Salivary 1,5-Anhydroglucitol and AGEs Are Associated with Postural Instability in Diabetic Foot Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Gait and Posture Evaluation
2.3. Analysis of Biomarkers
2.4. AGEs and 1,5-AG Analysis
2.5. Blood Glycated Hemoglobin
2.6. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Køhler, E.; Dalgas, U.; Buhl, C.S.; Brincks, J. Content and effects of balance training in people with diabetic peripheral neuropathy—A systematic review and meta-analysis. Physiother. Theory Pract. 2024, 41, 1083–1094. [Google Scholar] [CrossRef]
- Brognara, L.; Mazzotti, A.; Di Martino, A.; Faldini, C.; Cauli, O. Wearable Sensor for Assessing Gait and Postural Alterations in Patients with Diabetes: A Scoping Review. Medicina 2021, 57, 1145. [Google Scholar] [CrossRef]
- Omana, H.; Madou, E.; Montero-Odasso, M.; Payne, M.; Viana, R.; Hunter, S. The Effect of Dual-Task Testing on Balance and Gait Performance in Adults with Type 1 or Type 2 Diabetes Mellitus: A Systematic Review. Curr. Diabetes Rev. 2021, 17, e011020186496. [Google Scholar] [CrossRef]
- Petrofsky, J.; Lee, S.; Bweir, S. Gait characteristics in people with type 2 diabetes mellitus. Eur. J. Appl. Physiol. 2005, 93, 640–647. [Google Scholar] [CrossRef]
- Cavanagh, P.R.; Derr, J.A.; Ulbrecht, J.S.; Maser, R.E.; Orchard, T.J. Problems with Gait and Posture in Neuropathic Patients with Insulin-Dependent Diabetes Mellitus. Diabet. Med. 1992, 9, 469–474. [Google Scholar] [CrossRef]
- Allet, L.; Armand, S.; de Bie, R.A.; Pataky, Z.; Aminian, K.; Herrmann, F.R.; de Bruin, E.D. Gait alterations of diabetic patients while walking on different surfaces. Gait Posture 2009, 29, 488–493. [Google Scholar] [CrossRef]
- Petrofsky, J.; Laymon, M.; Lee, H. The influence of ageing and diabetic peripheral neuropathy on posture sway, tremor, and the time to achieve balance equilibrium. J. Sports Med. Phys. Fit. 2019, 59, 1011–1017. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wilson, R.S.; Schneider, J.A.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes mellitus and progression of rigidity and gait disturbance in older persons. Neurology 2004, 63, 996–1001. [Google Scholar] [CrossRef]
- Kwon, O.-Y.; Minor, S.D.; Maluf, K.S.; Mueller, M.J. Comparison of muscle activity during walking in subjects with and without diabetic neuropathy. Gait Posture 2003, 18, 105–113. [Google Scholar] [CrossRef]
- Yavuzer, G.; Yetkin, I.; Toruner, F.B.; Koca, N.; Bolukbasi, N. Gait deviations of patients with diabetes mellitus: Looking beyond peripheral neuropathy. Eura. Medicophys. 2006, 42, 127–133. [Google Scholar]
- Abate, M.; Schiavone, C.; Salini, V.; Andia, I. Management of limited joint mobility in diabetic patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2013, 6, 197–207. [Google Scholar] [CrossRef]
- Couppé, C.; Svensson, R.B.; Kongsgaard, M.; Kovanen, V.; Grosset, J.-F.; Snorgaard, O.; Bencke, J.; Larsen, J.O.; Bandholm, T.; Christensen, T.M.; et al. Human Achilles tendon glycation and function in diabetes. J. Appl. Physiol. 2016, 120, 130–137. [Google Scholar] [CrossRef]
- Karamanidis, K.; Arampatzis, A.; Mademli, L. Age-related deficit in dynamic stability control after forward falls is affected by muscle strength and tendon stiffness. J. Electromyogr. Kinesiol. 2008, 18, 980–989. [Google Scholar] [CrossRef]
- Petrofsky, J.; Lee, S.; Macnider, M.; Navarro, E. Autonomic, endothelial function and the analysis of gait in patients with type 1 and type 2 diabetes. Acta Diabetol. 2005, 42, 7–15. [Google Scholar] [CrossRef]
- Brognara, L.; Sempere-Bigorra, M.; Mazzotti, A.; Artioli, E.; Julián-Rochina, I.; Cauli, O. Wearable sensors-based postural analysis and fall risk assessment among patients with diabetic foot neuropathy. J. Tissue Viability 2023, 32, 516–526. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hong, Z.; Wu, L.; Zou, C.; Zheng, Y.; Chen, L.; Yin, L.; Qin, J. Influence of age on static postural control in adults with type 2 diabetes mellitus: A cross-sectional study. Front. Endocrinol. 2023, 14, 1242700. [Google Scholar] [CrossRef]
- Rasmussen, N.H.-H.; Dal, J.; Jensen, M.H.; Kvist, A.V.; van den Bergh, J.; Hirata, R.P.; Vestergaard, P. Impaired postural control in diabetes—A predictor of falls? Arch. Osteoporos. 2022, 18, 6. [Google Scholar] [CrossRef]
