Characteristics of Physical Exercise Programs and Their Effects on Quality of Life and Functional Capacity in Individuals with Chronic Obstructive Pulmonary Disease: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
3. Results
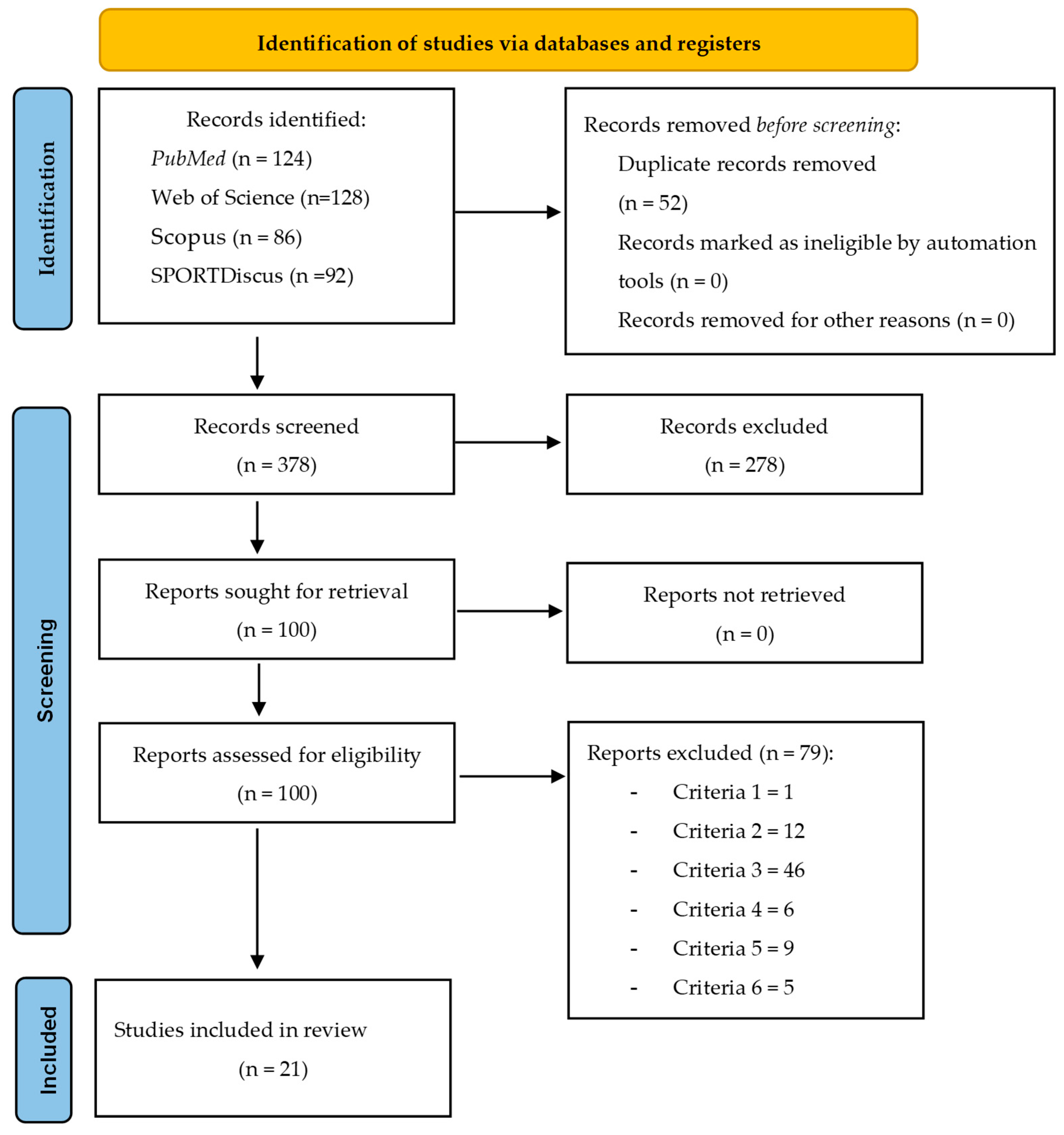
3.1. Selection of Sources of Evidence
3.2. Characteristics of Sources of Evidence
3.3. Risk of Bias Across Studies
3.4. Intervention Characteristics
| Study | Aims | Participants/Age/Sex | Measures or Tests of the Study |
|---|---|---|---|
| Amin et al. [31] | To test the feasibility of a community-based exercise program and its effect on individuals with moderate COPD. | N: 19 (CG: 10; EG: 9) Age: CG: 72.0 ± 10.1|EG: 66.8 ± 8.1 Sex (M/F): CG: 6/4|EG: 6/3 | Endurance time, SGRQ, body mass, body fat, Baseline Dyspnea Index, and total weight lifted. |
| Averna et al. [23] | To analyze the effects of a moderate-intensity exercise program in individuals with mild to moderate COPD. | N: 76 (EG: 56; CG: 20) Age: EG: 69 ± 5|CG: 71 ± 5 Sex (M/F): EG: 29/27|CG: 12/8 | Pulmonary function measures (FVC, FEV1, FEV1/FVC), systolic/diastolic blood pressure, heart rate, oxygen saturation, workload symptoms, and SGRQ. |
| Bertolino et al. [29] | To evaluate the effects of a home-based resistance exercise program with elastic tubing after supervised resistance training on peripheral muscle strength and quality of life in individuals with mild to severe COPD. | N: 16 (EG: 7; CG: 9) Age: 64.31 ± 8.14 Sex (M/F): 13/3 | CRDQ (dyspnea, emotion, mastery, and fatigue), maximal voluntary isometric contraction of knee extension, knee flexion, shoulder flexion, and shoulder abduction. |
| Borghi-Silva et al. [32] | To evaluate the effects of a 6-week aerobic exercise training program on autonomic modulation of heart rate in individuals with moderate to severe COPD. | N: 40 (EG: 20; CG: 14) Age: 67 ± 10 Sex (M/F): EG: 13/7|CG: 12/8 | Pulmonary function measures (tidal volume, respiratory rate, ventilation), VO2, heart rate, blood lactate, dyspnea (RPE, CR10 scale), 6MWT, time domain (rMSSD and rMSM), and frequency domain (LF, HF, and LF/HF). |
| Borghi-Silva et al. [33] | To analyze the effects of a physical training program on cardiac autonomic modulation by linear and nonlinear heart rate variability indices and exercise capacity in individuals with moderate to severe COPD | N: 20 (EG: 10; CG: 10) Age: EG: 67 ± 7|CG: 66 ± 10 Sex (M/F): EG: 7/3|CG: 5/5 | Pulmonary function measures (ventilation, VC, and FVC), heart rate, 6MWT, VO2, and VCO2. |
| Brønstad et al. [24] | To evaluate high-intensity knee extensor training on muscle function in individuals with mild to moderate COPD. | N: 12 (EG: 7; CG: 5) Age: EG: 67.6 ± 7.2|CG: 70.0 ± 4.6 Sex (M/F): ND | Mitochondrial respiration of the vastus lateralis muscle, VO2peak, work economy, knee-extensor testing (peak work, quadriceps muscle mass, femoral blood flow at 6 watts L·min−1, femoral blood flow at peak work, muscle VO2 at 6 watts, muscle VO2 at peak work, pulmonary VO2 at 6 watts, pulmonary VO2 at peak work, lactate at 6 watts, and lactate at peak work). |
| Calik-Kutukcu et al. [34] | To evaluate the effects of arm strength training on arm exercise capacity, activities of daily living, and occupational performance in individuals with moderate to severe COPD. | N: 42 (EG: 21; CG: 21) Age: EG: 58.38 ± 9.32|CG: 59.71 ± 9.3 Sex (M/F): EG: 16/5|CG: 11/10 | Hand grip strength, heart rate, SpO2, DSpO2, dyspnea (CR10 scale), arm fatigue (CR10 scale), leg fatigue (CR-10 scale), general fatigue (CR10 scale), peak workload, VO2peak, blood pressure, and upper extremity disability (Milliken Activity Daily Living Scale). |
| Clark et al. [22] | To analyze the effects of strength training on muscle function, isokinetic skeletal muscle, and cardiorespiratory capacity in individuals with mild COPD. | N: 43 (EG: 26; CG: 17) Age: 49 ± 11 Sex (M/F): EG: 15/1|CG: 10/7 | Pulmonary function measures (VEmax, VTmax, respiratory rate, FEV1, RV), isokinetic muscle strength of upper and lower limb using maximal contraction test, average peak torque and sustained repeated contractions, VO2max, heart rate maximum, endurance walk test, body mass index, PAO2, and Borg scale. |
