1. Introduction
Osteosarcoma is a malignant tumor originating from bone tissue, most commonly affecting the knee and shoulder regions. It represents the most prevalent primary bone malignancy in children and adolescents. The standard treatment for osteosarcoma involves a combination of chemotherapy and surgical resection, with early diagnosis being a critical determinant of treatment success [
1]. Despite being the most common primary bone cancer in the adolescent population, osteosarcoma is associated with a poor prognosis, particularly after metastasis. Although considerable progress has been made in understanding the disease, the five-year survival rate has improved only marginally, underscoring the limitations of current therapeutic approaches [
2,
3].
Cis and doxorubicin are among the most frequently used chemotherapeutic agents, either as monotherapy or in combination, for the treatment of osteosarcoma. Cis functions primarily by inducing DNA damage in tumor cells, thereby inhibiting cell proliferation and triggering apoptosis. However, a major challenge associated with Cis therapy is the emergence of drug resistance in some patients. This resistance reduces treatment efficacy, extends the duration of therapy, and compromises clinical outcomes [
4]. In light of these limitations, the development of novel therapeutic strategies, including combination treatments, is gaining increasing attention to overcome Cis resistance.
Over the past four decades, natural compounds have played a pivotal role in the discovery and development of anticancer agents. Bioactive molecules derived from natural sources, as well as their synthetic or semi-synthetic derivatives, have served as essential lead compounds in the design of more effective and targeted cancer therapies. Numerous studies have demonstrated that flavonoids play a significant role in cancer chemotherapy. These compounds interact with a wide range of genes and enzymes, modulating various molecular pathways involved in tumor progression. Their mechanisms of action include induction of cell cycle arrest, promotion of apoptosis, and inactivation of carcinogens [
5]. Hesperidin, a naturally occurring bioflavonoid belonging to the coumarin family, has garnered increasing attention for its anticancer potential. Coumarins are well-documented for their diverse biological activities, including anticancer effects. They have been reported to act as immunomodulators in malignant melanoma [
6], to exert antiproliferative effects in bladder cancer [
7], and to induce apoptosis in MG-67 osteosarcoma cells both in vitro and in vivo [
8]. For example, curcumol, a bioactive compound, has been shown to enhance the cytotoxic effects of Cis when used in combination therapy, significantly suppressing osteosarcoma tumor growth. Moreover, recent studies have identified the enzyme Otulin as a critical factor in the development of Cis resistance. Targeting Otulin may represent a promising strategy to overcome chemoresistance and improve therapeutic outcomes in osteosarcoma treatment [
9,
10].
Members of the Bcl-2 protein family are essential regulators of apoptotic cell death. Aberrant overexpression of pro-survival Bcl-2 family proteins or reduced expression of pro-apoptotic Bcl-2 family proteins, both of which suppress apoptosis, are frequently observed in various types of cancer. Due to their central role in the regulation of apoptosis, these proteins are considered promising targets for the development of new anticancer therapies [
11]. Bax, a pro-apoptotic protein within the Bcl-2 family, plays a critical role in the initiation of apoptosis. When Bax expression is dominant, it promotes apoptotic activity. In response to apoptotic signals, the synthesis of Bax increases, which neutralizes the anti-apoptotic effects of Bcl-2 and enhances the apoptotic response. Under normal physiological conditions, Bax is localized in the cytosol. However, during apoptosis, it translocates to the mitochondrial membrane, where it contributes to the formation of pores, leading to the release of cytochrome c and the activation of downstream apoptotic pathways. The loss of Bax function has been associated with the development of certain leukemias and colorectal cancers [
12].
In this study, we demonstrated that the combination of Hes and Cis exerts synergistic antitumor effects on osteosarcoma cells, as evidenced by cell proliferation, apoptosis, and migration assays. Specifically, our findings indicate that this combination enhances the sensitivity of osteosarcoma cells to Cis, suggesting its potential as a promising therapeutic strategy for osteosarcoma patients. Although numerous studies have reported the potent anticancer effects of phytochemicals with alternative therapeutic properties, biological challenges such as the selective targeting of cancer cells, efficient cellular delivery, and initiation of specific molecular interactions remain significant obstacles. These limitations highlight the importance of developing combination therapies, particularly those involving two or more agents, to achieve improved therapeutic outcomes. Currently, increasing attention is being directed toward combination treatments and the modulation of cell death pathways in cancer research. To our knowledge, this is the first study to demonstrate the therapeutic potential of a combined Hes and Cis treatment in osteosarcoma, providing novel insights into their synergistic mechanisms of action.
2. Material and Methods
2.1. Chemicals
Hesperidin (Sigma-Aldrich, H5254, ≥80%, 25 g), MTT, and RPMI-1640 medium were purchased from Sigma-Aldrich (Hamburg, Germany). Cisplatin (50 mg/100 mL, intravenous formulation) was obtained from Orna Farma (Koçak Pharma, Istanbul, Türkiye). Fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
2.2. Cell Culture
The human osteosarcoma cell line U2OS was obtained from Cytion (Catalog No. 300364, Eppelheim, Germany). Following verification, the cells were expanded for stock, cryopreserved, and cultured in 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA). Cell lines were passaged every three days and maintained for a maximum duration of two months. Short Tandem Repeat (STR) analysis was performed to authenticate the identity of all cell lines. Cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and incubated at 37 °C in a humidified atmosphere containing 5% CO2.