- Gorniak, S.L.; Lu, F.Y.; Lee, B.C.; Massman, P.J.; Wang, J. Cognitive impairment and postural control deficit in adults with Type 2 diabetes. Diabetes/Metab. Res. Rev. 2019, 35, e3089. [Google Scholar] [CrossRef]
- Ardelean, A.; Balta, D.-F.; Neamtu, C.; Neamtu, A.A.; Rosu, M.; Totolici, B. Personalized and predictive strategies for diabetic foot ulcer prevention and therapeutic management: Potential improvements through introducing Artificial Intelligence and wearable technology. Med. Pharm. Rep. 2024, 97, 419–428. [Google Scholar] [CrossRef]
- Khan, K.S.; Christensen, D.H.; Nicolaisen, S.K.; Gylfadottir, S.S.; Jensen, T.S.; Nielsen, J.S.; Thomsen, R.W.; Andersen, H. Falls and fractures associated with type 2 diabetic polyneuropathy: A cross-sectional nationwide questionnaire study. J. Diabetes Investig. 2021, 12, 1827–1834. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; St George, R.; Fitzpatrick, R.C. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch. Phys. Med. Rehabil. 2004, 85, 245–252. [Google Scholar] [CrossRef]
- Allet, L.; Armand, S.; Golay, A.; Monnin, D.; de Bie, R.A.; de Bruin, E.D. Gait characteristics of diabetic patients: A systematic review. Diabetes/Metab. Res. Rev. 2008, 24, 173–191. [Google Scholar] [CrossRef]
- Simoneau, G.G.; Ulbrecht, J.S.; Derr, J.A.; Becker, M.B.; Cavanagh, P.R. Postural Instability in Patients with Diabetic Sensory Neuropathy. Diabetes Care 1994, 17, 1411–1421. [Google Scholar] [CrossRef]
- Sacco, I.C.; Hamamoto, A.N.; Tonicelli, L.M.; Watari, R.; Ortega, N.R.; Sartor, C.D. Abnormalities of plantar pressure distribution in early, intermediate, and late stages of diabetic neuropathy. Gait Posture 2014, 40, 570–574. [Google Scholar] [CrossRef]
- Jørgensen, I.E.H.; Devantier, L.; Tankisi, H.; Andersen, H.; Khan, K.S. The impact of vestibular dysfunction on falls and postural instability in individuals with type 2 diabetes with and without diabetic polyneuropathy. PeerJ 2023, 11, e16382. [Google Scholar] [CrossRef]
- Ward, B.K.; Wenzel, A.; Kalyani, R.R.; Agrawal, Y.; Feng, A.L.; Polydefkis, M.; Ying, H.S.; Schubert, M.C.; Zuniga, M.G.; Della Santina, C.C.; et al. Characterization of Vestibulopathy in Individuals with Type 2 Diabetes Mellitus. Otolaryngol.–Head Neck Surg. 2015, 153, 112–118. [Google Scholar] [CrossRef]
- Li, J.; Jiang, J.; Zhang, Y.; Liu, B.; Zhang, L. Impairment of Vestibular Function and Balance Control in Patients with Type 2 Diabetes. Audiol. Neurotol. 2019, 24, 154–160. [Google Scholar] [CrossRef]
- Andreassen, C.S.; Jakobsen, J.; Andersen, H. Muscle Weakness. Diabetes 2006, 55, 806–812. [Google Scholar] [CrossRef]
- van Schie, C.H.; Vermigli, C.; Carrington, A.L.; Boulton, A. Muscle Weakness and Foot Deformities in Diabetes. Diabetes Care 2004, 27, 1668–1673. [Google Scholar] [CrossRef]
- Zochodne, D.W.; Toth, C. Diabetes and the Nervous System. In Aminoff’s Neurology and General Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 351–368. [Google Scholar] [CrossRef]
- Ferris, J.K.; Inglis, J.T.; Madden, K.M.; Boyd, L.A. Brain and Body: A Review of Central Nervous System Contributions to Movement Impairments in Diabetes. Diabetes 2020, 69, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Brognara, L.; Volta, I.; Cassano, V.M.; Navarro-Flores, E.; Cauli, O. The Association between Cognitive Impairment and Diabetic Foot Care: Role of Neuropathy and Glycated Hemoglobin. Pathophysiology 2020, 27, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Pop-Busui, R.; Devantier, L.; Kristensen, A.G.; Tankisi, H.; Dalgas, U.; Overgaard, K.; Andersen, H. Falls in individuals with type 2 diabetes; a cross-sectional study on the impact of motor dysfunction, postural instability and diabetic polyneuropathy. Diabet. Med. 2021, 38, e14470. [Google Scholar] [CrossRef] [PubMed]
- Toosizadeh, N.; Mohler, J.; Armstrong, D.G.; Talal, T.K.; Najafi, B. The Influence of Diabetic Peripheral Neuropathy on Local Postural Muscle and Central Sensory Feedback Balance Control. PLoS ONE 2015, 10, e0135255. [Google Scholar] [CrossRef]
- Brognara, L.; Mazzotti, A.; Zielli, S.O.; Arceri, A.; Artioli, E.; Traina, F.; Faldini, C. Wearable Technology Applications and Methods to Assess Clinical Outcomes in Foot and Ankle Disorders: Achievements and Perspectives. Sensors 2024, 24, 7059. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. 2011. Available online: https://iris.who.int/bitstream/handle/10665/70523/WHO_NMH_CHP_CPM_11.1_eng.pdf (accessed on 28 February 2025).