| Costes et al. [25] | To evaluate the effects of an exercise training program on baroreflex sensitivity in individuals with mild to moderate COPD. | N: 39 (EG: 21; CG: 18) Age: 62 ± 9|66 ± 1 Sex: ND | Pulmonary function measures (FEV1, VC, FEV1/VC, RV, TLC, ventilation), PAO2, PACO2, workload, VO2peak, VCO2, heart rate, spontaneous breathing, and head-up tilt (total frequency power, low frequency, high frequency). |
| Frei et al. [42] | To assess the effectiveness of the program and the experience of participants after 12 months in individuals with severe and very severe COPD who completed pulmonary rehabilitation. | N: 123 (EG: 61; CG: 62) Age: EG: 66.1 ± 8.3|CG: 67.4 ± 7.9 Sex (M/F): EG: 30/31|CG: 32/30 | Chronic Respiratory Questionnaire, 1 min sit-to-stand test, and 6MWT. |
| Gelinas et al. [26] | To analyze whether periodized aerobic exercise training could improve vascular structure and function in individuals with mild to moderate COPD. | N: 44 (EG: 24; CG:20) Age: EG: 69 ± 7|CG: 64 ± 5 Sex: ND | Pulmonary function measures (FEV1, FVC, FEV1/FVC ratio, TLC, RV, RV/TLC ratio, FRC, diffusion capacity for carbon monoxide, and diffusion capacity corrected for alveolar volume), endothelial function through brachial arterial flow-mediated dilatation, peripheral pulse wave velocity, carotid artery intima-media thickness, carotid compliance, distensibility, β-stiffness index, and VO2peak. |
| Gouzi et al. [27] | To compare changes in blood pressure during exercise in individuals with mild to moderate COPD, about muscle capillarization. | N: 84 (EG: 49; CG:35) Age: EG: 61.5 ± 7.9|CG: 62.1 ± 5.8 Sex (M/F): EG: 30/19|CG: 18/17 | Pulmonary function measure (FEV1), body mass index, 6MWT, VO2SL, resting and maximum systolic/diastolic blood pressure, muscle biopsies, capillary density, and capillary-to-fiber ratio. |
| Ko et al. [41] | Whether a short course of exercise training post-AECOPD, with periodic reinforcement exercise training and phone call reminders, reduces readmissions and increases physical activity in severe COPD individuals. | N: 136 (EG: 68; CG 68) Age: EG: 76 ± 8|CG: 74 ± 7 Sex (M/F): EG: 67/1|CG: 65/3 | The average number of steps per day, MET, average daily wearing time in minutes, percentage of daily wear time, percentage of time spent in sedentary intensity, light intensity, moderate intensity, and vigorous to very vigorous intensity. Pulmonary function measures (FEV1, FVC), body mass index, 6MWT, COPD assessment test, modified Medical Research Council score, and SGRQ. |
| Leite et al. [30] | To analyze the effects of 12-week aerobic training using continuous and interval sessions on autonomic modulation, mucociliary clearance, and aerobic function in individuals with severe, moderate, and mild COPD. | N: 16 (EG: 10; CG:6) Age: EG: 62|CG: 62.5 Sex: ND | Pulmonary function measures (FEV1, FVC, and FEV1/FVC ratio), heart rate variability measures (standard deviation of regular RR intervals, root mean square of the difference between the adjacent regular RR intervals in a time interval, low frequency power, low frequency power in normalized units, high frequency power, high frequency power in normalized units, LF/HF ratio), SpO2, subjective perception of effort, VO2peak, and gas exchange threshold. |
| Liu et al. [35] | To analyze the effects of a supervised endurance exercise training program in a home setting, offering convenience and prolonged benefits, in individuals with moderate to severe COPD. | N: 48 (EG: 24; CG: 24) Age: EG: 71.4 ± 1.7|CG: 72.8 ± 1.3 Sex: M | Pulmonary function measures (FVC, FEV1, FEV1/FVC, inspiratory capacity), incremental shuttle walk test, breathlessness (Borg scale), Short-Form-12 quality-of-life questionnaire. |
| Marrara et al. [36] | To evaluate the responsiveness of the 6MST to an aerobic physical training program and to determine its efficacy regarding spirometry variables during the 6MST, as well as physical performance, sensation of dyspnea, and SpO2 during the 6MST and 6MWT, in individuals with moderate to severe COPD. | N: 36 (EG: 21; CG:15) Age: EG: 70.5 ± 8.5|CG: 68.3 ± 8.7 Sex: ND | Pulmonary function measures (FEV1, FVC, FEV1/FVC, maximal voluntary ventilation), 6MST, VO2peak, VO2, ventilatory equivalent for CO2 at rest, steps climbed, work efficiency, dyspnea (modified Borg scale), SpO2 levels, 6MWT, and dyspnea (modified Borg scale). |
