifentanil (starting dose: 0.5 ng/mL, and titrated as needed). Regarding sedation depth, we targeted a BIS of 60–80 and a Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score ≤ 3 [15]. ABGA was repeated 5 min after oxygen delivery (T1). When the SpO2 decreased to <95%, a triple airway maneuver (head tilt, jaw thrust, and mouth opening) was applied, as oxygen reserves rapidly deplete at SpO2 levels < 94%, which confers the risk of a swift drop to <90% [16]. If the SpO2 further decreased to <90% despite intervention, an airway device was inserted and manual ventilation initiated. All patients were transferred to the post-anesthesia care unit (PACU) for post-procedural monitoring.
2.3. Ablation Procedure
After planning sonography, either percutaneous RFA or microwave ablation (MWA) was performed. Artificial ascites (500–1000 mL of 5% dextrose) were induced as needed to enhance ultrasonographic visualization by separating the liver from adjacent structures and to reduce the risk of thermal injury by providing a fluid barrier during ablation. The choice of ablation modality and electrode type (Cool-tip™, Jet-tip®, or Proteus®) was determined by the interventional radiologist. RFA was conducted using a 200 W generator (Mygen M-2004, RF Medical, Seoul, Republic of Korea) with impedance control for 8 to 18 min. For microwave ablation (MWA), a single 13 G antenna was used with a 150 W generator (Emprint™ HP, Covidien, Minneapolis, MN, USA) for 6–10 min. Additional time was provided for patients with multiple hepatic masses.
2.4. Outcome Measurements
The primary outcome of this study was the intra-procedural PaO2 that was measured at T1. The PaO2 provides a direct, objective measurement of alveolar oxygenation and gas exchange efficiency, and this makes it an appropriate primary outcome for evaluating the effectiveness of oxygen delivery during procedural sedation. Secondary outcomes included the incidence of hypoxia, the ∆P/F ratio and ∆PaCO2 between T0 and T1, changes in the RR, and patient satisfaction.
Hypoxia was defined as an SpO2 < 95% for procedural safety, and severe hypoxia was defined as an SpO2 < 90% despite airway maneuvers and the need for mask ventilation. Based on our institutional protocol to enable early recognition of airway compromise during deep sedation, the 95% threshold was selected. Unlike procedures performed in the lateral or semi-lateral position, percutaneous liver RFA is conducted in the supine position, which significantly increases the risk of glossoptosis. Furthermore, concurrent opioid administration due to severe procedural pain elevates the risk of respiratory depression. Therefore, a conservative SpO2 threshold was adopted to identify subtle ventilatory impairment at an early stage and enhance patient safety.
For the calculation of the P/F ratio, the FiO2 was assumed to be 1.0 in the HFNC group and 0.5 in the facemask group, based on estimated oxygen delivery via a simple facemask at 6 L/min. Patient satisfaction was assessed using a modified Iowa Satisfaction with Anesthesia Scale (ISAS) [17]. The ISAS comprises 11 items rated on a 6-point scale ranging from −3 to +3, with total scores ranging from −33 to +33. Higher scores indicate greater overall satisfaction. The scale has been validated in monitored anesthesia care and has been applied in procedural sedation settings [18].
2.5. Sample Size Calculation
The sample size was calculated using G*Power 3.1 (α = 0.05, power = 0.8), based on a previous study by Heinrich et al., which reported PaO2 values as medians (IQR): 406 (362–446) mmHg for the HFNC and 335 (292–389) mmHg for the facemask [19]. These were converted to means ± SD using Meta-Converter (https://meta-converter.com/conversions/mean-sd-iqr, accessed on 5 April 2021), yielding 405 ± 71.3 mmHg and 339 ± 82.3 mmHg, respectively. Based on this, a sample size of 23 per group was required; allowing for a 10% dropout, 26 participants per group (total n = 52) were enrolled.