- Boulton, A.J. Management of Diabetic Peripheral Neuropathy. Clin. Diabetes 2005, 23, 9–15. [Google Scholar] [CrossRef]
- Bönhof, G.J.; Herder, C.; Ziegler, D. Diagnostic Tools, Biomarkers, and Treatments in Diabetic polyneuropathy and Cardiovascular Autonomic Neuropathy. Curr. Diabetes Rev. 2022, 18, e120421192781. [Google Scholar] [CrossRef]
- Carmichael, J.; Fadavi, H.; Ishibashi, F.; Shore, A.C.; Tavakoli, M. Advances in Screening, Early Diagnosis and Accurate Staging of Diabetic Neuropathy. Front. Endocrinol. 2021, 12, 671257. [Google Scholar] [CrossRef]
- Fujita, Y.; Murakami, T.; Nakamura, A. Recent Advances in Biomarkers and Regenerative Medicine for Diabetic Neuropathy. Int. J. Mol. Sci. 2021, 22, 2301. [Google Scholar] [CrossRef]
- Buse, J.B.; Freeman, J.L.R.; Edelman, S.V.; Jovanovic, L.; McGill, J.B. Serum 1,5-Anhydroglucitol (GlycoMark™): A Short-Term Glycemic Marker. Diabetes Technol. Ther. 2003, 5, 355–363. [Google Scholar] [CrossRef]
- Yamanouchi, T.; Tachibana, Y.; Akanuma, H.; Minoda, S.; Shinohara, T.; Moromizato, H.; Miyashita, H.; Akaoka, I. Origin and disposal of 1,5-anhydroglucitol, a major polyol in the human body. Am. J. Physiol. Metab. 1992, 263, E268–E273. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, T.; Minoda, S.; Yabuuchi, M.; Akanuma, Y.; Akanuma, H.; Miyashita, H.; Akaoka, I. Plasma 1,5-Anhydro-D-Glucitol as New Clinical Marker of Glycemic Control in NIDDM Patients. Diabetes 1989, 38, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, T.; Ogata, N.; Tagaya, T.; Kawasaki, T.; Sekino, N.; Funato, H.; Akaoka, I.; Miyashita, H. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet 1996, 347, 1514–1518. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Dunseath, G.J.; Owens, D.R. The Loss of Postprandial Glycemic Control Precedes Stepwise Deterioration of Fasting with Worsening Diabetes. Diabetes Care 2007, 30, 263–269. [Google Scholar] [CrossRef]
- Nowatzke, W.; Sarno, M.J.; Birch, N.C.; Stickle, D.F.; Eden, T.; Cole, T.G. Evaluation of an assay for serum 1,5-anhydroglucitol (GlycoMark™) and determination of reference intervals on the Hitachi 917 analyzer. Clin. Chim. Acta 2004, 350, 201–209. [Google Scholar] [CrossRef]
- Mook-Kanamori, D.O.; Selim, M.M.E.-D.; Takiddin, A.H.; Al-Homsi, H.; Al-Mahmoud, K.A.S.; Al-Obaidli, A.; Zirie, M.A.; Rowe, J.; Yousri, N.A.; Karoly, E.D.; et al. 1,5-Anhydroglucitol in Saliva Is a Noninvasive Marker of Short-Term Glycemic Control. J. Clin. Endocrinol. Metab. 2014, 99, E479–E483. [Google Scholar] [CrossRef] [PubMed]
- Burman, M.D.; Bag, S.; Ghosal, S.; Bhowmik, S. Glycation of Proteins and Its End Products: From Initiation to Natural Product-Based Therapeutic Preventions. ACS Pharmacol. Transl. Sci. 2025, 8, 636–653. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; Ke, W.; Li, X.; Xiao, H.; Li, Y. Association between the soluble receptor for advanced glycation end products and diabetes mellitus: Systematic review and meta-analysis. BMC Endocr. Disord. 2024, 24, 232. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.E.; Regalado, J.C.; Malacara, J.M.; Nava, L.E.; Wróbel-Zasada, K.; Castro-Rivas, A.; Wróbel, K. Advanced glycosylation end products in skin, serum, saliva and urine and its association with complications of patients with Type 2 diabetes mellitus. J. Endocrinol. Investig. 2005, 28, 223–230. [Google Scholar] [CrossRef]