| Pothirat et al. [40] | To determine the long-term efficacy of an intensive cycle ergometer exercise program on various clinical parameters of individuals with moderate, severe, or very severe COPD. | N: 41 (EG: 27; CG: 14) Age: EG: 71.9 ± 6.4|CG: 72.0 ± 7.9 Sex (M/F): EG: 12:15|CG: 7:7% | Pulmonary function measures (FEV1 and FEV1/FVC), as well as upper and lower limb muscle strength, were measured using a handheld dynamometer. Maximum inspiratory pressure, 6 min walk test (6MWT), Modified Medical Research Council scale, Transitional Dyspnea Index questionnaire, SGRQ, and survival rates were also assessed. |
| Varas et al. [28] | To assess the effects of a community-based pulmonary rehabilitation program designed to increase physical activity in participants with mild to moderate COPD. | N: 40 (EG: 21; CG: 19) Age: EG: 69.5 ± 7.4|CG: 64.8 ± 9.1 Sex (M): EG: 18/3|CG: 13/6 | Endurance shuttle test, steps/day recorder device, modified Baecke questionnaire, and SGRQ. |
| Wang et al. [37] | To investigate whether a home-based exercise training program can reduce inflammatory biomarkers in individuals with moderate to severe COPD. | N: 26 (EG: 12; CG: 14) Age: EG: 71.4 ± 1.9|CG: 71.9 ± 2.7 Sex: M | Pulmonary function measures (FEV1 and FEV1/FVC ratio), shuttle walk test, elbow flexion and knee extension muscle strength through a dynamometer, serum C-reactive protein, and the concentration of interleukin-8. |
| Wootton et al. [38] | To evaluate the effect on health-related quality of life (HRQoL) of adding ongoing feedback to a 12-month unsupervised maintenance walking program in individuals with moderate to severe COPD. | N: 95 (EG: 49; CG: 46) Age: EG: 70|CG: 69 Sex (M/F): EG: 25/24|CG: 30/16 | Pulmonary function measures (FEV1, FVC, FEV1/FVC, TLC, FRC, RV, and RV/TLC), carbon monoxide diffusion capacity, SGRQ, the Chronic Respiratory Disease Questionnaire, 6MWT, incremental shuttle walk test, and endurance shuttle walk test. |
| Zambom-Ferraresi et al. [39] | To compare the effects of 12-week training periods involving resistance training only with the impact of 12-week training periods involving combined resistance and endurance training on strength, endurance performance, and quality of life in individuals with moderate to severe COPD. | N: 36 (EG1: 14; EG2: 14; CG: 8) Age: EG1: 68 ± 7|EG2: 68 ± 7|CG: 69 ± 5 Sex: ND | Pulmonary function measures (FEV1, FVC, FEV1/FVC, TLC, IC, and IC/TLC), maximum inspiratory pressure in cm H2O (MIP), maximum expiratory pressure in cm H2O (MEP), maximal power output (50% of one repetition maximum in the leg press), maximal strength (in the leg-press and chest-press), maximal dynamic strength, maximal cycle exercise test, and the BODE index. |
| Reference | Reporting | Ext. Validity | Int. Validity | Int. Validity-Cofounding | Power | Final | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | Score | |
| Amin et al. [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 22 |
| Averna et al. [23] | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 19 |
| Bertolini et al. [29] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 18 |
| Borghi-Silva et al. [32] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 20 |
| Borghi-Silva et al. [33] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 21 |
| Brønstad et al. [24] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 18 |
| Calik-Kutukcu et al. [34] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 22 |
| Clark et al. [22] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 18 |
| Costes et al. [25] | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 18 |
| Frei et al. [42] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 25 |
| Gelinas et al. [26] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 23 |
| Gouzi et al. [27] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 18 |
| Ko et al. [41] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 23 |
| Leite et al. [30] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 19 |
| Liu et al. [35] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 21 |
| Marrara et al. [36] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 19 |
| Pothirat et al. [40] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 21 |
| Varas et al. [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 25 |
| Wang et al. [37] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 20 |