2.6. Statistical Analysis
Continuous variables are presented as the mean ± standard deviation or the median with interquartile range, and categorical variables as frequencies and percentages. Between-group comparisons were performed using Student’s t-test or the Mann–Whitney U test for continuous data, and the chi-square test or Fisher’s exact test for categorical data, as appropriate.
To compare the intra-procedural PaO2 between the groups, an analysis of covariance (ANCOVA) was performed with the baseline PaO2 as a covariate. Similarly, the ΔP/F ratio was adjusted for pre-procedural values using ANCOVA. Linear regression analysis was used to evaluate changes in PaCO2, adjusting for potential confounders including age, body mass index (BMI), chronic obstructive pulmonary disease (COPD), asthma, and smoking status. Given the multiple secondary outcomes—including hypoxia incidence, ΔP/F ratio, ΔPaCO2, RR, and patient satisfaction—the Bonferroni correction was applied to control for type I errors. For repeated measurements of RR, a generalized linear mixed model (GLMM) with random intercepts was fitted. The model included group, time, and group × time interaction as fixed effects, and subject as a random effect. An unstructured covariance matrix was used without assuming homogeneity across groups. Model assumptions, including the normality of residuals and homogeneity of variance, were assessed for all parametric tests. When assumptions were not fully met, a ranked ANCOVA was performed as a non-parametric alternative to confirm the robustness of the results. As the findings were consistent, the original parametric results were retained for reporting. All statistical analyses were performed using SPSS Statistics version 29.0.2.0 (IBM Corp., Armonk, NY, USA), and a two-sided p-value < 0.05 was considered statistically significant.
3. Results
A total of 136 patients scheduled for RFA under MAC between July and November 2021 were screened. Of these, 40 did not meet the inclusion criteria, 39 declined to participate, and 5 were excluded due to changes in their therapeutic plan. Finally, 52 patients were randomized; however, 1 patient in the facemask group was excluded because of clotted blood samples. Therefore, 51 patients (25 and 26 in the facemask and HFNC groups, respectively) were included in the final analysis (Figure 1). The baseline characteristics were comparable between groups (Table 1), despite significantly different pre-procedure PaO2 levels (p = 0.037). Using ANCOVA with the pre-procedural PaO2 as a covariate, the adjusted mean PaO2 during the procedure was significantly higher in the HFNC group (298.8 ± 18.6 mmHg; 95% confidence interval [CI]: 261.4–336.2) compared to the facemask group (194.2 ± 19.0 mmHg; 95% CI: 156.0–232.3, p < 0.001; Figure 2A). Similarly, the adjusted mean ΔPaO2 (T1–T0) was significantly greater in the HFNC group (203.4 ± 18.6 vs. 98.8 ± 19.0; difference = 104.6, p < 0.001; Figure 2B).
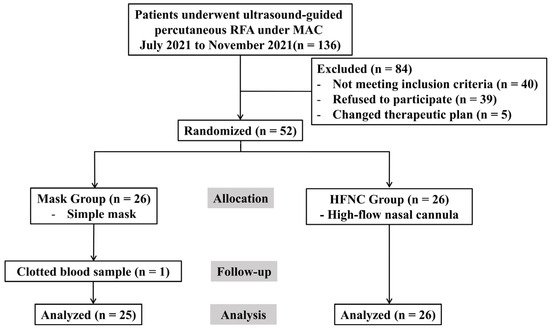
Figure 1.
A flow chart of the study inclusion process according to the CONSORT statement. RFA, radiofrequency ablation; MAC, monitored anesthesia care; HFNC, high-flow nasal cannula.

Table 1.
The baseline characteristics and clinical variables.
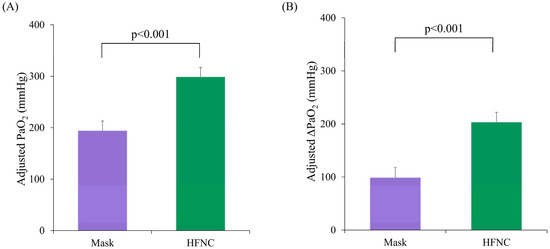
Figure 2.