- Heier, M.; Margeirsdottir, H.D.; Gaarder, M.; Stensæth, K.H.; Brunborg, C.; Torjesen, P.A.; Seljeflot, I.; Hanssen, K.F.; Dahl-Jørgensen, K. Soluble RAGE and atherosclerosis in youth with type 1 diabetes: A 5-year follow-up study. Cardiovasc. Diabetol. 2015, 14, 126. [Google Scholar] [CrossRef]
- Rezaei, M.; Rabizadeh, S.; Mirahmad, M.; Hajmiri, M.S.; Nakhjavani, M.; Hemmatabadi, M.; Shirzad, N. The association between advanced glycation end products (AGEs) and ABC (hemoglobin A1C, blood pressure, and low-density lipoprotein cholesterol) control parameters among patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2022, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Alqerban, A. Levels of proinflammatory chemokines and advanced glycation end products in patients with type-2 diabetes mellitus undergoing fixed orthodontic treatment. Angle Orthod. 2021, 91, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Băbţan, A.M.; Ilea, A.; Boşca, B.A.; Crişan, M.; Petrescu, N.B.; Collino, M.; Sainz, R.M.; Gerlach, J.Q.; Câmpian, R.S. Advanced Glycation End Products As Biomarkers in Systemic Diseases: Premises and Perspectives of Salivary Advanced Glycation End Products. Biomark. Med. 2019, 13, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Lapolla, A.; Odetti, P.; Fogarty, J.; Monnier, V.M. Pentosidine Formation in Skin Correlates with Severity of Complications in Individuals with Long-Standing IDDM. Diabetes 1992, 41, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.J.C.; Light, N.D.; Bailey, A.J. Evidence for glucose-mediated covalent cross-linking of collagen after glycosylation in vitro. Biochem. J. 1985, 225, 745–752. [Google Scholar] [CrossRef]
- Vincent, A.M.; Perrone, L.; Sullivan, K.A.; Backus, C.; Sastry, A.M.; Lastoskie, C.; Feldman, E.L. Receptor for Advanced Glycation End Products Activation Injures Primary Sensory Neurons via Oxidative Stress. Endocrinology 2007, 148, 548–558. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A.; Archibald, V.; Dines, K.C.; Maxfield, E.K. Anti-oxidant and pro-oxidant effects on nerve conduction velocity, endoneurial blood flow and oxygen tension in non-diabetic and streptozotocin-diabetic rats. Diabetologia 1994, 37, 449–459. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A.; Dines, K.C.; Maxfield, E.K.; Carey, F.; Mirrlees, D.J. Aldose reductase inhibition, nerve perfusion, oxygenation and function in streptozotocin-diabetic rats: Dose-response considerations and independence from a myo-inositol mechanism. Diabetologia 1994, 37, 651–663. [Google Scholar] [CrossRef]