| Wootton et al. [38] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 22 |
| Zambom-Ferraresi et al. [39] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 20 |
| Study | Program Duration, Frequency, Session Duration | Training Program | Main Outcomes |
|---|---|---|---|
| Amin et al. [31] | Program duration: 12 weeks Frequency: 2× week Session duration: 30 min plus resistance training | EG training program:
| The community-based exercise program was both feasible and effective in improving aerobic capacity (endurance time) and reducing the transition dyspnea index. |
| Averna et al. [23] | Program duration: 12 weeks Frequency: 3× week Session duration: 60 min | EG training program:
| Favorable modifications in respiratory and cardiovascular parameters were observed, including improved exercise tolerance, reduced blood pressure values at rest and during exertion, increased forced vital capacity, higher oxygen saturation levels during exercise, and enhanced quality of life related to overall health. |
| Bertolini et al. [29] | Program duration: 16 weeks Frequency: 3× week Session duration: ND | EG training program (unsupervised):
| The exercise training program maintained the gains made in the previous supervised training on the strength of the upper and lower limbs and CRDQ domains, with no additional benefits. |
| Borghi-Silva et al. [32] | Program duration: 6 weeks Frequency: 3× week Session duration: 30 min. | EG training program:
| Significant improvements in VO2peak, blood lactate, minute ventilation, dyspnea at peak exercise, sympathetic activity, and parasympathetic activity at rest. Moreover, reductions in respiratory rate and increases in tidal volume were observed during exercise. |
| Borghi-Silva et al. [33] | Program duration: 6 versus 12 weeks Frequency: 3× week Session duration: 35 min | EG training program: High-intensity workload relative to each subject’s aerobic capacity (walking).
| After 6 weeks, HRV indices, aerobic capacity (as measured by walking distance), peak heart rate, and lactate levels improved. However, after 12 weeks, the majority of the variables continued to show additional improvements. Short-term rehabilitation is an adequate time to modify outcomes, as cardiac modulation and exercise capacity are beneficial. |
| Brønstad et al. [24] | Program duration: 6 weeks Frequency: 3× week Session duration: ND |
| High-intensity aerobic interval training of a limited muscle group restored work performance and oxidative capacity of the quadriceps muscle. |
| Calik-Kutukcu et al. [34] | Program duration: 8 weeks (23 sessions) Frequency: 3× week Session duration: ND | EG training program:
| The upper extremity muscle strength training alone increased upper extremity muscle strength, arm exercise capacity, performance in activities of daily living, and participants’ satisfaction with their performance in these activities. Additionally, it decreased dyspnea and fatigue perceptions during supported arm exercises and dyspnea perception during activities of daily living. |
| Clark et al. [22] | Program duration: 12 weeks Frequency: 2× week Session duration: ND | EG training program:
| There was a significant reduction in skeletal muscle endurance and strength compared with healthy but sedentary individuals. Moreover, skeletal muscle training resulted in a highly significant improvement in exercise endurance during treadmill walking, without any central cardiorespiratory changes, and correlated with improved skeletal muscle function measures. |
| Costes et al. [25] | Program duration: 8 weeks Frequency: 3× week Session duration: 35/45 min | Endurance exercise training program:
| Exercise training was associated with an increase in spontaneous baroreflex sensitivity, indicating cardiovascular benefits. It increased tolerance and the ability to sustain a high ventilation level without changes in pulmonary volumes or blood gas levels. |
| Frei et al. [42] | Program duration: 12 months Frequency: 6× week Session duration: 20 min | EG training program:
| The home exercise program did not affect dyspnea but improved 1MSTST performance and patient-perceived fitness. |
| Gelinas et al. [26] | Program duration: 8 weeks (24 sessions) Frequency: 3× week Session duration: 20–45 min | EG training program: Supervised training on the cycle ergometer.