A comparison of the arterial oxygen partial pressure (PaO2) between the facemask (Mask) and HFNC groups during liver radiofrequency ablation. (A) The intra-procedural adjusted mean PaO2 between the Mask and HFNC groups during the procedure, analyzed using analysis of covariance (ANCOVA) with the baseline PaO2 as a covariate. (B) The changes in PaO2 (ΔPaO2) from the baseline to intra-procedure between the two groups. The adjusted ΔPaO2 values were analyzed using ANCOVA. The error bars represent the standard error of the mean (SE). HFNC, high-flow nasal cannula.
Hypoxia occurred in 14 patients (27.5%), including 8 (32.0%) in the facemask group and 6 (23.0%) in the HFNC group (p = 0.475) (Figure 3). Severe hypoxia occurred more frequently in the facemask group (24.0% vs. 11.5%), corresponding to a risk ratio of 0.48 (95% CI: 0.13–1.72). However, the difference was not statistically significant (p = 0.291). Other ABGA parameters, including ΔP/F ratio and ΔPaCO2, did not differ significantly between groups (Table 2). Patient satisfaction, as assessed by the ISAS, was also similar between groups (Table 2).
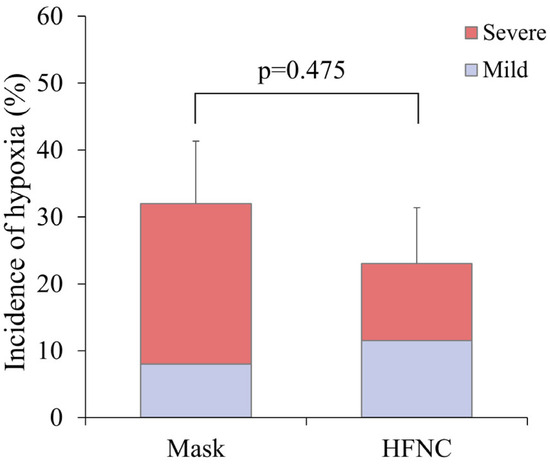
Figure 3.
A comparison of the incidence of hypoxia between the facemask (Mask) and HFNC groups during liver radiofrequency ablation. Hypoxia was categorized as mild (SpO2 < 95%) or severe (SpO2 < 90% despite triple airway maneuver). The stacked bar graph illustrates the proportion of mild and severe hypoxia in each group. While the incidence of severe hypoxia was higher in the Mask group, the overall incidence of hypoxia did not differ significantly between the two groups (p = 0.475).

Table 2.
A comparison of the adjusted outcomes and patient satisfaction between the Mask and HFNC groups.
At T0, the mean RR was 13.7 ± 2.4 and 14.7 ± 2.5 breaths/min in the HFNC and facemask groups, respectively (p = 0.131) (Table 3). At T1 and T2, the mean RR was significantly higher in the HFNC group (T1: 12.2 ± 3.0 vs. 9.8 ± 4.7 breaths/min, p = 0.036; T2: 14.2 ± 4.5 vs. 11.0 ± 4.9 breaths/min, p = 0.031). A generalized linear mixed model (GLMM) revealed a significant group × time interaction (p = 0.003) and a main effect of time (p = 0.024). Within-group analysis demonstrated significant RR reductions in the facemask group from T0 to T1 (p < 0.001) and from T0 to T2 (p = 0.002), whereas RR in the HFNC group remained stable (Table 3, Figure 4).

Table 3.
Time-course changes in respiratory rate (breath/min).