2.3. MTS Assay
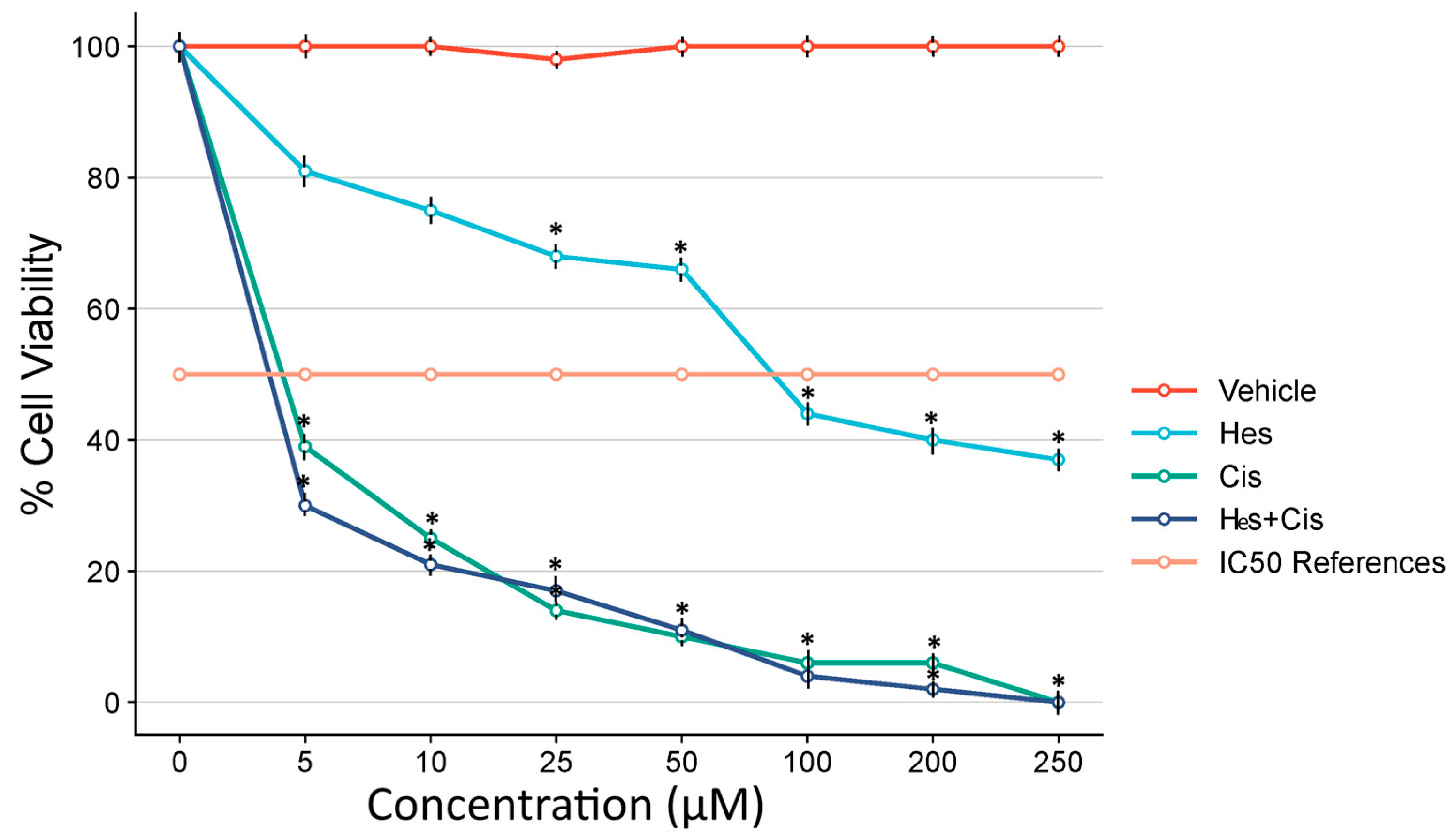
To evaluate cytotoxicity in cells treated with Hes and Cis, the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) was obtained from Promega Corporation, Madison, WI, USA. This assay utilizes the reagent 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium to measure cell viability. A total of 5 × 103 human osteosarcoma U2OS cells were seeded into 96-well plates, incubated overnight, and subsequently treated with increasing concentrations of Hes (0, 5, 25, 50, 100, 150, 200, and 250 µM) and Cis (0, 10, 20, 30, 40, 50, 60, and 70 µM). Following 48 h of incubation, the MTS reagent was added to each well in accordance with the manufacturer’s protocol. Absorbance was measured at 492 nm using a Multiskan GO microplate reader (Thermo Scientific, Waltham, MA, USA).
2.4. Determination of IC50
Based on the results of the MTS assay, the IC50 values for Hes and Cis in both control and experimental groups were determined using Combenefit v2.021 software to assess the effectiveness of each compound at varying concentrations.
