Comparison of Ultrasound Versus Ultrasound with Nerve Stimulator-Guided Infraclavicular Block Anesthesia Methods in Pediatric Patients
Abstract
1. Introduction
2. Material and Method
2.1. Study Population
2.2. Sample Size
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, A.; Colen, D.L.; Fox, J.P.; Chang, B.; Lin, I.C. Pediatric hand and upper extremity injuries presenting to emergency departments in the United States: Epidemiology and health care–associated costs. Hand 2019, 16, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Schaldenbrand, K.; Wallis, B.; De Oliveira, G.S., Jr. Regional anesthesia to improve pain outcomes in pediatric surgical patients: A qualitative systematic review of randomized controlled trials. Br. J. Anaesth. 2014, 113, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, Y.; Liu, S.; Cao, Y. Minimum effective volume of 0.2% ropivacaine for ultrasound-guided axillary brachial plexus block in preschool-age children. Sci. Rep. 2021, 11, 17002. [Google Scholar] [CrossRef] [PubMed]
- Marhofer, P.; Sitwohl, C.; Greher, M.; Kapral, S. Ultrasound guidance for infraclavicular brachial plexus anesthesia in children. Anaesthesia 2004, 59, 642–646. [Google Scholar] [CrossRef]
- Pušnik, L.; Radochová, B.; Janáček, J.; Saudek, F.; Serša, I.; Cvetko, E.; Umek, N.; Snoj, Ž. Fascicle differentiation of upper extremity nerves on high-resolution ultrasound with multimodal microscopic verification. Sci. Rep. 2025, 15, 557. [Google Scholar] [CrossRef]
- Reysner, T.; Wieczorowska-Tobis, K.; Mularski, A.; Kowalski, G.; Daroszewski, P.; Reysner, M. Revolutionizing pediatric surgery: The transformative role of regional anesthesia—A narrative review. Surgeries 2025, 6, 9. [Google Scholar] [CrossRef]
- Altınay, M.; Türk, H.Ş.; Ediz, N.; Talmac, M.A.; Oba, S. Our ultrasound guided brachial plexus block experiences for upper extremity surgeries in pediatric patients. Med. Bull. Sisli Etfal Hosp. 2020, 54, 231–235. [Google Scholar] [CrossRef]
- Amiri, H.R.; Espandar, R. Upper extremity surgery in younger children under ultrasound-guided supraclavicular brachial plexus block: A case series. J. Child Orthop. 2011, 5, 5–9. [Google Scholar] [CrossRef]
- İnce, İ.; Aksoy, M.; Dostbil, A.; Tuncer, K. Can we use a lower volume of local anesthetic for infraclavicular brachial plexus nerve block under ultrasound guidance in children? J. Clin. Anesth. 2017, 41, 132–136. [Google Scholar] [CrossRef]