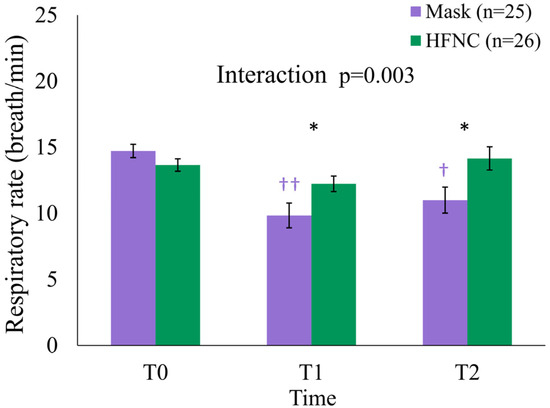
Figure 4.
The changes in respiratory rate (RR) over time in the facemask (Mask) and HFNC groups during liver radiofrequency ablation. The data are presented as the mean ± standard error. Significant group-by-time interaction was observed (p = 0.003, generalized linear mixed model (GLMM)). Between-group differences were significant at T1 and T2 (* p = 0.036 and * p = 0.031, respectively). Within-group analysis showed that, in the Mask group, RR decreased at T1 (†† p < 0.001) and T2 († p = 0.002) compared to T0, with no significant changes in the HFNC group. Symbols: * p < 0.05; † p < 0.05, †† p < 0.001 (within group). HFNC, high-flow nasal cannula; T0, pre-procedure; T1, intra-procedure; T2, post-procedure.
4. Discussion
This randomized controlled trial demonstrated that the HFNC significantly improved intra-procedural arterial oxygenation compared to a simple facemask during percutaneous liver RFA under MAC. The marked difference in ΔPaO2 highlights the dynamic effect of the HFNC in enhancing alveolar oxygenation beyond baseline levels. However, despite this physiological improvement, the incidence of hypoxia (SpO2 < 95%) did not differ significantly between groups, raising questions about the clinical translation of improved oxygenation.
The lack of difference in hypoxia incidence likely reflects the multifactorial nature of oxygen desaturation during deep sedation. Notably, our institutional protocol mandated predefined interventions when SpO2 dropped below 95%, which included a triple airway maneuver to rapidly restore oxygen saturation. These early responses may have mitigated desaturation severity and reduced between-group differences. In fact, most desaturation events resolved promptly without escalation to invasive support; only two patients required manual ventilation, and none required intubation. Taken together, these observations imply that upper airway obstruction, rather than inadequate oxygen delivery, may be a dominant cause of hypoxia during MAC. Glossoptosis induced by sedation, especially in the supine position with opioid co-administration, can precipitate airway collapse and compromise ventilation despite high FiO2 delivery [20,21]. This is consistent with previous observations that the HFNC improves oxygenation but may not prevent desaturation when upper airway patency is lost [6,7].
Although the HFNC theoretically offers continuous positive airway pressure (PEEP)-like effects through high flow rates, the flow setting of 30 L/min in our study was likely insufficient to generate clinically meaningful airway pressure. Prior studies indicate that flow rates ≥ 40–60 L/min are required to produce measurable PEEP and facilitate alveolar recruitment [22,23]. However, higher flows were not feasible during RFA due to patient discomfort and interference with procedural accuracy. Therefore, while this moderate flow setting reflects real-world procedural constraints, it may have limited the physiological potential of the HFNC. Still, the significant PaO2 improvement observed in the HFNC group suggests enhanced oxygenation even without optimal PEEP. Another consideration is the role of gas reabsorption atelectasis associated with high FiO2 delivery. Deep sedation and supine positioning predispose patients to alveolar collapse, and the administration of 100% oxygen via the HFNC may exacerbate this through nitrogen washout and resorption. This phenomenon may counteract the benefits of increased FiO2 and help explain the unchanged incidence of hypoxia [24].
Although the observed difference in severe hypoxia (11.5% in the HFNC group vs. 24.0% in the facemask group) corresponds to a risk ratio of 0.48, the wide 95% CI (0.13–1.72) reflects substantial uncertainty. While not statistically significant, the trend toward reduced severe hypoxia in the HFNC group may be clinically relevant and warrants further evaluation in larger, adequately powered studies.