| Eight weeks of aerobic training improved cardiorespiratory fitness, as measured by VO2peak, reduced dyspnea, and lowered blood pressure, but had a non-significant effect on other established markers of cardiovascular disease risk. |
| Gouzi et al. [27] | Program duration: 6 weeks Frequency: 3× week Session duration: 1 h–30 min | Endurance training:
| Participants showed slight improvements in VO2peak. |
| Ko et al. [41] | Program duration: 4–8 weeks (continuing exercise at home for 1 year) Frequency: 1–2× weekly Session duration: 2 h |
| A short course of exercise training following acute exacerbations of COPD, combined with periodic reinforcement and phone call reminders, reduced the frequency of exacerbations and increased the time to readmission for acute exacerbations of COPD. However, the program did not improve physical activities and exercise tolerance at 12 months. |
| Leite et al. [30] | Program duration: 12 weeks Frequency: 3× week Session duration: ND | EG training program performed on the treadmill:
| Aerobic training (continuous and interval sessions) positively influenced the high frequency index (ms2), VO2peak, and anaerobic threshold. |
| Liu et al. [35] | Program duration: 3 months Frequency: daily Session duration: ND | EG training program:
| Participants in the cell phone group improved their shuttle walking test distance and duration after eight weeks. The improvements in shuttle walking test distance, inspiratory capacity, and quality-of-life questionnaire scoring improved even more at the end of the 12 weeks of training, with fewer acute exacerbations and hospitalizations. |
| Marrara et al. [36] | Program duration: 6 weeks Frequency: 3× week Session duration: 35 min | EG training program:
| The 6 min step test improved physical performance and reduced the sensation of dyspnea. |
| Pothirat et al. [40] | Program duration: 24 months Frequency: 2× per week Session duration: 30–60 min | EG exercise program:
| All individuals showed strength increases in the four trained muscle groups. There were also improvements in the level of dyspnea, endurance, and quality of life over the 12-month follow-up period. |
| Varas et al. [28] | Program duration: 8 weeks Frequency: 5× per week Session duration: 30–60 min | EG training program:
| There were significant improvements in the shuttle run test, number of steps, and Baecke scores of the physical activity questionnaire, while the St. George’s Respiratory Questionnaire scores decreased. These results remained evident after 3 and 12 months of follow-up. |
| Wang et al. [37] | Program duration: 6 months Frequency: daily Session duration: ND | EG training program:
| A mobile-phone-based system can provide an efficient home endurance exercise training program with improved exercise capacity, strength of limb muscles, and a decrease in serum C-reactive protein and interleukin-8. |
| Wootton et al. [38] | Program duration: 2 months Frequency: 3× per week Session duration: ND | EG and CG training program:
| Following a 2-month supervised walking training program, ongoing feedback was no more effective than no feedback in maintaining quality of life during a 12-month unsupervised walking program. |
| Zambom-Ferraresi et al. [39] | Program duration: 12 weeks Frequency: 2× week Session duration: 90 min | Resistance training alone:
| The combined program produced greater improvements in muscle power output and exercise capacity than the resistance training program alone. Specifically, muscle power and maximal exercise capacity improved, while heart rate and blood lactate at a given submaximal workload decreased. Identical improvements were found in aerobic capacity for both training groups. |
4. Discussion
4.1. Cardiorespiratory Training
4.2. Strength Training
4.3. Combined Training
4.4. Limitations, Strengths, and Suggestions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levine, S.M.; Marciniuk, D.D. Global Impact of Respiratory Disease: What Can We Do, Together, to Make a Difference? Chest 2022, 161, 1153–1154. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Sethi, S.; Sethi, R.; Eschberger, K.; Lobbins, P.; Cai, X.; Grant, B.J.B.; Murphy, T.F. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 176, 356–361. [Google Scholar] [CrossRef]
- Strachan, D.P. Causes and control of chronic respiratory disease: Looking beyond the smokescreen. J. Epidemiol. Community Health 1992, 46, 177–179. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Houben-Wilke, S.; Janssen, D.J.A.; Franssen, F.M.E.; Vanfleteren, L.E.G.W.; Wouters, E.F.M.; Spruit, M.A. Contribution of individual COPD assessment test (CAT) items to CAT total score and effects of pulmonary rehabilitation on CAT scores. Health Qual. Life Outcomes 2018, 16, 205. [Google Scholar] [CrossRef]