- Bosenberg, A. Regional anesthesia in children: An update. S. Afr. J. Anaesth. Analg. 2013, 19, 282–288. [Google Scholar] [CrossRef]
- De Windt, A.C.; Asehnoune, K.; Roquilly, A.; Guillaud, C.; Le Roux, C.; Pinaud, M.; Lejus, C. An opioid-free anesthetic using nerve blocks enhances rapid recovery after minor hand surgery in children. Eur. J. Anaesthesiol. 2010, 27, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Splinter, W.M.; Thomson, M.E. Somatic paravertebral block decreases opioid requirements in children undergoing an appendectomy. Can. J. Anesth. 2010, 57, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Zouheir, M.N.; Martin, R.; Mariam, E.R.; Fouad, M.Z.; Mohamad, A.A.T.; Per, A.L. Nerve stimulator-guided paravertebral blockade combined with sevoflurane sedation versus general anesthesia with systemic analgesia for post herniorrhaphy pain relief in children: A prospective randomized trial. Anesthesiology 2005, 103, 600–605. [Google Scholar]
- Chiono, J.; Raux, O.; Briguier, S. Bilateral suprazygomatic maxillary nerve block for cleft palate repair in children: A prospective, randomized, double-masked study versus placebo. Anesthesiology 2014, 120, 1362–1369. [Google Scholar] [CrossRef]
- Walker, B.J.; Long, J.; Madhankumar, S. Complications in pediatric regional anesthesia: An analysis of more than 100,000 blocks from the Pediatric Regional Anaesthesia Network. Anesthesiology 2018, 129, 721–732. [Google Scholar] [CrossRef]
- Allison, C.E.; Aronson, D.C.; Geukers, V.G.; Van Den Berg, R.; Schlack, W.S.; Hollmann, M.W. Paraplegia after thoracotomy under combined general and epidural anesthesia in a child. Paediatr. Anaesth. 2008, 18, 539–542. [Google Scholar] [CrossRef]
- Ponde, V. Recent trends in paediatric regional anaesthesia. Indian J. Anaesth. 2019, 63, 746–753. [Google Scholar] [CrossRef]
- Sandhu, N.S.; Capan, L.M. Ultrasound-guided infraclavicular brachial plexus block. Br. J. Anaesth. 2002, 89, 254–259. [Google Scholar] [CrossRef]
- Kokki, H.; Ylönen, P.; Laisalmi, M.; Heikkinen, M.; Reinikainen, M. Isobaric ropivacaine 5 mg/ml for spinal anesthesia in children. Anesth. Analg. 2005, 100, 66–70. [Google Scholar] [CrossRef]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, H.; Degenhardt, E. Guidelines on the management of postoperative pain. J. Pain 2016, 7, 131–157. [Google Scholar] [CrossRef]