These findings suggest that the physiological improvements observed with the HFNC may have potential clinical relevance in high-risk patients, such as those with impaired pulmonary function, obesity, or obstructive sleep apnea, where even modest improvements in oxygenation and respiratory stability could help prevent desaturation events and respiratory compromise. Further studies that focus on high-risk populations are needed to confirm the definitive impact of the HFNC on patient prognosis.
Moreover, the FiO2 delivered via the HFNC is theoretically fixed but may be diluted by ambient air due to mouth breathing or irregular respiratory patterns, particularly under sedation. Studies have shown that FiO2 dilution increases with open-mouth breathing and high inspiratory flow demands, potentially limiting effective oxygen delivery [22,25]. In our study, although we assumed FiO2 values of 1.0 (HFNC) and 0.5 (facemask) for P/F ratio calculations, these estimates are subject to variability and should be interpreted cautiously.
Interestingly, RR remained more stable in the HFNC group throughout the procedure. This contrasts with the facemask group, which exhibited a reduction in RR that persisted post-procedurally. A stable RR is clinically meaningful, as irregular or suppressed respiratory patterns may lead to hypoventilation and hypoxemia. While sedation level and opioid dosing were equivalent across groups, the observed difference may reflect the physiological benefits of the HFNC, such as reduced inspiratory workload and improved comfort [26,27,28].
Despite this, we observed no significant difference in arterial PaCO2 levels between groups. This aligns with previous reports in settings such as bronchoscopy, thoracic surgery, and COPD care, where the HFNC had minimal impact on PaCO2 due to its limited effect on minute ventilation [14,16,25,29,30,31,32]. While the HFNC can flush out nasopharyngeal dead space and aid CO2 elimination, deep sedation-induced hypoventilation likely blunts its effectiveness in this context [26,31]. Patient satisfaction, assessed using the validated ISAS, was similarly high in both groups, suggesting that the method of oxygen delivery did not negatively affect patient experience under deep sedation. The lack of difference in satisfaction may be explained by several factors. Although previous studies have demonstrated improved comfort with the HFNC compared to non-invasive ventilation or other oxygen delivery methods [33,34], the large-bore nasal cannula may have caused mild discomfort in our patients. Moreover, the relatively short procedural duration and the use of deep sedation likely reduced patients’ awareness of external stimuli, making it difficult for them to perceive differences between oxygen delivery methods. These factors, in combination, may have contributed to the absence of a measurable difference in satisfaction between the groups. Future studies may explore the potential impact of the HFNC on procedural success and workflow efficiency, such as reducing airway interventions or procedural interruptions. In addition, cost effectiveness analyses that consider the prevention of post-procedural complications and recovery-related healthcare costs may further support the practical value of the HFNC in clinical practice.
This study has several limitations. First, the small sample size was powered only for the primary outcome (PaO2) and not for secondary outcomes such as hypoxia, ΔP/F ratio, ΔPaCO2, or satisfaction. A post hoc power analysis indicated that the statistical power for these variables was insufficient (e.g., 11.1% for hypoxia, 5.1% for ΔPaCO2), introducing the possibility of a type II error and limiting definitive conclusions. Further multicenter studies with larger sample sizes are warranted to validate these findings and to optimize clinical protocols. Second, ABGA was collected at a fixed intra-procedural timepoint rather than during actual desaturation events. This limits its utility in assessing real-time respiratory compromise and weakens its correlation with SpO2-based hypoxia. Future studies should consider transcutaneous gas monitoring or real-time ABG sampling during desaturation episodes to better capture dynamic gas exchange. Lastly, although we assumed an FiO2 of 0.5 for the facemask and 1.0 for the HFNC group based on conventional estimates, interindividual variability in FiO2 delivery remains a limitation. Our study aimed to evaluate whether the HFNC, which generally provides higher and more consistent FiO2, improves oxygenation compared to the standard oxygen therapy commonly used during deep sedation. However, given the practical limitations of HFNC application during liver RFA, future studies comparing the HFNC with other high-flow oxygen delivery methods, such as non-rebreather masks, may help to identify more effective alternatives.