- da Silva, H.E.; Zipperer, A. A correlação entre o desempenho físico funcional de membros inferiores e a gravidade da doença pulmonar obstrutiva crônica. Fisioter. Em Mov. 2013, 26, 379–387. [Google Scholar] [CrossRef]
- Lacasse, Y.; Martin, S.; Lasserson, T.J.; Goldstein, R.S. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. A Cochrane systematic review. Eura Medicophys 2007, 43, 475–485. [Google Scholar] [CrossRef]
- Iepsen, U.W.; Jørgensen, K.J.; Ringbæk, T.; Hansen, H.; Skrubbeltrang, C.; Lange, P. A combination of resistance and endurance training increases leg muscle strength in COPD: An evidence-based recommendation based on systematic review with meta-analyses. Chron. Respir. Dis. 2015, 12, 132–145. [Google Scholar] [CrossRef]
- Topcuoğlu, C.; Sağlam, M.; Yağlı, N.V. Comparison of the effects of high and low-moderate load lower limb resistance training on muscle strength and exercise capacity in individuals with COPD: A systematic review and meta-analysis. Heart Lung 2024, 64, 107–116. [Google Scholar] [CrossRef]
- O’Shea, S.D.; Taylor, N.F.; Paratz, J.D. Progressive resistance exercise improves muscle strength and may improve elements of performance of daily activities for people with COPD: A systematic review. Chest 2009, 136, 1269–1283. [Google Scholar] [CrossRef]
- Puhan, M.A.; Schünemann, H.J.; Frey, M.; Scharplatz, M.; Bachmann, L.M. How should COPD patients exercise during respiratory rehabilitation? Comparison of exercise modalities and intensities to treat skeletal muscle dysfunction. Thorax 2005, 60, 367–375. [Google Scholar] [CrossRef]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef]
- Bolton, C.E.; Bevan-Smith, E.F.; Blakey, J.D.; Crowe, P.; Elkin, S.L.; Garrod, R.; Greening, N.J.; Heslop, K.; Hull, J.H.; Man, W.D.-C.; et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013, 68, ii1–ii30. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2017. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Rico-González, M.; Pino-Ortega, J.; Clemente, F.; Los Arcos, A. Guidelines for performing systematic reviews in sports science. Biol. Sport. 2021, 39, 463–471. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Marquet, K.; Liesenborgs, A.; Bergs, J.; Vleugels, A.; Claes, N. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: A systematic review and meta-analysis. Crit. Care 2015, 19, 63. [Google Scholar] [CrossRef]
- HaiBo, T.; Xin, K.; ShiHeng, L.; Lin, L. Comparison of Ahmed glaucoma valve implantation and trabeculectomy for glaucoma: A systematic review and meta- Analysis. PLoS ONE 2015, 10, e0118142. [Google Scholar] [CrossRef]
- Clark, C.J.; Cochrane, L.M.; Mackay, E.; Paton, B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur. Respir. J. 2000, 15, 92–97. [Google Scholar] [CrossRef]
- Averna, T.; Brunelli, S.; Delussu, A.S.; Porcacchia, P.; Lucarelli, E.; Polidori, L.; Traballesi, M. Effects of a moderately intensive, 12-week training program on participants over 60 years of age with chronic obstructive pulmonary disease | Effetti di un programma di allenamento ad intensita moderata delia durata di 12 settimane in soggetti sopra i 60. Med. Dello Sport 2009, 62, 299–313. [Google Scholar]
- Brønstad, E.; Rognmo, O.; Tjonna, A.E.; Dedichen, H.H.; Kirkeby-Garstad, I.; Håberg, A.K.; Ingul, C.B.; Wisløff, U.; Steinshamn, S. High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur. Respir. J. 2012, 40, 1130–1136. [Google Scholar] [CrossRef]
- Costes, F.; Roche, F.; Pichot, V.; Vergnon, J.M.; Garet, M.; Barthelemy, J.C. Influence of exercise training on cardiac baroreflex sensitivity in patients with COPD. Eur. Respir. J. 2004, 23, 396–401. [Google Scholar] [CrossRef]
- Gelinas, J.C.; Lewis, N.C.; Harper, M.I.; Melzer, B.; Agar, G.; Rolf, J.D.; Eves, N.D. Aerobic exercise training does not alter vascular structure and function in chronic obstructive pulmonary disease. Exp. Physiol. 2017, 102, 1548–1560. [Google Scholar] [CrossRef]
- Gouzi, F.; Maury, J.; Bughin, F.; Blaquière, M.; Ayoub, B.; Mercier, J.; Perez-Martin, A.; Pomiès, P.; Hayot, M. Impaired training-induced adaptation of blood pressure in COPD patients: Implication of the muscle capillary bed. Int. J. COPD 2016, 11, 2349–2357. [Google Scholar] [CrossRef]
- Varas, A.B.; Córdoba, S.; Rodríguez-Andonaegui, I.; Rueda, M.R.; García-Juez, S.; Vilaró, J. Effectiveness of a community-based exercise training programme to increase physical activity level in patients with chronic obstructive pulmonary disease: A randomized controlled trial. Physiother. Res. Int. 2018, 23, e1740. [Google Scholar] [CrossRef]