2.5. Antagonist–Synergist Effect
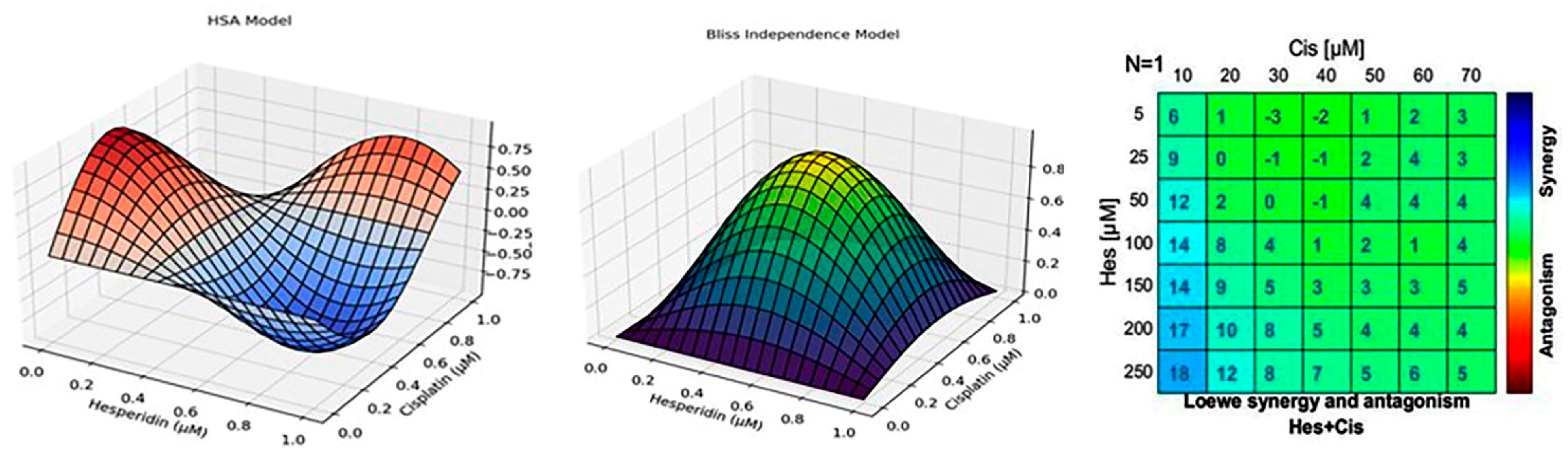
To evaluate the antagonistic or synergistic interaction between Hes and Cis, eight different concentrations of each compound were prepared using serial dilution, resulting in 64 distinct combination conditions. Following 48 h of incubation, cell viability was assessed using the MTS assay. The dose–response curves for each compound were established in 96-well plates with five replicates per condition. The data obtained from the MTS assay were analyzed using Combenefit software (Cambridge, UK) to determine potential synergistic or antagonistic effects between the two agents. After identifying the IC50 values, experimental groups were established using the determined IC50 doses for U2OS cells. The IC50 values were calculated through probit analysis using the SPSS version 20 statistical software package. The resulting IC50 doses were subsequently used in all further experimental procedures.