Despite these limitations, this study addresses oxygenation strategies during sedation with combined sedatives and opioids for painful therapeutic procedures such as liver RFA. The deeper sedation level and supine positioning increase the risk of upper airway obstruction due to glossoptosis and hypoventilation, which heighten the risk of hypoxemia. These high-risk conditions have been less extensively examined in prior studies on oxygenation during procedural sedation. Our findings suggest that although the HFNC improves oxygenation, careful selection of flow rates is needed to balance oxygenation and procedural feasibility in this setting.
5. Conclusions
In conclusion, the HFNC improved intra-procedural arterial oxygenation and stabilized RR in patients undergoing liver RFA under MAC, despite the lack of observed differences in clinical endpoints such as hypoxia incidence or patient satisfaction. These findings support the physiological utility of the HFNC in this setting and highlight the need for further studies examining optimized flow rates, individualized FiO2 delivery, and patient subgroups at higher risk of desaturation.
Author Contributions
Conceptualization, K.-w.J. and J.-P.Y.; methodology, K.-w.J.; software, H.P.; validation, J.-U.Y. and H.P.; formal analysis, K.-w.J. and J.-P.Y.; investigation, J.-P.Y. and G.W.K.; resources, J.-U.Y.; data curation, G.W.K.; writing—original draft preparation, J.-P.Y.; writing—review and editing, K.-w.J.; visualization, K.-w.J. and J.-P.Y.; supervision, K.-w.J.; project administration, J.-P.Y.; funding acquisition, J.-P.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the 2024 research grant from Pusan National University Yangsan Hospital. No external funding was received.
Institutional Review Board Statement
This prospective, randomized, controlled study was approved by the Institutional Review Board of Asan Medical Center (approval number: 2021-0714, 13 May 2021) and registered at the Clinical Research Information Service (registration number: KCT0006221, 17 May 2021). Written informed consent was obtained from all patients prior to enrollment. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank ACE Statistical Consulting for their assistance with the statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RFA | Radiofrequency ablation |
| MAC | Monitored anesthesia care |
| HFNC | High-flow nasal cannula |
| FiO2 | Fraction of inspired oxygen |
| PEEP | Positive end-expiratory pressure |
| SpO2 | Peripheral oxygen saturation |
| PaO2 | Arterial oxygen partial pressure |
| PaCO2 | Arterial partial pressure of carbon dioxide |
| RR | Respiratory rate |
| BIS | Bispectral index |
| ABGA | Arterial blood gas analysis |
| P/F | Ratio of arterial partial pressure of oxygen to fraction of inspired oxygen |
| ISAS | Iowa Satisfaction with Anesthesia Scale |
| ANCOVA | Analysis of covariance |
| COPD | Chronic obstructive pulmonary disease |
| CI | Confidence interval |
References
- Tatli, S.; Tapan, U.; Morrison, P.R.; Silverman, S.G. Radiofrequency ablation: Technique and clinical applications. Diagn. Interv. Radiol. 2012, 18, 508–516. [Google Scholar] [CrossRef] [PubMed]
- McGhana, J.P.; Dodd, G.D., 3rd. Radiofrequency ablation of the liver: Current status. Am. J. Roentgenol. 2001, 176, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, D.; Kim, D.K.; Chung, I.S.; Bang, Y.J.; Kim, K.; Kim, M.; Choi, J.W. Comparison of respiratory effects between dexmedetomidine and propofol sedation for ultrasound-guided radiofrequency ablation of hepatic neoplasm: A randomized controlled trial. J. Clin. Med. 2021, 10, 3040. [Google Scholar] [CrossRef]