- Brownlee, M.; Vlassara, H.; Cerami, A. Trapped Immunoglobulins on Peripheral Nerve Myelin From Patients with Diabetes Mellitus. Diabetes 1986, 35, 999–1003. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, L.-H.; Wang, X.-H.; Wang, C.-H.; Yu, C.; Zhang, X.-L.; Ning, L.-Y.; Huang, H.-Y.; Su, J.-B.; Wang, X.-Q. Plasma 1,5-anhydro-d-glucitol is associated with peripheral nerve function and diabetic peripheral neuropathy in patients with type 2 diabetes and mild-to-moderate hyperglycemia. Diabetol. Metab. Syndr. 2022, 14, 24. [Google Scholar] [CrossRef]
- Rajaobelina, K.; Farges, B.; Nov, S.; Maury, E.; Cephise-Velayoudom, F.; Gin, H.; Helmer, C.; Rigalleau, V. Skin autofluorescence and peripheral neuropathy four years later in type 1 diabetes. Diabetes/Metab. Res. Rev. 2017, 33, e2832. [Google Scholar] [CrossRef]
- Yaffe, K.; Lindquist, K.; Schwartz, A.V.; Vitartas, C.; Vittinghoff, E.; Satterfield, S.; Simonsick, E.M.; Launer, L.; Rosano, C.; Cauley, J.A.; et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 2011, 77, 1351–1356. [Google Scholar] [CrossRef]
- Sempere-Bigorra, M.; Brognara, L.; Julian-Rochina, I.; Mazzotti, A.; Cauli, O. Relationship between deep and superficial sensitivity assessments and gait analysis in diabetic foot patients. Int. Wound J. 2023, 20, 3023–3034. [Google Scholar] [CrossRef]
- Posada-Ordax, J.; Cosin-Matamoros, J.; Losa-Iglesias, M.E.; Becerro-De-Bengoa-Vallejo, R.; Esteban-Gonzalo, L.; Martin-Villa, C.; Calvo-Lobo, C.; Rodriguez-Sanz, D. Accuracy and Repeatability of Spatiotemporal Gait Parameters Measured with an Inertial Measurement Unit. J. Clin. Med. 2021, 10, 1804. [Google Scholar] [CrossRef] [PubMed]
- Bugané, F.; Benedetti, M.; Casadio, G.; Attala, S.; Biagi, F.; Manca, M.; Leardini, A. Estimation of spatial-temporal gait parameters in level walking based on a single accelerometer: Validation on normal subjects by standard gait analysis. Comput. Methods Programs Biomed. 2012, 108, 129–137. [Google Scholar] [CrossRef]
- Amatachaya, S.; Naewla, S.; Srisim, K.; Arrayawichanon, P.; Siritaratiwat, W. Concurrent validity of the 10-meter walk test as compared with the 6-minute walk test in patients with spinal cord injury at various levels of ability. Spinal Cord 2014, 52, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the Reliability and Validity of a Shorter Walk Test Compared with the 10-Meter Walk Test for Measurements of Gait Speed in Healthy, Older Adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.L.; Pin, T.W. Reliability, validity and minimal detectable change of 2-minute walk test, 6-minute walk test and 10-meter walk test in frail older adults with dementia. Exp. Gerontol. 2019, 115, 9–18. [Google Scholar] [CrossRef]
- Brognara, L.; Arceri, A.; Zironi, M.; Traina, F.; Faldini, C.; Mazzotti, A. Gait Spatio-Temporal Parameters Vary Significantly Between Indoor, Outdoor and Different Surfaces. Sensors 2025, 25, 1314. [Google Scholar] [CrossRef]
- Pasquini, A.; Jacopetti, M.; Pogliacomi, F.; Ramazzina, I.; Costantino, C. Neuromuscular recovery in ACL reconstruction with Bone-Tendon-Patellar-Bone and Semitendinosus-Gracilis autograft. Acta Biomed. 2017, 88, 62–68. [Google Scholar] [CrossRef]