In addition to the Chou–Talalay CI and HSA models, the Bliss Independence and Loewe Additivity models were also applied using Combenefit software to validate the observed interaction between Hes and Cis. These models offer complementary perspectives on drug synergy by evaluating expected versus observed effects. The inclusion of multiple interaction models enhances the methodological robustness of our findings and addresses variability in synergy interpretation across different frameworks.
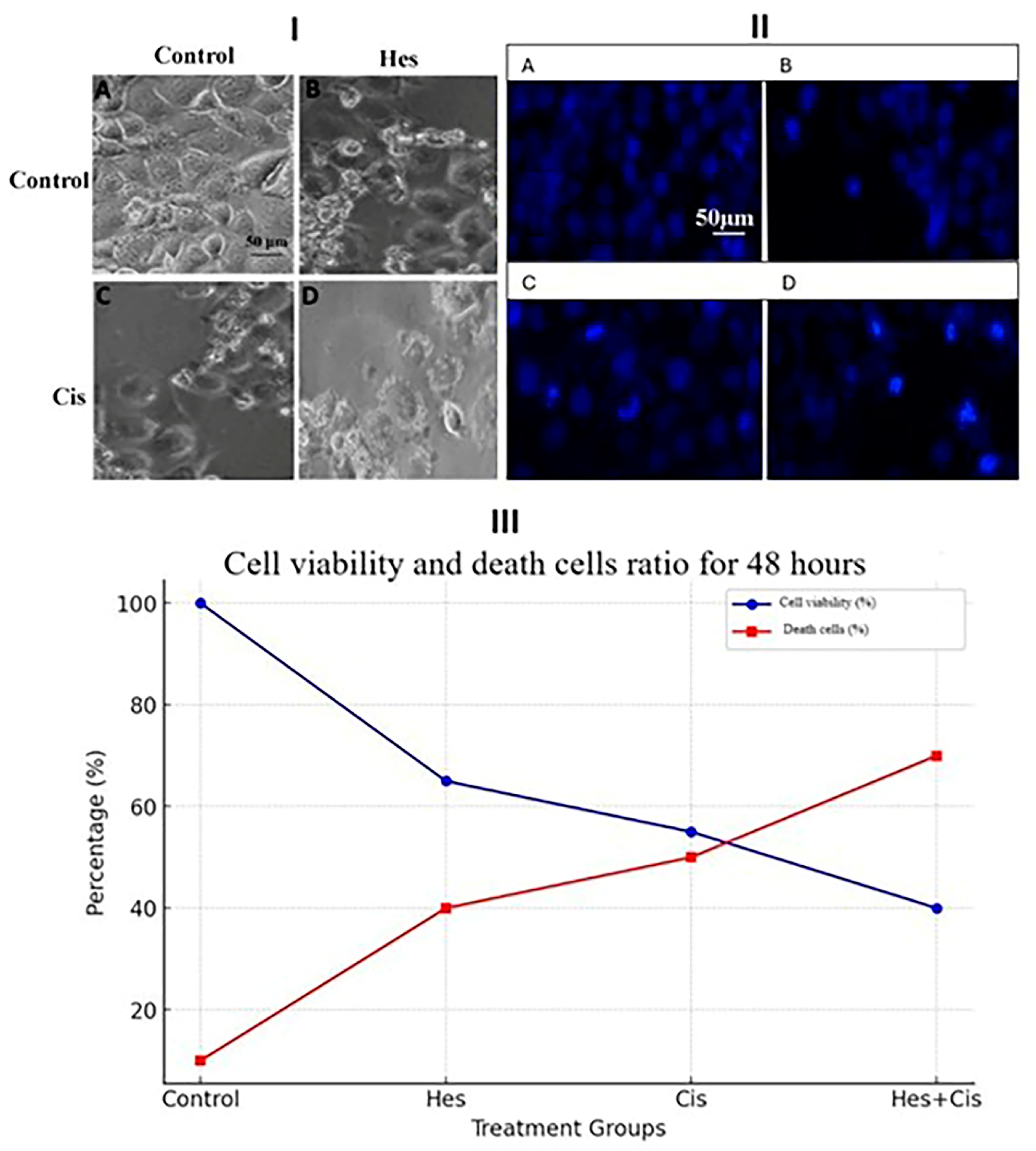
2.6. Hoechst 33342 Fluorescent Staining
The cell death induced by Hes and Cis in U2OS cells was assessed using Hoechst 33342 (Thermo Scientific, Waltham, MA, USA), a fluorescent dye that specifically stains nuclei and highlights apoptotic features through morphological changes such as chromatin condensation and nuclear fragmentation. Osteosarcoma cells were seeded in 24-well plates at a density of 5 × 104 cells per well and incubated under standard conditions. After 24 h, cells were treated with Hes IC50, Cis IC50, and Hes IC50 + Cis IC50 concentrations, based on the 48 h IC50 values previously determined. Live-cell staining was performed in accordance with the manufacturer’s protocol, and cells were