- Savarese, J.J.; Tabler, N.G., Jr. Multimodal analgesia as an alternative to the risks of opioid monotherapy in surgical pain management. J. Healthc. Risk Manag. 2017, 37, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Elvir, O.R.; White, P.F. The role of multimodal analgesia in pain management after ambulatory surgery. Curr. Opin. Anaesth. 2010, 23, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Fettiplace, M.; Joudeh, L.; Bungart, B.; Boretsky, K. Local anesthetic dosing and toxicity of pediatric truncal catheters: A narrative review of published practice. Reg. Anesth. Pain Med. 2023, 49, 59–66. [Google Scholar] [CrossRef] [PubMed]

| US | US + NS | p | ||
|---|---|---|---|---|
| Age | 0.774 | |||
| Mean ± SD | 9.29 ± 3.41 | 9.16 ± 3.42 | ||
| Min–Max (Median) | 2–15 (9.5) | 2–15 (9.5) | ||
| 95% CI | 8.68, 9.90 | 8.55, 9.77 | ||
| Gender | Male n (%) | 82 (68.3) | 81 (67.5) | 0.890 |
| Female n (%) | 38 (31.7) | 39 (32.5) | ||
| ASA | No Comorbidity n (%) | 115 (95.8) | 117 (97.5) | 0.722 |
| Comorbidity n (%) | 5 (4.2) | 3 (2.5) | ||
| Height | 0.917 | |||
| Mean ± SD | 137.3 ± 20.5 | 136.4 ± 20.4 | ||
| Min–Max (Median) | 85–177 (138) | 86–172 (139) | ||
| 95% CI | 133.63, 140.97 | 132.75, 140.05 | ||
| Weight | 0.774 | |||
| Mean ± SD | 36.2 ± 14.6 | 36.5 ± 14.5 | ||
| Min–Max (Median) | 10–63 (33.5) | 10–60 (36) | ||
| 95% CI | 33.59, 38.81 | 33.91, 39.09 | ||
| BMI | 0.306 | |||
| Mean ± SD | 18.1 ± 2.8 | 18,5 ± 3.0 | ||
| Min–Max (Median) | 12.7–25.1 (18) | 12.9–24 (18.4) | ||
| 95% CI | 17.60, 18.60 | 17.96, 19.04 | ||
| US | US + NS | p | ||
|---|---|---|---|---|
| Surgical Procedure Area | Upper Arm (%) | 35 (29.2) | 36 (30.0) | 0.769 |
| Forearm n (%) | 65 (54.2) | 68 (56.7) | ||
| Hand n (%) | 20 (16.6) | 16 (13.3) | ||
| Sedation | Light (midazolam only) n (%) | 72 (60.6) | 68 (56.7) | 0.784 |
| Deep (midazolam + ketamine) n (%) | 48 (39.4) | 52 (43.3) | ||
| Processing Time (min) | <0.001 * | |||
| Mean ± SD | 6.1 ± 0.8 | 8.31 ± 0.82 | ||
| Min–Max (Median) | 5–8 (6) | 7–10 (8) | ||
| 95% CI | 5.96, 6.24 | 8.16, 8.46 | ||
| Surgery Time (min) | 0.732 | |||
| Mean ± SD | 62.4 ± 11.3 | 62.4 ± 9.5 | ||
| Min–Max (Median) | 30–82 (66) | 34–76 (66) | ||
| 95% CI | 60.38, 64.42 | 60.70, 64.10 | ||
| US | US + NS | p | |
|---|---|---|---|
| FLACC n | 42 | 44 | |
| Mean ± SD | 2.36 ± 0.87 | 2.30 ± 0.85 | 0.892 |
| Min–Max (Median) | 2–7 (3) | 2–7 (3) | 0.837 |