- Carral, J.M.C.; Pallin, E.; Orbegozo, A.; Pérez, C.A. Effects of Three Different Chair-Based Exercise Programs on People Older Than 80 Years. Rejuvenation Res. 2017, 20, 411–419. [Google Scholar] [CrossRef]
- Vila, M.H.; Pérez, R.; Mollinedo, I.; Cancela, J.M. Analysis of Gait for Disease Stage in Patients with Parkinson’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 720. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Fransson, P.; Lush, D.; Gomez, S. The effect of foam surface properties on postural stability assessment while standing. Gait Posture 2008, 28, 649–656. [Google Scholar] [CrossRef]
- Paulus, W.; Straube, A.; Brandt, T.H. Visual postural performance after loss of somatosensory and vestibular function. J. Neurol. Neurosurg. Psychiatry 1987, 50, 1542–1545. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.M.; Yan, S.F.; Yan, S.; Schmidt, A.M. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res. Rev. 2002, 1, 1–15. [Google Scholar] [CrossRef]
- Casadei, G.; Filippini, M.; Brognara, L. Glycated Hemoglobin (HbA1c) as a Biomarker for Diabetic Foot Peripheral Neuropathy. Diseases 2021, 9, 16. [Google Scholar] [CrossRef]
- Vouillarmet, J.; Maucort-Boulch, D.; Michon, P.; Thivolet, C. Advanced Glycation End Products Assessed by Skin Autofluorescence: A New Marker of Diabetic Foot Ulceration. Diabetes Technol. Ther. 2013, 15, 601–605. [Google Scholar] [CrossRef]
- Akyüz, S.; Mutlu, A.B.B.; Guven, H.E.; Başak, A.M.; Yilmaz, K.B. Elevated HbA1c level associated with disease severity and surgical extension in diabetic foot patients. Turk. J. Trauma Emerg. Surg. 2023, 29, 1013–1018. [Google Scholar] [CrossRef]
- Eckert, A.J.; Zimny, S.; Altmeier, M.; Dugic, A.; Gillessen, A.; Bozkurt, L.; Götz, G.; Karges, W.; Wosch, F.J.; Kress, S.; et al. Factors associated with diabetic foot ulcers and lower limb amputations in type 1 and type 2 diabetes supported by real-world data from the German/Austrian DPV registry. J. Diabetes 2024, 16, e13531. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Brem, H.; Ehrlich, P.; Zhang, J.-G.; Cai, W.; Li, Z.; Croitoru, A.; Thung, S.; Vlassara, H. Adverse Effects of Dietary Glycotoxins on Wound Healing in Genetically Diabetic Mice. Diabetes 2003, 52, 2805–2813. [Google Scholar] [CrossRef]
- Sullivan, E.V.; Rose, J.; Rohlfing, T.; Pfefferbaum, A. Postural sway reduction in aging men and women: Relation to brain structure, cognitive status, and stabilizing factors. Neurobiol. Aging 2009, 30, 793–807. [Google Scholar] [CrossRef]
- Chau, R.M.; Ng, T.K.; Kwan, R.L.; Choi, C.H.; Cheing, G.L. Risk of fall for people with diabetes. Disabil. Rehabil. 2013, 35, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
- Masdeu, J.C. Gait and balance disorders. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 939–955. [Google Scholar] [CrossRef]