incubated at 37 °C for 30 min. Fluorescent images were then acquired using a DAPI filter at 20× objective magnification with the EVOS® FL Imaging System was obtained from Thermo Fisher Scientific, Waltham, MA, USA.
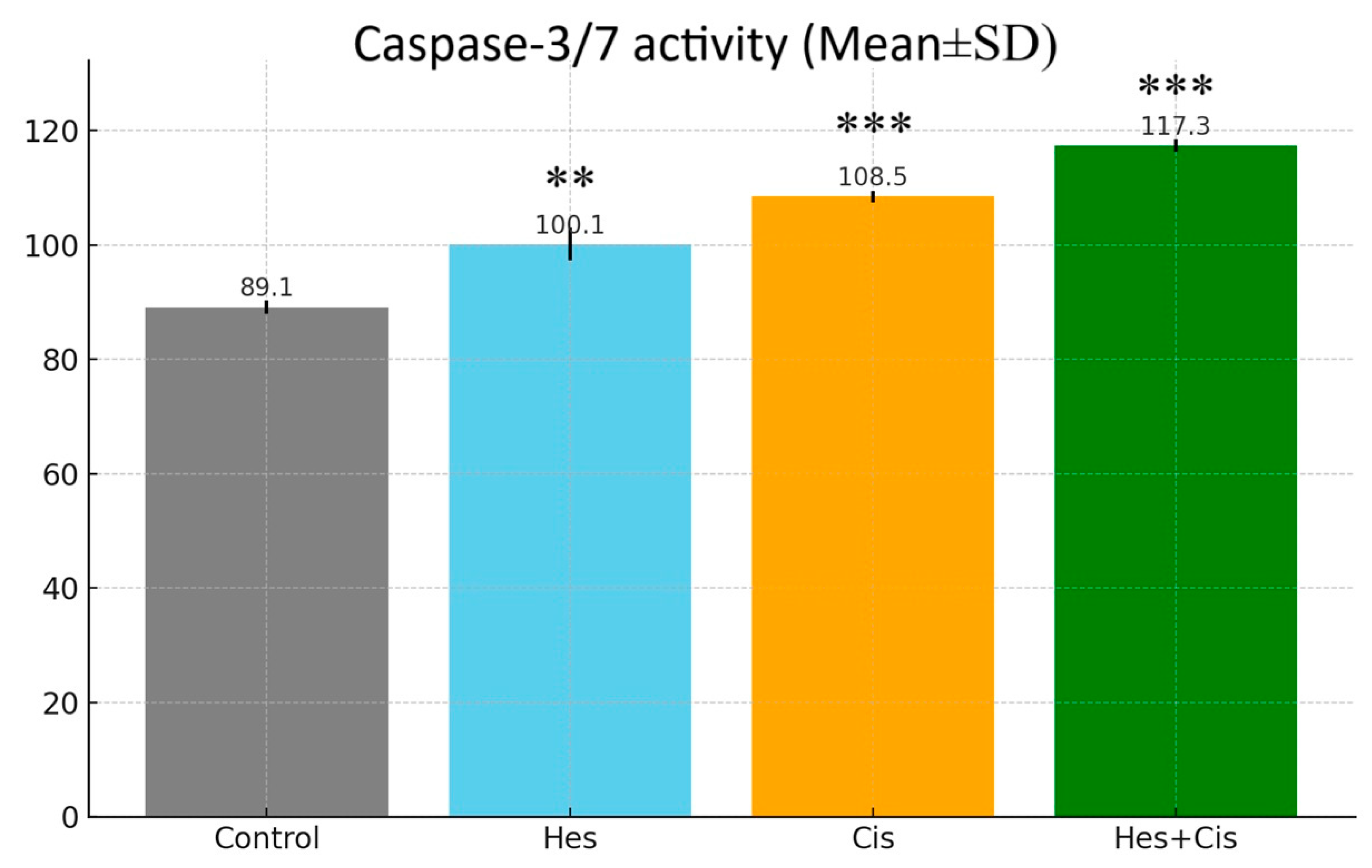
2.7. Caspase-3/7 Activity Assay
The Caspase-3/7 Glo Assay kit from Promega (Madison, WI, USA) was used to confirm the activity in the apoptosis pathway. Cells were treated in 96-well plates; caspase reagent was added, according to the kit procedure, at the end of the incubation and the cells were then left in the dark for 1 h at room temperature. Luminescence values were recorded with the assistance of a microplate reader and compared to the control group.
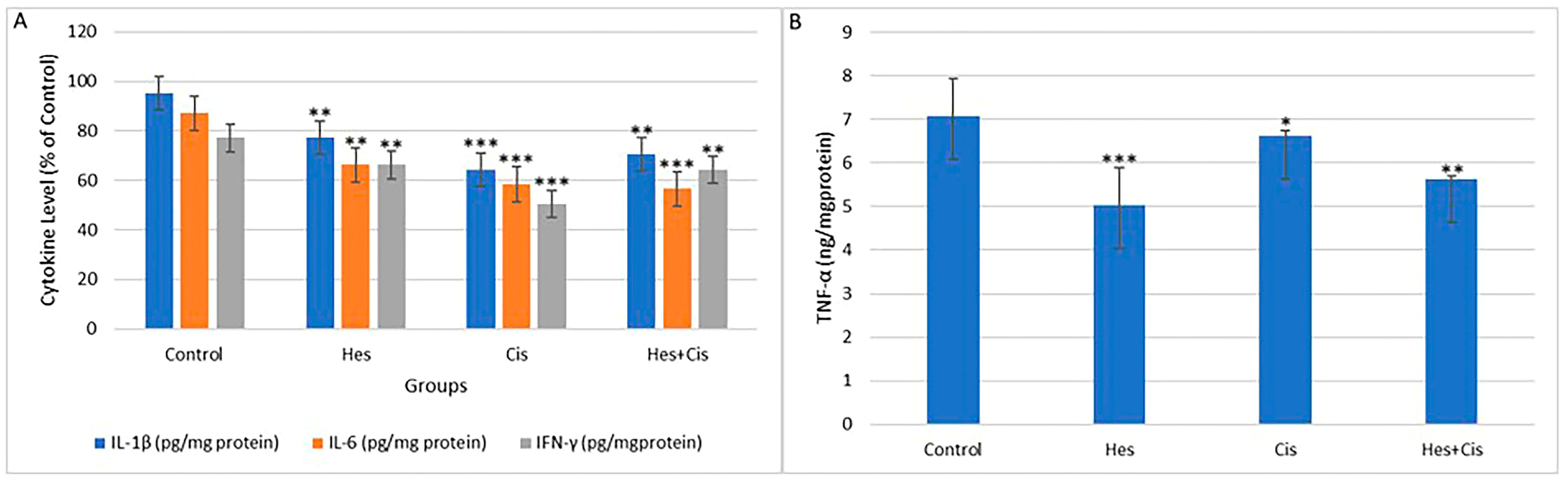
2.8. Inflammatory Cytokines (TNF-α, IFN-γ, IL-1, IL-6)
To evaluate the anti-inflammatory effects of crocin on MCF-7 cells, the levels of TNF-α (Invitrogen, Cat. No. 13-7341-81), IL-1β (Invitrogen, Cat. No. PA1-84913), IL-6 (Cat. No. EH2IL6), and IFN-γ (Invitrogen, Waltham, MA, USA, Cat. No. 14-7311-81) were measured spectrophotometrically at 540 nm.