- Sato, K.; Taniki, N.; Kanazawa, R.; Shimizu, M.; Ishii, S.; Ohama, H.; Takawa, M.; Nagamatsu, H.; Imai, Y.; Shiina, S. Efficacy and safety of deep sedation in percutaneous radiofrequency ablation for hepatocellular carcinoma. Adv. Ther. 2019, 36, 344–354. [Google Scholar] [CrossRef]
- Ghisi, D.; Fanelli, A.; Tosi, M.; Nuzzi, M.; Fanelli, G. Monitored anesthesia care. Minerva Anestesiol. 2005, 71, 533–538. [Google Scholar]
- Kimura, S.; Sone, M.; Sugawara, S.; Itou, C.; Ozawa, M.; Sato, T.; Matsui, Y.; Arai, Y.; Kusumoto, M. Safety of propofol sedation administered by interventional radiologists for radiofrequency ablation in patients with hepatocellular carcinoma. Jpn. J. Radiol. 2024, 42, 1290–1297. [Google Scholar] [CrossRef]
- Bhananker, S.M.; Posner, K.L.; Cheney, F.W.; Caplan, R.A.; Lee, L.A.; Domino, K.B. Injury and liability associated with monitored anesthesia care: A closed claims analysis. Anesthesiology 2006, 104, 228–234. [Google Scholar] [CrossRef]
- Sharma, V.K.; Nguyen, C.C.; Crowell, M.D.; Lieberman, D.A.; de Garmo, P.; Fleischer, D.E. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest. Endosc. 2007, 66, 27–34. [Google Scholar] [CrossRef]
- Arrowsmith, J.B.; Gerstman, B.B.; Fleischer, D.E.; Benjamin, S.B. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest. Endosc. 1991, 37, 421–427. [Google Scholar] [CrossRef]
- Spoletini, G.; Alotaibi, M.; Blasi, F.; Hill, N.S. Heated humidified high-flow nasal oxygen in adults: Mechanisms of action and clinical implications. Chest 2015, 148, 253–261. [Google Scholar] [CrossRef]
- Ghoshal, A.G. Hypoxemia and oxygen therapy. J. Assoc. Chest Physicians 2020, 8, 42–47. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, X.; Li, L.; Wei, M.; Zhao, B.; Wang, X.; Pan, Z.; Tian, J.; Yu, W.; Su, D. High-flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: A randomized multicenter clinical trial. Gastrointest. Endosc. 2019, 90, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Renda, T.; Corrado, A.; Iskandar, G.; Pelaia, G.; Abdalla, K.; Navalesi, P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br. J. Anaesth. 2018, 120, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Douglas, N.; Ng, I.; Nazeem, F.; Lee, K.; Mezzavia, P.; Krieser, R.; Steinfort, D.; Irving, L.; Segal, R. A randomised controlled trial comparing high-flow nasal oxygen with standard management for conscious sedation during bronchoscopy. Anaesthesia 2018, 73, 169–176. [Google Scholar] [CrossRef]
- Sheahan, C.G.; Mathews, D.M. Monitoring and delivery of sedation. Br. J. Anaesth. 2014, 113 (Suppl. S2), ii37–ii47. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, C.Y.; Lee, K.; Kim, N.; Lee, S.; Oh, Y.J. Comparison of high-flow nasal oxygenation and standard low-flow nasal oxygenation during rigid bronchoscopy: A randomized controlled trial. Korean J. Anesthesiol. 2025, 78, 39–47. [Google Scholar] [CrossRef]
- Dexter, F. Iowa Satisfaction with Anesthesia Scale. Korean J. Anesthesiol. 2012, 62, 297. [Google Scholar] [CrossRef]
- Sohn, H.M.; Ryu, J.H. Monitored anesthesia care in and outside the operating room. Korean J. Anesthesiol. 2016, 69, 319–326. [Google Scholar] [CrossRef]
- Heinrich, S.; Horbach, T.; Stubner, B.; Prottengeier, J.; Irouschek, A.; Schmidt, J. Benefits of heated and humidified high flow nasal oxygen for preoxygenation in morbidly obese patients undergoing bariatric surgery: A randomized controlled study. J. Obes. Bariatrics 2014, 1, 7. [Google Scholar]