| 95% CI | 2.22, 2.46 | 2.23, 2.51 | |
| VAS n | 78 | 76 | |
| Mean ± SD | 2.21 ± 0.83 | 2.18 ± 0.76 | 0.892 |
| Min–Max (Median) | 1–7 (3) | 1–7 (3) | 0.959 |
| 95% CI | 2.11, 2.32 | 2.09, 2.27 |
| US | US + NS | p | |
|---|---|---|---|
| FLACC 1st hr | 0.559 | ||
| Mean ± SD | 1.10 ± 0.30 | 1.14 ± 0.35 | |
| Min–Max (Median) | 1–2 (1) | 1–2 (1) | |
| 95% CI | 1.05, 1.15 | 1.08, 1.20 | |
| FLACC 3rd hr | 0.845 | ||
| Mean ± SD | 1.17 ± 0.44 | 1.18 ± 0.45 | |
| Min–Max (Median) | 1–3 (1) | 1–3 (1) | |
| 95% CI | 1.09, 1.25 | 1.10, 1.26 | |
| FLACC 6th hr | 0.958 | ||
| Mean ± SD | 1.43 ± 0.94 | 1.45 ± 1.00 | |
| Min–Max (Median) | 1–5 (1) | 1–5 (1) | |
| 95% CI | 1.26, 1.60 | 1.27, 1.63 | |
| FLACC 9th hr | 0.704 | ||
| Mean ± SD | 3.12 ± 1.19 | 2.98 ± 1.07 | |
| Min–Max (Median) | 1–6 (3) | 1–5 (3) | |
| 95% CI | 2.91, 3.33 | 2.79, 3.17 | |
| FLACC 12th hr | 0.467 | ||
| Mean ± SD | 3.17 ± 1.29 | 3.32 ± 1.20 | |
| Min–Max (Median) | 1–7 (3) | 2–7 (3) | |
| 95% CI | 2.94, 3.40 | 3.11, 3.53 | |
| FLACC 15th hr | 0.469 | ||
| Mean ± SD | 3.33 ± 1.28 | 3.55 ± 1.30 | |
| Min–Max (Median) | 1–6 (3) | 2–7 (3) | |
| 95% CI | 3.10, 3.56 | 3.32, 3.78 | |
| FLACC 24th hr | 1.000 | ||
| Mean ± SD | 2.52 ± 0.67 | 2.48 ± 0.59 | |
| Min–Max (Median) | 2–5 (2) | 1–4 (2) | |
| 95% CI | 2.40, 2.64 | 2.37, 2.59 |
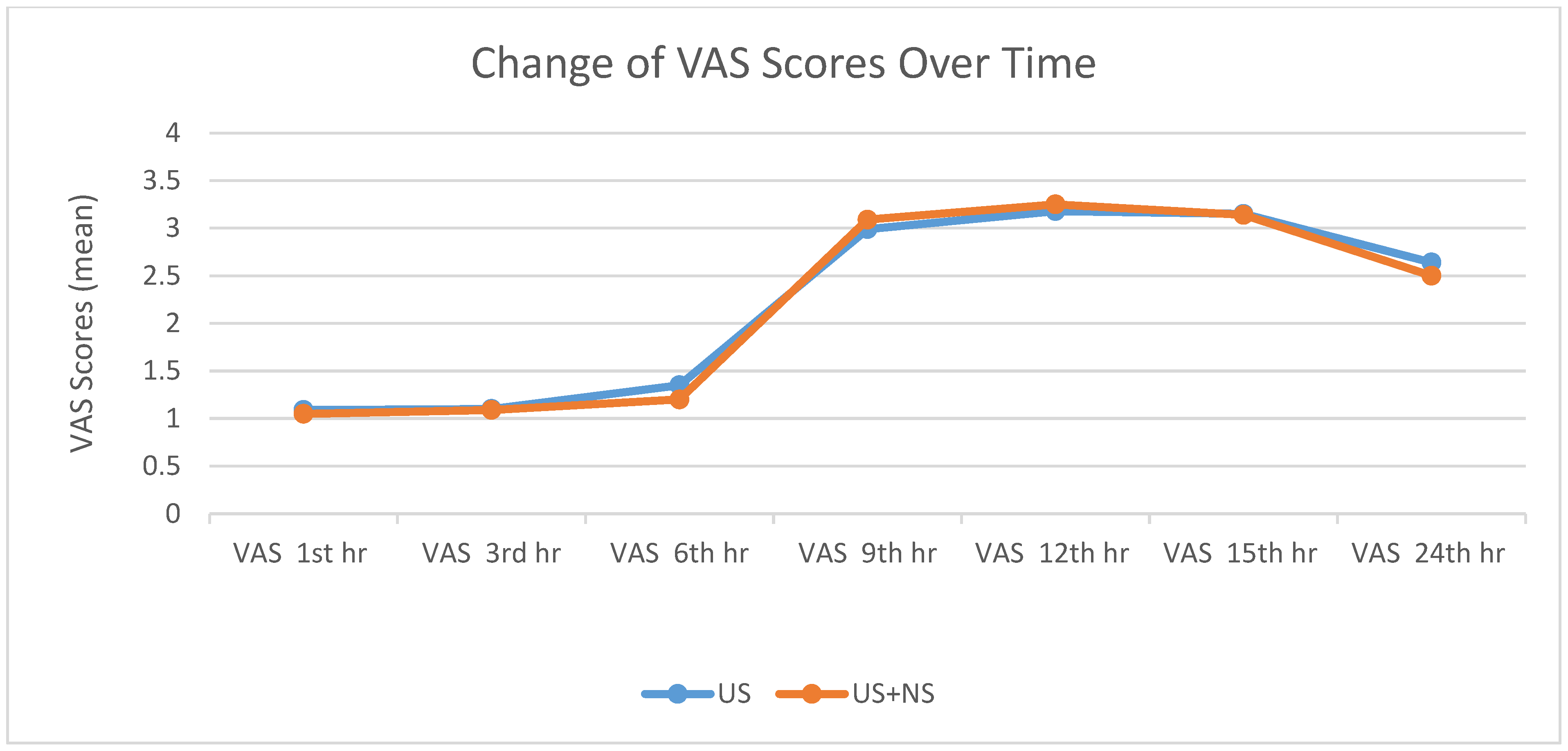
| US | US + NS | p | |
|---|---|---|---|
| VAS 1st hr | 0.375 | ||
| Mean ± SD | 1.09 ± 0.29 | 1.05 ± 0.22 | |
| Min–Max (Median) | 1–2 (1) | 1–2 (1) | |
| 95% CI | 1.04, 1.14 | 1.01, 1.09 | |
| VAS 3rd hr | 0.830 | ||
| Mean ± SD | 1.10 ± 0.31 | 1.09 ± 0.29 | |
| Min–Max (Median) | 1–2 (1) | 1–2 (1) | |
| 95% CI | 1.04, 1.16 | 1.04, 1.14 | |
| VAS 6th hr | 0.560 | ||
| Mean ± SD | 1.35 ± 0.77 | 1.20 ± 0.43 | |
| Min–Max (Median) | 1–4 (1) | 1–3 (1) | |
| 95% CI | 1.21, 1.49 | 1.12, 1.28 | |
| VAS 9th hr | 0.569 | ||
| Mean ± SD | 2.99 ± 1.26 | 3.09 ± 1.20 | |
| Min–Max (Median) | 1–6 (3) | 1–6 (3) | |
| 95% CI | 2.76, 3.22 | 2.88, 3.30 | |
| VAS 12th hr | 0.713 | ||
| Mean ± SD | 3.18 ± 1.29 | 3.25 ± 1.28 | |
| Min–Max (Median) | 1–7 (3) | 2–7 (3) | |
| 95% CI | 2.95, 3.41 | 3.02, 3.48 | |
| VAS 15th hr | 0.861 | ||
| Mean ± SD | 3.15 ± 1.21 | 3.14 ± 1.15 | |
| Min–Max (Median) | 1–5 (3) | 1–5 (3) | |
| 95% CI | 2.93, 3.37 | 2.93, 3.35 | |
| VAS 24th hr | 0.176 | ||
| Mean ± SD | 2.64 ± 0.66 | 2.50 ± 0.60 | |