2.9. Gene Expression
Quantitative gene expression analysis was conducted to evaluate the effects of Hes and Cis on pro-apoptotic and anti-apoptotic gene expression in U2OS cells. For this purpose, specific primer sets targeting the genes of interest were designed. The β-actin gene was used as an internal reference to normalize gene expression levels. Relative changes in gene expression were calculated using the threshold cycle (Ct) values.
2.10. Total RNA Isolation
For total RNA isolation, osteosarcoma cells were seeded into 6-well plates and treated with Hes IC50, Cis IC50, and the Hes IC50 + Cis IC50 combination. Following treatment, the cells were harvested by adding 1 mL of cold phosphate-buffered saline (PBS) to each well. Total RNA was isolated using a commercial kit, in accordance with the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA). The purity and concentration of the RNA were assessed using an Optizen NanoQ micro-volume spectrophotometer (Mecasys, Yuseong-gu, Republic of Korea). RNA samples were then diluted with ultrapure water to a final concentration of 750 ng/10 μL. Subsequently, complementary DNA (cDNA) synthesis was performed using the corresponding kit, following the manufacturer’s protocol (Bio-Rad, Hercules, CA, USA). The synthesized cDNA samples were stored at –20 °C, while the remaining RNA samples were preserved at –86 °C for further analyses.
2.11. RT-qPCR
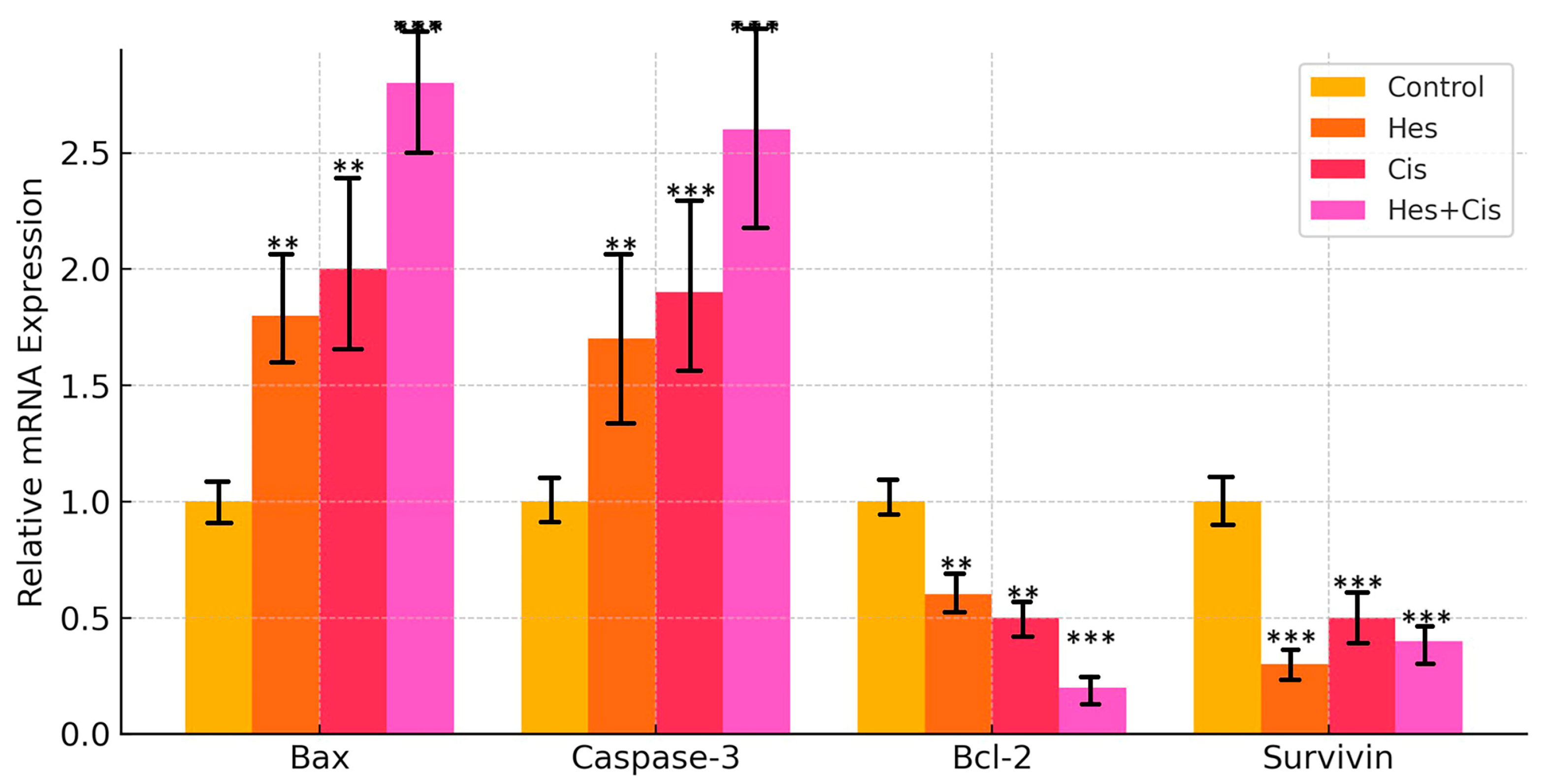
Complementary DNAs (cDNAs) were amplified using the SYBR Green-based quantitative PCR kit (2× qPCRBIO SyGreen Mix Lo-ROX Kit, PCR Biosystems, London, UK). The expression levels of Bax, Bcl-2, Caspase-3, and Survivin—key genes involved in the apoptotic pathway—were assessed in control and treatment groups of osteosarcoma (U2OS) cells using the real-time quantitative PCR (RT-qPCR) method. β-Actin was employed as the reference housekeeping gene for normalization. Gene amplification and quantification were performed using a Bio-Rad CFX96 real-time PCR system. The untreated group served as the negative control. The forward and reverse primer sequences used in the RT-qPCR are listed in
Table 1. The qPCR cycling conditions were as follows: initial denaturation at 95 °C for 2 min, followed by 40 cycles at 95 °C for 5 s, 45 cycles at 66 °C for 45 s, 45 cycles at 74 °C for 2 min, and a final extension step at 72 °C for 5 min. Relative mRNA expression levels were calculated using the 2
−ΔΔCt method. All cDNA and standard samples were analyzed under identical conditions and in triplicate to minimize intra-group variability. Expression of endogenous β-actin mRNA was used as an internal control for both calibration and normalization.