- Hillman, D.R.; Platt, P.R.; Eastwood, P.R. The upper airway during anaesthesia. Br. J. Anaesth. 2003, 91, 31–39. [Google Scholar] [CrossRef]
- Nishimura, M. High-flow nasal cannula oxygen therapy in adults: Physiological benefits, indication, clinical benefits, and adverse effects. Respir. Care 2016, 61, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Parke, R.L.; Eccleston, M.L.; McGuinness, S.P. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir. Care 2011, 56, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Lucangelo, U.; Vassallo, F.G.; Marras, E.; Ferluga, M.; Beziza, E.; Comuzzi, L.; Berlot, G.; Zin, W.A. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit. Care Res. Pract. 2012, 2012, 506382. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Liang, P.C.; Chuang, Y.H.; Huang, Y.J.; Lin, P.J.; Wu, C.Y. Effects of high-flow nasal oxygen during prolonged deep sedation on postprocedural atelectasis: A randomised controlled trial. Eur. J. Anaesthesiol. 2020, 37, 1025–1031. [Google Scholar] [CrossRef]
- Yang, H.; Huang, D.; Luo, J.; Liang, Z.; Li, J. The use of high-flow nasal cannula in patients with chronic obstructive pulmonary disease under exacerbation and stable phases: A systematic review and meta-analysis. Heart Lung 2023, 60, 116–126. [Google Scholar] [CrossRef]
- Mündel, T.; Feng, S.; Tatkov, S.; Schneider, H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J. Appl. Physiol. 2013, 114, 1058–1065. [Google Scholar] [CrossRef]
- Rittayamai, N.; Tscheikuna, J.; Rujiwit, P. High-flow nasal cannula versus conventional oxygen therapy after endotracheal extubation: A randomized crossover physiologic study. Respir. Care 2014, 59, 485–490. [Google Scholar] [CrossRef]
- Mauri, T.; Turrini, C.; Eronia, N.; Grasselli, G.; Volta, C.A.; Bellani, G.; Pesenti, A. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am. J. Respir. Crit. Care Med. 2017, 195, 1207–1215. [Google Scholar] [CrossRef]
- Wang, M.L.; Hung, M.H.; Chen, J.S.; Hsu, H.H.; Cheng, Y.J. Nasal high-flow oxygen therapy improves arterial oxygenation during one-lung ventilation in non-intubated thoracoscopic surgery. Eur. J. Cardiothorac. Surg. 2018, 53, 1001–1006. [Google Scholar] [CrossRef]
- Huang, X.; Du, Y.; Ma, Z.; Zhang, H.; Jun, L.; Wang, Z.; Lin, M.; Ni, F.; Li, X.; Tan, H.; et al. High-flow nasal cannula oxygen versus conventional oxygen for hypercapnic chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. Clin. Respir. J. 2021, 15, 437–444. [Google Scholar] [CrossRef]
- Duan, L.; Xie, C.; Zhao, N. Effect of high-flow nasal cannula oxygen therapy in patients with chronic obstructive pulmonary disease: A meta-analysis. J. Clin. Nurs. 2022, 31, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khanna, P.; Chowdhury, S.R.; Haritha, D.; Sarkar, S. The impact of high-flow nasal cannula vs other oxygen delivery devices during bronchoscopy under sedation: A systematic review and meta-analyses. Indian. J. Crit. Care Med. 2022, 26, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Roca, O.; Hernández, G.; Díaz-Lobato, S.; Carratalá, J.M.; Gutiérrez, R.M.; Masclans, J.R.; Spanish Multidisciplinary Group of High Flow Supportive Therapy in Adults (HiSpaFlow). Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit. Care 2016, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Som, A.; Bhattacharjee, S.; Arora, M.K.; Baidya, D.K. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: A meta-analysis and systematic review. J. Crit. Care 2016, 35, 138–144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).