- Gu, Y.; Dennis, S.M. Are falls prevention programs effective at reducing the risk factors for falls in people with type-2 diabetes mellitus and peripheral neuropathy: A systematic review with narrative synthesis. J. Diabetes Complicat. 2017, 31, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, A.; Justine, M.; Mustafah, N.M.; Jamil, N.; Manaf, H. Postural Control and Gait Performance in the Diabetic Peripheral Neuropathy: A Systematic Review. BioMed Res. Int. 2016, 2016, 9305025. [Google Scholar] [CrossRef] [PubMed]
- Hewston, P.; Deshpande, N. Falls and Balance Impairments in Older Adults with Type 2 Diabetes: Thinking Beyond Diabetic Peripheral Neuropathy. Can. J. Diabetes 2016, 40, 6–9. [Google Scholar] [CrossRef]
- Pizzigalli, L.; Cremasco, M.M.; Mulasso, A.; Rainoldi, A. The contribution of postural balance analysis in older adult fallers: A narrative review. J. Bodyw. Mov. Ther. 2016, 20, 409–417. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Ekoru, K.; Doumatey, A.; Bentley, A.R.; Chen, G.; Zhou, J.; Shriner, D.; Fasanmade, O.; Okafor, G.; Eghan, B.; Agyenim-Boateng, K.; et al. Type 2 diabetes complications and comorbidity in Sub-Saharan Africans. eClinicalMedicine 2019, 16, 30–41. [Google Scholar] [CrossRef]
- Ang, L.; Jaiswal, M.; Martin, C.; Pop-Busui, R. Glucose Control and Diabetic Neuropathy: Lessons from Recent Large Clinical Trials. Curr. Diabetes Rep. 2014, 14, 528. [Google Scholar] [CrossRef]
- Bonnet, C.; Carello, C.; Turvey, M.T. Diabetes and Postural Stability: Review and Hypotheses. J. Mot. Behav. 2009, 41, 172–190. [Google Scholar] [CrossRef]
- Brown, S.J.; Handsaker, J.C.; Bowling, F.L.; Boulton, A.J.; Reeves, N.D. Diabetic peripheral neuropathy compromises balance during daily activities. Diabetes Care 2015, 38, 1116–1122. [Google Scholar] [CrossRef]
- Piras, A.; Perazzolo, M.; Scalinci, S.Z.; Raffi, M. The effect of diabetic retinopathy on standing posture during optic flow stimulation. Gait Posture 2022, 95, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Sawacha, Z.; Spolaor, F.; Guarneri, G.; Contessa, P.; Carraro, E.; Venturin, A.; Avogaro, A.; Cobelli, C. Abnormal muscle activation during gait in diabetes patients with and without neuropathy. Gait Posture 2012, 35, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W. AGEs and Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2010, 51, 4867–4874. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Kim, W.J.; Park, C.Y.; Park, S.E.; Rhee, E.J.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Kim, S.W.; Park, H.S.; Kim, Y.J.; et al. Serum 1,5-anhydroglucitol is associated with diabetic retinopathy in Type 2 diabetes. Diabet. Med. 2012, 29, 1184–1190. [Google Scholar] [CrossRef]
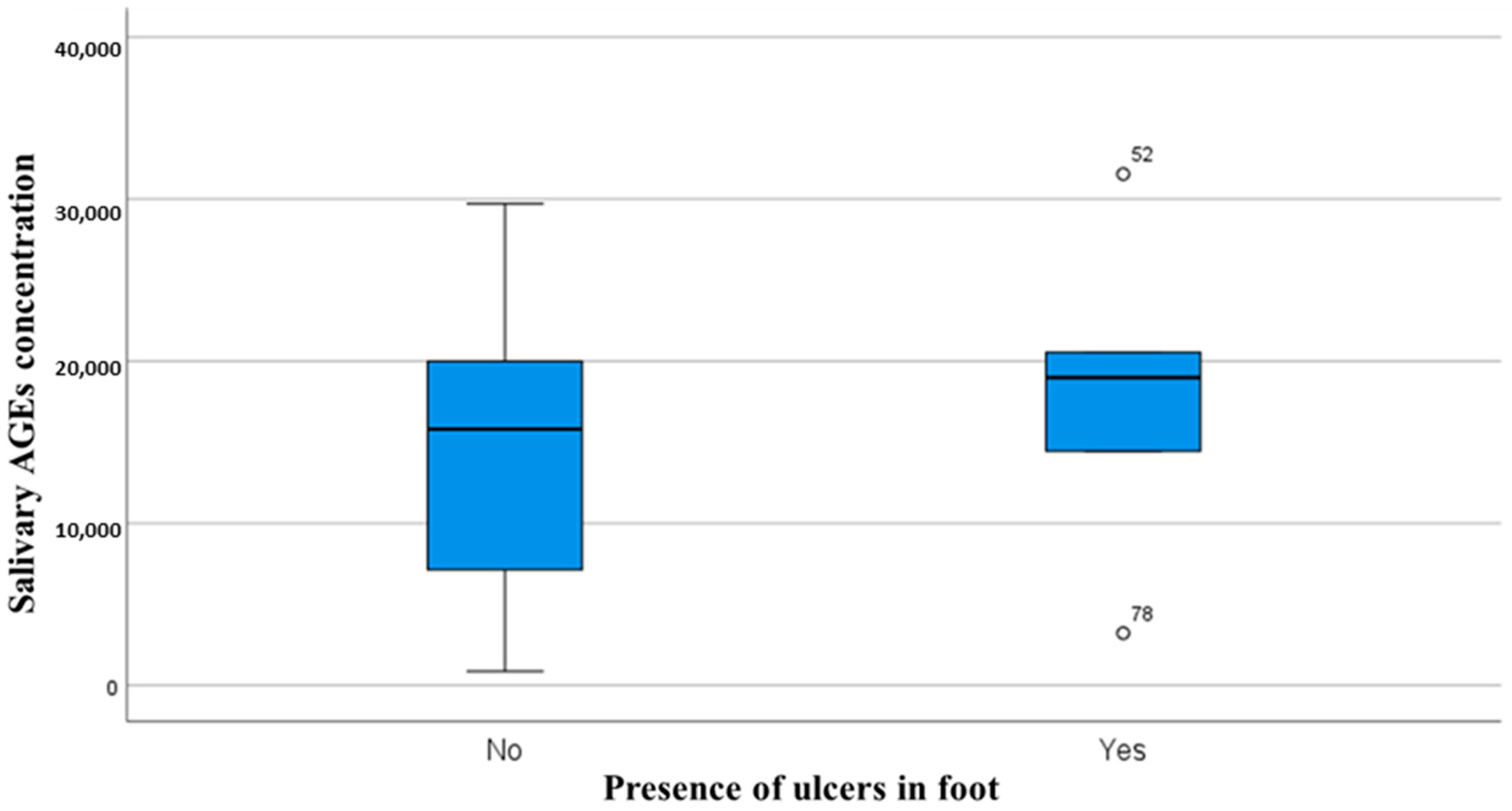
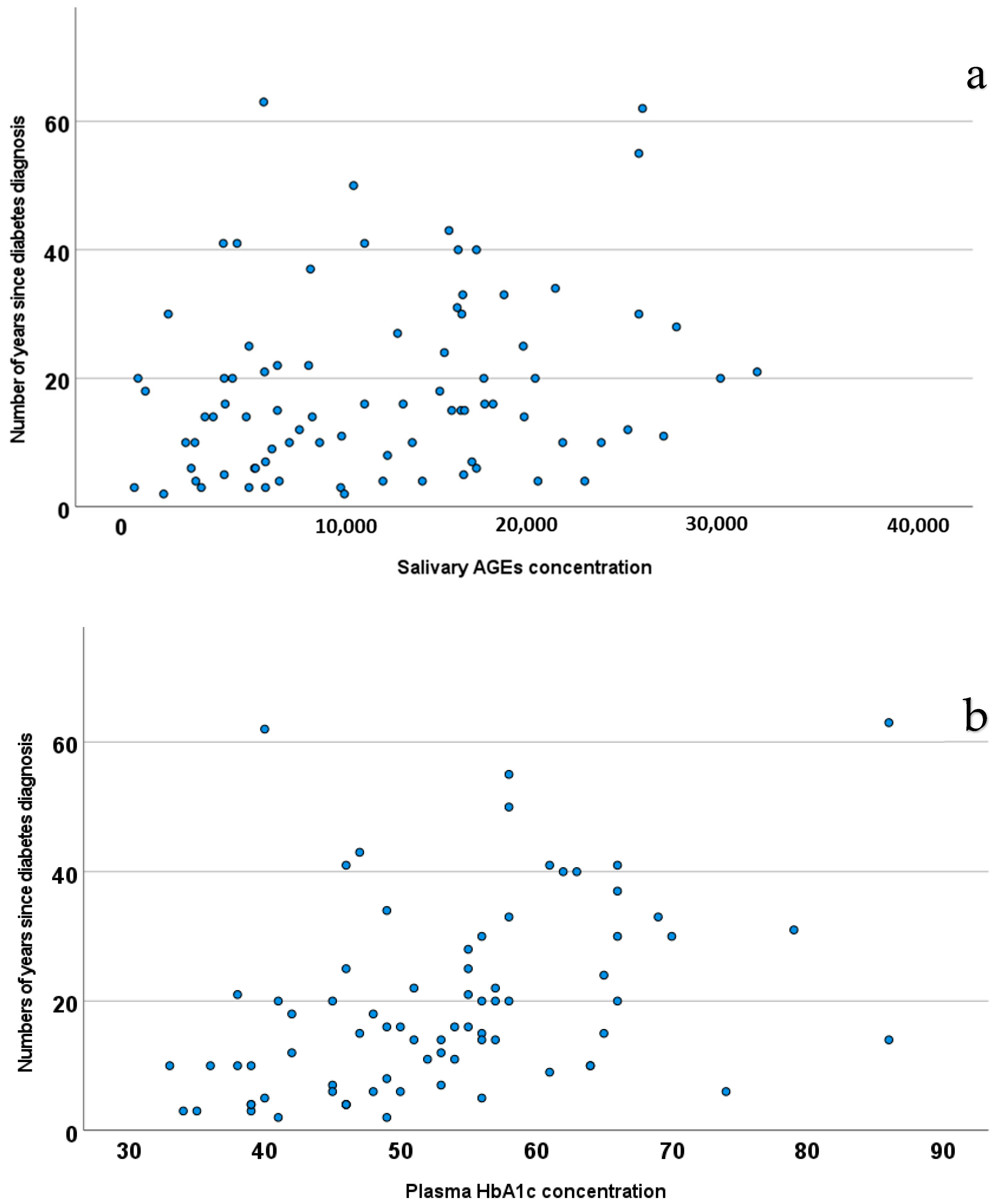
| Salivary 1,5-Anhydroglucitol Concentration | Salivary AGE Concentration | |||
|---|---|---|---|---|
| Correlation Coefficients | p Values | Correlation Coefficients | p Values | |
| Age | 0.074 | 0.635 | 0.106 | 0.472 |
| Body mass index (BMI) | −0.012 | 0.937 | −0.129 | 0.383 |
| Glycemia (fasting glucose concentration in plasma) | −0.269 | 0.108 | 0.234 | 0.141 |