2.12. Statistical Analysis
The differences in cell viability ratios, as determined by the MTS assay, and the mean gene expression levels obtained from RT-qPCR analyses were evaluated using two-way analysis of variance (ANOVA). Post hoc comparisons were conducted using the Tukey Honestly Significant Difference (HSD) test to identify statistically significant differences between groups. For pairwise comparisons, the independent samples t-test was applied, provided that the assumption of data homogeneity was met. All statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY, USA), with a p-value of <0.05 considered statistically significant.
4. Discussion
In the clinical management of osteosarcoma, Cis-based chemotherapy remains a cornerstone of treatment; however, its efficacy is often limited by the development of drug resistance and adverse side effects. Consequently, the identification of novel therapeutic strategies, including combination therapies, is essential to improve treatment outcomes. In this study, we demonstrated that Hes and Cis exert a synergistic effect on U2OS osteosarcoma cells by enhancing apoptosis through the mitochondrial pathway. Our results showed that both Hes and Cis significantly upregulated the expression of the pro-apoptotic genes Bax and Caspase-3, while downregulating the anti-apoptotic genes Bcl-2 and Survivin, indicating the activation of apoptotic mechanisms. Moreover, the combination treatment resulted in a more pronounced reduction in cell proliferation compared to either agent alone, suggesting that Hes may sensitize osteosarcoma cells to Cis-induced apoptosis. While the Chou–Talalay model is a standard method for assessing combination indices, the use of the Bliss and Loewe models in this study provides additional layers of validation. Their integration reinforces the conclusion that the Hes–Cis combination exerts a reproducible synergistic effect that is not limited to a single modeling approach. This methodological diversity strengthens the translational potential of our findings. Furthermore, the calculated CI values (
Table 2) corroborated the observed synergistic interactions across multiple analytical models, confirming the robustness and reproducibility of the combination effect between Hes and Cis.
These findings underscore the potential of Hes as a chemosensitizer in osteosarcoma therapy and support further investigation into its clinical applicability. In clinical practice, Cis-based therapy remains a cornerstone in the treatment of osteosarcoma. Despite numerous combination therapies being evaluated over the past four decades, the long-term survival rate of osteosarcoma patients has shown little improvement [
14,
15]. This highlights a critical bottleneck in current chemotherapy approaches that must be addressed [
16]. In this study, we propose a novel and effective combination therapy for osteosarcoma. Hes was found to sensitize osteosarcoma cells to Cis-induced proliferation suppression in a time- and dose-dependent manner over a 48 h period. Furthermore, the combined treatment of Hes and Cis significantly induced apoptosis. Our findings demonstrate that Hes exerts a strong synergistic effect when used in combination with Cis. These results offer promising implications for improving therapeutic strategies in osteosarcoma patients.
In this study, the cytotoxic effect of Hes on apoptosis in U2OS osteosarcoma cells was demonstrated through its combined application with Cis. The apoptosis induced by Hes and Cis was activated via stimulation of Bax, Bcl-2, and Caspase-3, thereby initiating the intrinsic apoptotic pathway through mitochondrial activation. While Hes and Cis upregulated the expression of pro-apoptotic proteins (Bax and Caspase-3), they concurrently downregulated the expression of anti-apoptotic proteins (Bcl-2 and Survivin). These proteins are known to regulate intracellular apoptotic pathways and may enhance the sensitivity of U2OS cells to treatment. Notably, the findings from this study are consistent with those reported in studies using the MG-63 osteosarcoma cell line [
8]. A significant increase in the expression of both early and late apoptotic markers was observed following single and combined treatments with Hes and Cis (
p < 0.001), further supporting the potential of this combination in inducing apoptosis in osteosarcoma cells.
