Virtual Reality Distraction for Reducing Pain and Anxiety During Percutaneous Cardiovascular Interventions: A Systematic Review and Meta-Analysis with Trial Sequential Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Data Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias and Certainty of Evidence
2.7. Statistical Analysis
3. Results
3.1. Search Results and Study Selection
3.2. Characteristics of Included Studies
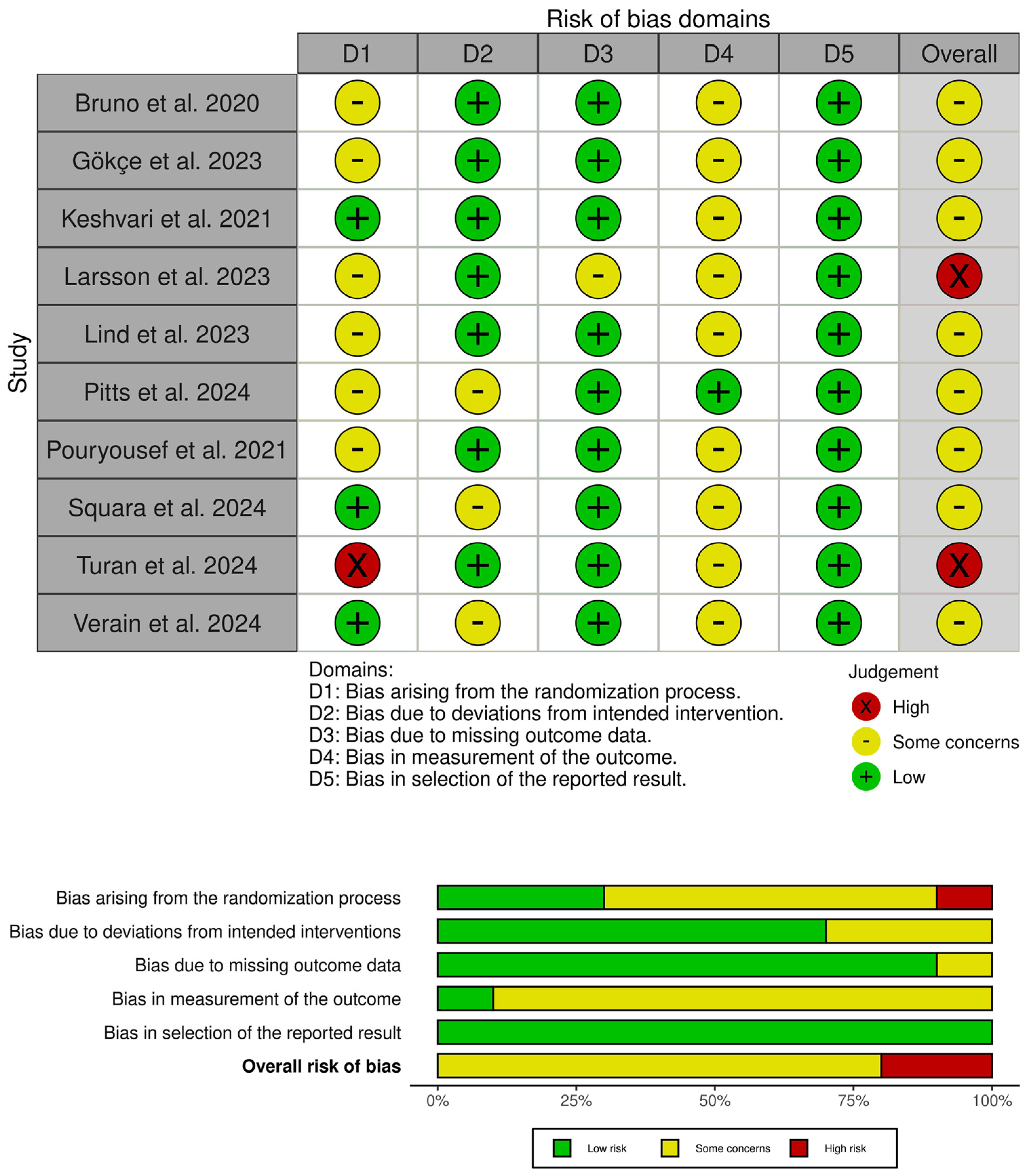
3.3. Risk of Bias and Certainty of Evidence
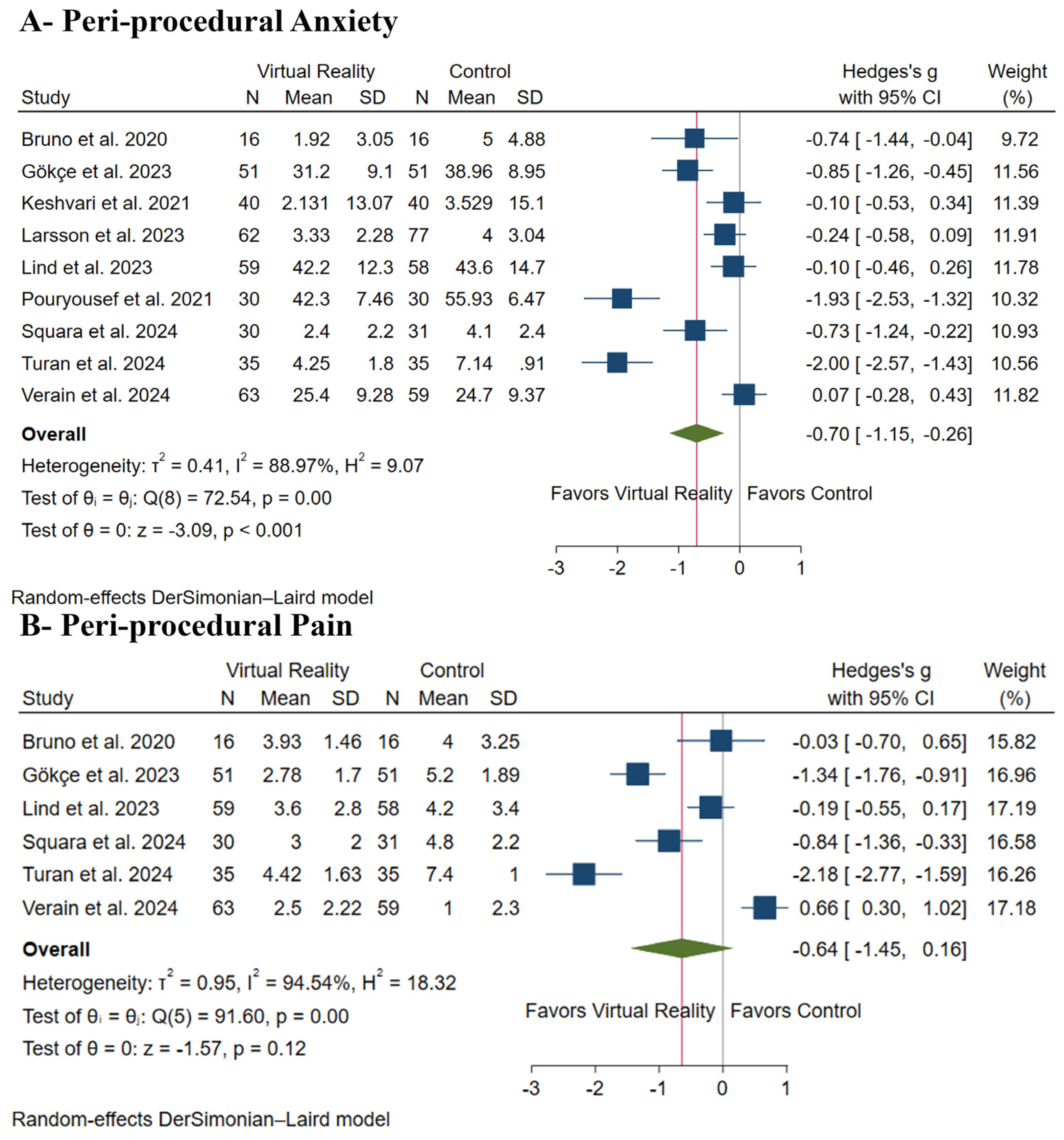
3.4. Primary Outcomes: Peri-Procedural Anxiety and Pain
3.5. Secondary Outcomes
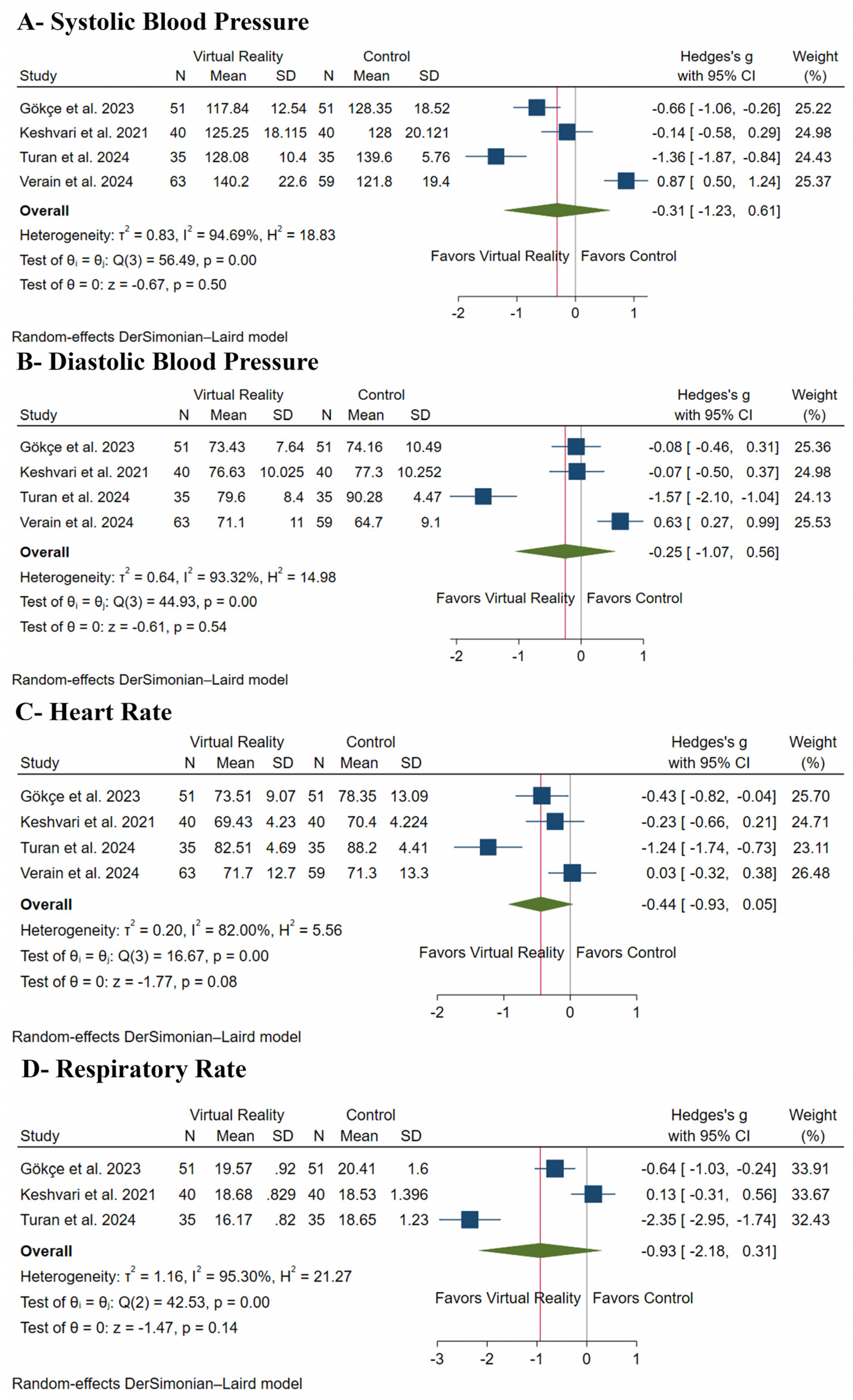
3.5.1. Vital Signs
3.5.2. Procedure Duration
3.6. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El Mathari, S.; Hoekman, A.; Kharbanda, R.K.; Sadeghi, A.H.; de Lind van Wijngaarden, R.; Götte, M.; Klautz, R.J.; Kluin, J. Virtual Reality for Pain and Anxiety Management in Cardiac Surgery and Interventional Cardiology. JACC Adv. 2024, 3, 100814. [Google Scholar] [CrossRef] [PubMed]
- Younes, O.; Amer, R.; Fawzy, H.; Shama, G. Psychiatric disturbances in patients undergoing open-heart surgery. Middle East Curr. Psychiatry 2019, 26, 4. [Google Scholar] [CrossRef]
- Ayasrah, S.M.; Ahmad, M.M. Educational video intervention effects on periprocedural anxiety levels among cardiac catheterization patients: A randomized clinical trial. Res. Theory Nurs. Pract. 2016, 30, 70–84. [Google Scholar] [CrossRef]