- Bertolini, G.N.; Ramos, D.; Leite, M.R.; De Carvalho, L.C.S., Jr.; Freire, A.P.C.F.; De Lima, F.F.; Silva, B.S.d.A.; Pastre, C.M.; Ramos, E.M.C. Effects of a home-based exercise program after supervised resistance training in patients with chronic obstructive pulmonary disease. Medicina 2016, 49, 331–337. [Google Scholar] [CrossRef]
- Leite, M.R.; Kalva-Filho, C.A.; Freire, A.P.C.F.; Silva, B.S.d.A.; Nicolino, J.; Toledo-Arruda, A.; Papoti, M.; Vanderlei, L.C.M.; Ramos, D.; Ramos, E.M.C. Effects of 12 weeks of aerobic training on autonomic modulation, mucociliary clearance, and aerobic parameters in patients with COPD. Int. J. COPD 2015, 10, 2549–2557. [Google Scholar] [CrossRef]
- Amin, S.; Abrazado, M.; Quinn, M.; Storer, T.W.; Tseng, C.-H.; Cooper, C.B. Un estudio controlado del entrenamiento con ejercicios basados en la comunidad en pacientes con EPOC moderada. BMC Pulm. Med. 2014, 14, 125. [Google Scholar]
- Borghi-Silva, A.; Arena, R.; Castello, V.; Simões, R.P.; Martins, L.E.B.; Catai, A.M.; Costa, D. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir. Med. 2009, 103, 1503–1510. [Google Scholar] [CrossRef]
- Borghi-Silva, A.; Mendes, R.G.; Tirmer, R.; Oliveira, C.R.; Fregonezi, G.A.F.; Resqueti, V.R.; Arena, R.; Sampaio-Jorge, L.M.; Costa, D. Potential effect of 6 versus 12-weeks of physical training on cardiac autonomic function and exercise capacity in chronic obstructive pulmonary disease. Eur. J. Phys. Rehabil. Med. 2015, 51, 211–221. [Google Scholar]
- Calik-Kutukcu, E.; Arikan, H.; Saglam, M.; Vardar-Yagli, N.; Oksuz, C.; Inal-Ince, D.; Savci, S.; Duger, T.; Coplu, L. Arm strength training improves activities of daily living and occupational performance in patients with COPD. Clin. Respir. J. 2017, 11, 820–832. [Google Scholar] [CrossRef]
- Liu, W.T.; Wang, C.H.; Lin, H.C.; Lin, S.M.; Lee, K.Y.; Lo, Y.L.; Hung, S.-H.; Chang, Y.-M.; Chung, K.; Kuo, H.-P. Efficacy of a cell phone-based exercise programme for COPD. Eur. Respir. J. 2008, 32, 651–659. [Google Scholar] [CrossRef]
- Marrara, K.T.; Marino, D.M.; Jamami, M.; Delfino De Oliveira Junior, A.; Amorim, V. Lorenzo PDi Responsiveness of the six-minute step test to a physical training program in patients with COPD* Responsividade do teste do degrau de seis minutos a um programa de treinamento físico em pacientes com, D.P.O.C.J. Bras. Pneumol. 2012, 38, 579–587. [Google Scholar] [CrossRef]
- Wang, C.H.; Chou, P.C.; Joa, W.C.; Chen, L.F.; Sheng, T.F.; Ho, S.C.; Lin, H.-C.; Huang, C.-D.; Chung, F.-T.; Chung, K.F.; et al. Mobile-phone-based home exercise training program decreases systemic inflammation in COPD: A pilot study. BMC Pulm. Med. 2014, 14, 142. [Google Scholar] [CrossRef]
- Wootton, S.L.; McKeough, Z.; Ng, C.L.W.; Jenkins, S.; Hill, K.; Eastwood, P.R.; Hillman, D.; Jenkins, C.; Cecins, N.; Spencer, L.; et al. Effect on health-related quality of life of ongoing feedback during a 12-month maintenance walking programme in patients with COPD: A randomized controlled trial. Respirology 2018, 23, 60–67. [Google Scholar] [CrossRef]
- Zambom-Ferraresi, F.; Cebollero, P.; Gorostiaga, E.M.; Hernández, M.; Hueto, J.; Cascante, J.; Rezusta, L.; Val, L.; Anton, M.M. Effects of combined resistance and endurance training versus resistance training alone on strength, exercise capacity, and quality of life in patients with COPD. J. Cardiopulm. Rehabil. Prev. 2015, 35, 446–453. [Google Scholar] [CrossRef]
- Pothirat, C.; Chaiwong, W.; Phetsuk, N.; Liwsrisakun, C.; Bumroongkit, C.; Deesomchok, A.; Theerakittikul, T. Long-term efficacy of intensive cycle ergometer exercise training program for advanced COPD patients. Int. J. COPD 2015, 10, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.W.S.; Tam, W.; Siu, E.H.S.; Chan, K.P.; Ngai, J.C.L.; Ng, S.S.; Chan, T.O.; Hui, D.S. Effect of short-course exercise training on the frequency of exacerbations and physical activity in patients with COPD: A randomized controlled trial. Respirology 2021, 26, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Radtke, T.; Dalla Lana, K.; Brun, P.; Sigrist, T.; Spielmanns, M.; Beyer, S.; Riegler, T.F.; Büsching, G.; Spielmanns, S.; et al. Effectiveness of a Long-term Home-Based Exercise Training Program in Patients With COPD After Pulmonary Rehabilitation: A Multicenter Randomized Controlled Trial. Chest 2022, 162, 1277–1286. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Paredi, P.; Kharitonov, S.A.; Leak, D.; Ward, S.; Cramer, D.; Barnes, P.J. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000, 162, 369–373. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.J.H.; Pahor, M.; Lauretani, F.; Corsi, A.M.; Rhys Williams, G.; Guralnik, J.M.; Ferrucci, L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 242–248. [Google Scholar] [CrossRef]