| HbA1c (glycated hemoglobin concentration in plasma) | −0.203 | 0.221 | 0.394 | 0.009 |
| Speed | 0.217 | 0.173 | 0.207 | 0.168 |
| Cadence | −0.073 | 0.651 | −0.033 | 0.83 |
| Stride_Length_centimeters | 0.278 | 0.078 | 0.13 | 0.389 |
| Stance_Duration | −0.046 | 0.773 | 0.042 | 0.781 |
| Swing_Duration | −0.024 | 0.884 | 0.102 | 0.998 |
| Double_Support_duration | −0.022 | 0.89 | 0.056 | 0.713 |
| Single_Support_Duration | −0.024 | 0.884 | 0.102 | 0.998 |
| mStep_Length_LR_cm | 0.227 | 0.153 | 0.161 | 0.286 |
| mStep_Duration_LR_ms | 0.047 | 0.772 | 0.048 | 0.752 |
| mStep_Duration_LR_s | 0.052 | 0.748 | 0.042 | 0.784 |
| mStance_Duration_LR | −0.071 | 0.659 | 0.047 | 0.755 |
| mSwing_Duration_LR | 0.011 | 0.946 | −0.004 | 0.978 |
| EO_SP_AP_axis | −0.253 | 0.106 | −0.136 | 0.36 |
| EO_SP_ML_axis | −0.099 | 0.534 | −0.114 | 0.444 |
| EO_Sway_Area | −0.077 | 0.629 | −0.194 | 0.191 |
| EO_Foam_SP_AP_axis | −0.196 | 0.214 | −0.209 | 0.159 |
| EO_Foam_SP_ML_axis | −0.251 | 0.109 | −0.031 | 0.834 |
| EO_Foam_Sway_Area | −0.173 | 0.273 | −0.119 | 0.424 |
| EO_Tandem_SP_AP_axis | −0.163 | 0.301 | 0.108 | 0.47 |
| EO_Tandem_SP_ML_axis | −0.11 | 0.488 | 0.056 | 0.706 |
| EO_Tandem_Sway_Area | −0.031 | 0.848 | 0.086 | 0.565 |
| EO_TF_SP_AP_axis | −0.087 | 0.585 | 0.143 | 0.337 |
| EO_TF_SP_ML_axis | −0.097 | 0.542 | 0.133 | 0.371 |
| EO_TF_Sway_Area | −0.073 | 0.648 | 0.106 | 0.479 |
| EC_SP_AP_axis | −0.071 | 0.653 | −0.244 | 0.099 |
| EC_SP_ML_axis | −0.132 | 0.406 | −0.04 | 0.79 |
| EC_Sway_Area | −0.069 | 0.664 | −0.104 | 0.486 |
| EC_Foam_SP_AP_axis | −0.013 | 0.935 | −0.13 | 0.382 |
| EC_Foam_SP_ML_axis | 0.067 | 0.671 | −0.022 | 0.883 |
| EC_Foam_Sway_Area | 0.015 | 0.926 | −0.068 | 0.649 |
| EC_Tandem_SP_AP_axis | 0.217 | 0.167 | −0.057 | 0.705 |
| EC_Tandem_SP_ML_axis | 0.365 | 0.017 | 0.052 | 0.728 |
| EC_Tandem_Sway_Area | 0.334 | 0.031 | −0.107 | 0.476 |
| EC_TF_SP_AP_axis | 0.182 | 0.256 | 0.419 | 0.004 |
| EC_TF_SP_ML_axis | 0.209 | 0.183 | 0.436 | 0.002 |
| EC_TF_Sway_Area | 0.192 | 0.224 | 0.387 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brognara, L.; Sempere-Bigorra, M.; Cauli, O. Salivary 1,5-Anhydroglucitol and AGEs Are Associated with Postural Instability in Diabetic Foot Patients. Medicina 2025, 61, 968. https://doi.org/10.3390/medicina61060968
Brognara L, Sempere-Bigorra M, Cauli O. Salivary 1,5-Anhydroglucitol and AGEs Are Associated with Postural Instability in Diabetic Foot Patients. Medicina. 2025; 61(6):968. https://doi.org/10.3390/medicina61060968
Chicago/Turabian StyleBrognara, Lorenzo, Mar Sempere-Bigorra, and Omar Cauli. 2025. "Salivary 1,5-Anhydroglucitol and AGEs Are Associated with Postural Instability in Diabetic Foot Patients" Medicina 61, no. 6: 968. https://doi.org/10.3390/medicina61060968
APA StyleBrognara, L., Sempere-Bigorra, M., & Cauli, O. (2025). Salivary 1,5-Anhydroglucitol and AGEs Are Associated with Postural Instability in Diabetic Foot Patients. Medicina, 61(6), 968. https://doi.org/10.3390/medicina61060968