In recent years, combining natural compounds with conventional therapeutic agents has attracted considerable attention, not only in cancer research but also in studies involving various disease models [
17,
18]. The incorporation of natural agents into combination therapies for cancer has been shown to exert chemoprotective effects against anticancer drugs while simultaneously enhancing their anti-proliferative activity [
8,
19,
20]. A review of the literature revealed that Hes has previously been applied to the MG-63 osteosarcoma cell line; however, no study to date has investigated the effects of Hes alone or in combination with Hes + Cis on U2OS cells [
21]. This study is the first to demonstrate the anti-proliferative effects of the Hes + Cis combination in U2OS osteosarcoma cells.
In this study, single doses of Cis and Hes were initially administered and their IC50 values determined. Subsequently, the combination index was calculated, and various combination doses were applied to evaluate their effects on cell viability. Consequently, a synergistic effect was observed with the combination of 106 μM Hes and 4.83 μM Cis.
The present study demonstrated that oxidative stress was elevated in the group treated with Hes and Cis, likely due to a reduction in inflammatory cytokine levels. As a result, the expression of Bax, a pro-apoptotic gene, was upregulated. Numerous studies have reported that oxidative stress mediators originating from the cytosol and mitochondria induce apoptosis by activating apoptotic genes such as p53 [
22]. Furthermore, the combination treatment triggered apoptosis through the activation of Caspase-3. In certain cases, apoptosis may also be initiated in response to misfolded proteins, a process associated with endoplasmic reticulum (ER) stress. However, further studies are required to elucidate this mechanism. It is crucial to investigate agents that enhance PARP cleavage following the activation of effector caspases such as Caspase-3, -6, and -7. Some studies have demonstrated that curcumin can induce PARP cleavage [
23].
Previous studies have reported that coumarins exhibit immunomodulatory activity against malignant melanoma [
6]. In a study on bladder cancer, labeled coumarins were shown to suppress cell proliferation, highlighting their potential utility in clinical applications [
7]. Notably, this activity was observed even in the absence of UV radiation. Coumarins have also been found to affect the adhesion and motility of certain neoplastic cells; moreover, their impact on an invasive mouse melanoma cell line has been described. The processes of proliferation and survival signaling in cancer cells involve complex interactions among growth factors such as epidermal growth factor (EGF), transforming growth factor (TGF)-α, and TGF-β [
24]. Studies conducted on various cell lines have shown that Hes exerts anticarcinogenic effects by inhibiting cell proliferation. In the present study, it was observed that Hes, when combined with Cis, significantly downregulated the mRNA expression of Bcl-2, a key gene associated with cellular proliferation. These findings are in agreement with those of previous studies [
25,
26,
27]. In the treatment of cancers such as osteosarcoma, chemotherapy and radiotherapy remain indispensable. Therefore, the development of novel therapeutic strategies is of great importance [
28].
While the current study provides robust in vitro evidence for the synergistic antitumor effects of Hes and Cis in osteosarcoma cells, several important limitations must be acknowledged. First, the experiments were conducted in conventional 2D monolayer cultures, which do not replicate the complex three-dimensional architecture of the tumor microenvironment. Recent studies have demonstrated that tumor progression is strongly influenced by the biophysical properties of the ECM, including its stiffness and spatial organization, which modulate cellular behaviors through mechanotransduction and integrin signaling [
29]. These properties are not adequately modeled in 2D cultures, which may limit the translational relevance of the observed drug responses. Second, the tumor ECM, often referred to as the oncomatrix, undergoes dynamic remodeling during cancer development and metastasis, influencing cell adhesion, signaling, and drug sensitivity. The evolving biochemical and mechanical landscape of the oncomatrix plays a crucial role in tumor heterogeneity and resistance mechanisms but was not addressed in this study [
30]. Third, although multiple drug synergy models including the Chou–Talalay, Bliss, Loewe, and HSA models were applied to validate the interaction between Hes and Cis, these computational frameworks do not incorporate the physiological feedback loops, ECM interactions, or pharmacokinetic dynamics that are present in vivo. Future studies using 3D tumor spheroids, biomimetic hydrogels, or animal models are needed to confirm and extend these findings under more physiologically relevant conditions.
Future perspectives for the application of Hes in osteosarcoma treatment are promising yet require further exploration. Based on the synergistic antitumor effects observed in this study, future research should prioritize validating the efficacy and safety of Hes and Cis combination therapy in appropriate in vivo osteosarcoma models. Additionally, it will be important to investigate the pharmacokinetics, optimal dosing schedules, and potential toxicity profiles of this combination to ensure its clinical applicability. Molecular studies are also needed to clarify the upstream regulators and downstream signaling pathways beyond Bax and Bcl-2 modulation, including the roles of mitochondrial integrity, oxidative stress responses, and endoplasmic reticulum stress mechanisms. Furthermore, assessing the effects of Hes in combination with other chemotherapeutic agents could expand its therapeutic potential. Finally, the development of advanced drug delivery systems such as nanoparticle-based formulations may improve the bioavailability, stability, and tumor-targeting efficiency of Hes, supporting its transition from experimental research to clinical application in osteosarcoma management.