- Delewi, R.; Vlastra, W.; Rohling, W.J.; Wagenaar, T.C.; Zwemstra, M.; Meesterman, M.G.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; de Winter, R.J.; et al. Anxiety levels of patients undergoing coronary procedures in the catheterization laboratory. Int. J. Cardiol. 2017, 228, 926–930. [Google Scholar] [CrossRef]
- Choinière, M.; Watt-Watson, J.; Victor, J.C.; Baskett, R.J.; Bussières, J.S.; Carrier, M.; Cogan, J.; Costello, J.; Feindel, C.; Guertin, M.-C.; et al. Prevalence of and risk factors for persistent postoperative nonanginal pain after cardiac surgery: A 2-year prospective multicentre study. CMAJ Can. Med. Assoc. J. 2014, 186, E213–E223. [Google Scholar] [CrossRef]
- Guimarães-Pereira, L.; Reis, P.; Abelha, F.; Azevedo, L.F.; Castro-Lopes, J.M. Persistent postoperative pain after cardiac surgery: A systematic review with meta-analysis regarding incidence and pain intensity. Pain 2017, 158, 1869–1885. [Google Scholar] [CrossRef]
- Miozzo, A.P.; Stein, C.; Bozzetto, C.B.; Plentz, R.D.M. Massage therapy reduces pain and anxiety after cardiac surgery: A systematic review and meta-analysis of randomized clinical trials. Clin. Trials Regul. Sci. Cardiol. 2016, 23–24, 1–8. [Google Scholar] [CrossRef]