- Ramos, E.M.C.; de Toledo-Arruda, A.C.; Fosco, L.C.; Bonfim, R.; Bertolini, G.N.; Guarnier, F.A.; Cecchini, R.; Pastre, C.M.; Langer, D.; Gosselink, R.; et al. The effects of elastic tubing-based resistance training compared with conventional resistance training in patients with moderate chronic obstructive pulmonary disease: A randomized clinical trial. Clin. Rehabil. 2014, 28, 1096–1106. [Google Scholar] [CrossRef]
- Rennard, S.I.; Drummond, M.B. Early chronic obstructive pulmonary disease: Definition, assessment, and prevention. Lancet 2015, 385, 1778–1788. [Google Scholar] [CrossRef]
- Soriano, J.B.; Polverino, F.; Cosio, B.G. What is early COPD and why is it important? Eur. Respir. J. 2018, 52, 1801448. [Google Scholar] [CrossRef]
| Database | Specificities of the Databases | Search Strategy |
|---|---|---|
| PubMed | None to report | ((((“exercise”[MeSH Terms] OR “exercise”[All Fields] OR “exercises”[All Fields] OR “exercise therapy”[MeSH Terms] OR (“exercise”[All Fields] AND “therapy”[All Fields]) OR “exercise therapy”[All Fields] OR “exercise s”[All Fields] OR “exercised”[All Fields] OR “exerciser”[All Fields] OR “exercisers”[All Fields] OR “exercising”[All Fields]) AND “prescription*”[All Fields]) OR ((“education”[MeSH Subheading] OR “education”[All Fields] OR “training”[All Fields] OR “education”[MeSH Terms] OR “train”[All Fields] OR “train s”[All Fields] OR “trained”[All Fields] OR “training s”[All Fields] OR “trainings”[All Fields] OR “trains”[All Fields]) AND “program*”[All Fields]) OR ((“exercise”[MeSH Terms] OR “exercise”[All Fields] OR “exercises”[All Fields] OR “exercise therapy”[MeSH Terms] OR (“exercise”[All Fields] AND “therapy”[All Fields]) OR “exercise therapy”[All Fields] OR “exercise s”[All Fields] OR “exercised”[All Fields] OR “exerciser”[All Fields] OR “exercisers”[All Fields] OR “exercising”[All Fields]) AND “periodization*”[All Fields])) AND (“pulmonary disease, chronic obstructive”[MeSH Terms] OR (“pulmonary”[All Fields] AND “disease”[All Fields] AND “chronic”[All Fields] AND “obstructive”[All Fields]) OR “chronic obstructive pulmonary disease”[All Fields] OR (“chronic”[All Fields] AND “obstructive”[All Fields] AND “pulmonary”[All Fields] AND “disease”[All Fields]) OR “copd*”[All Fields])) |
| Web of Science | None to report | ((((ALL=(exercise prescription*)) OR ALL=(training program*)) OR ALL=(exercise periodization*)) AND ALL=(chronic obstructive pulmonary disease )) OR ALL=(COPD*) |
| Scopus | Search for title and abstract also includes keywords | (TITLE-ABS-KEY (exercise AND prescription*) OR TITLE-ABS-KEY training AND program*) OR TITLE-ABS-KEY (exercise AND periodization*) AND TITLE-ABS-KEY (chronic AND obstructive AND pulmonary AND disease) OR TITLE-ABS-KEY (copd*) |
| SPORTDiscus | Search for title only | ((exercise prescription*OR training program* OR exercise periodization*) AND (chronic obstructive pulmonary disease OR COPD*)). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, R.; Brito, J.P.; Ceylan, H.İ.; Soares, M.d.B.; Martins, A.D.; Vasconcelos, T.; Moutão, J.; Alves, S. Characteristics of Physical Exercise Programs and Their Effects on Quality of Life and Functional Capacity in Individuals with Chronic Obstructive Pulmonary Disease: A Scoping Review. Medicina 2025, 61, 970. https://doi.org/10.3390/medicina61060970
Oliveira R, Brito JP, Ceylan Hİ, Soares MdB, Martins AD, Vasconcelos T, Moutão J, Alves S. Characteristics of Physical Exercise Programs and Their Effects on Quality of Life and Functional Capacity in Individuals with Chronic Obstructive Pulmonary Disease: A Scoping Review. Medicina. 2025; 61(6):970. https://doi.org/10.3390/medicina61060970
Chicago/Turabian StyleOliveira, Rafael, João Paulo Brito, Halil İbrahim Ceylan, Maria de Brito Soares, Alexandre Duarte Martins, Tiago Vasconcelos, João Moutão, and Susana Alves. 2025. "Characteristics of Physical Exercise Programs and Their Effects on Quality of Life and Functional Capacity in Individuals with Chronic Obstructive Pulmonary Disease: A Scoping Review" Medicina 61, no. 6: 970. https://doi.org/10.3390/medicina61060970
APA StyleOliveira, R., Brito, J. P., Ceylan, H. İ., Soares, M. d. B., Martins, A. D., Vasconcelos, T., Moutão, J., & Alves, S. (2025). Characteristics of Physical Exercise Programs and Their Effects on Quality of Life and Functional Capacity in Individuals with Chronic Obstructive Pulmonary Disease: A Scoping Review. Medicina, 61(6), 970. https://doi.org/10.3390/medicina61060970