| Min–Max (Median) | 1–5 (3) | 1–5 (2) | |
| 95% CI | 2.52, 2.76 | 2.39, 2.61 |
| US | US + NS | p | ||
|---|---|---|---|---|
| MBD | 0.460 | |||
| Mean ± SD | 6.20 ± 0.95 | 6.29 ± 0.88 | ||
| Min–Max (Median) | 3–8 (6) | 3–8 (6) | ||
| 95% CI | 6.03, 6.37 | 6.13, 6.45 | ||
| SBD | 0.381 | |||
| Mean ± SD | 9.38 ± 2.13 | 9.53 ± 2.05 | ||
| Min–Max (Median) | 5–15 (9) | 5–16 (9) | ||
| 95% CI | 9.00, 9.76 | 9.16, 9.90 | ||
| Intraoperative and postoperative patients given additional opioids n (%) | 0 (0) | 0 (0) | −1 | |
| Intraoperative and postoperative patients without additional opioids n (%) | 120 (100) | 120 (100) | 1 | |
| Post Operative Non-Opioid Analgesia | not given n (%) | 6 (5.0) | 8 (6.7) | 0.582 |
| given n (%) | 114 (95.0) | 112 (93.3) | ||
| Time of first postoperative analgesic administration | 0.100 | |||
| Mean ± SD | 9.38 ± 2.13 | 9.68 ± 2.06 | ||
| Min–Max (Median) | 5–15 (9) | 5–16 (9) | ||
| 95% CI | 9.00, 9.76 | 9.31, 10.05 | ||
| Total number of analgesics given in the first 24 h postoperatively | 0.819 | |||
| Mean ± SD | 1.89 ± 0.59 | 1.88 ± 0.60 | ||
| Min–Max (Median) | 1–3 (2) | 1–3 (2) | ||
| 95% CI | 1.78, 2.00 | 1.77, 1.99 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şengel, A.; Büyükfirat, E.; Seçilmiş, S.; Altay, N.; Atlas, A.; Şengül, A. Comparison of Ultrasound Versus Ultrasound with Nerve Stimulator-Guided Infraclavicular Block Anesthesia Methods in Pediatric Patients. Medicina 2025, 61, 985. https://doi.org/10.3390/medicina61060985
Şengel A, Büyükfirat E, Seçilmiş S, Altay N, Atlas A, Şengül A. Comparison of Ultrasound Versus Ultrasound with Nerve Stimulator-Guided Infraclavicular Block Anesthesia Methods in Pediatric Patients. Medicina. 2025; 61(6):985. https://doi.org/10.3390/medicina61060985
Chicago/Turabian StyleŞengel, Abdulhakim, Evren Büyükfirat, Selçuk Seçilmiş, Nuray Altay, Ahmet Atlas, and Abdullah Şengül. 2025. "Comparison of Ultrasound Versus Ultrasound with Nerve Stimulator-Guided Infraclavicular Block Anesthesia Methods in Pediatric Patients" Medicina 61, no. 6: 985. https://doi.org/10.3390/medicina61060985
APA StyleŞengel, A., Büyükfirat, E., Seçilmiş, S., Altay, N., Atlas, A., & Şengül, A. (2025). Comparison of Ultrasound Versus Ultrasound with Nerve Stimulator-Guided Infraclavicular Block Anesthesia Methods in Pediatric Patients. Medicina, 61(6), 985. https://doi.org/10.3390/medicina61060985