- Saab, O.; Al-Obaidi, H.; Merza, N.; Bhagat, U.; Al-Sagban, A.; Algodi, M.; Abuelazm, M.; El-Serag, H. The Impact of Visual Distraction Interventions on Patients’ Pain and Anxiety During Colonoscopy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Gastroenterol. 2024. [Google Scholar] [CrossRef]
- Varon, J.; Marik, P.E. Perioperative hypertension management. Vasc. Health Risk Manag. 2008, 4, 615–627. [Google Scholar] [CrossRef]
- Goldberg, M.E.; Weaver, F.A. Strategies for managing perioperative hypertension. Crit. Care Clin. 2007, 23 (Suppl. S1), 7–21. [Google Scholar]
- Baytar, A.A.; Bollucuo, K. Effect of virtual reality on preoperative anxiety in patients undergoing septorhinoplasty. Braz. J. Anesthesiol. (Engl. Ed.) 2023, 73, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Hughes, O.; MacQuhae, F.; Rakosi, A.; Herskovitz, I.; Kirsner, R.S. Stress and wound healing. In Stress and Skin Disorders; Springer: Berlin/Heidelberg, Germany, 2016; pp. 185–207. [Google Scholar] [CrossRef]
- Munafò, M.R.; Stevenson, J. Anxiety and surgical recovery. J. Psychosom. Res. 2001, 51, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Sloot, S.; Boland, J.; Snowden, J.A.; Ezaydi, Y.; Foster, A.; Gethin, A.; Green, T.; Chopra, L.; Verhagen, S.; Vissers, K.; et al. Side effects of analgesia may significantly reduce quality of life in symptomatic multiple myeloma: A cross-sectional prevalence study. Support. Care Cancer 2015, 23, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, B.; Abuelazm, M.T.; Swed, S.; Gamal, M.; Atef, M.; Al-Zeftawy, M.A.; Noori, M.A.; Lutz, A.; Volgman, A.S. The efficacy of nitroglycerin to prevent radial artery spasm and occlusion during and after transradial catheterization: A systematic review and meta-analysis of randomized controlled trials. Clin. Cardiol. 2022, 45, 1171–1183. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Raisakis, K.; Hahalis, G.; Kaoukis, A.; Kossyvakis, C.; Avramides, D.; Pappas, L.; Panagopoulou, V.; Pyrgakis, V.; et al. Moderate procedural sedation and opioid analgesia during transradial coronary interventions to prevent spasm: A prospective randomized study. JACC Cardiovasc. Interv. 2013, 6, 267–273. [Google Scholar] [CrossRef]
- Apfelbaum, J.; Gross, J.B.; Connis, R.T.; Agarkar, M.; Arnold, D.E.; Coté, C.J.; Tung, A. Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018: A Report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology 2018, 128, 437–479. [Google Scholar] [CrossRef]
- Squara, F.; Bateau, J.; Scarlatti, D.; Bun, S.-S.; Moceri, P.; Ferrari, E. Virtual Reality for the Management of Pain and Anxiety in Patients Undergoing Implantation of Pacemaker or Implantable Cardioverter Defibrillator: A Randomized Study. J. Med. Syst. 2024, 48, 28. [Google Scholar] [CrossRef]
- Indovina, P.; Barone, D.; Gallo, L.; Chirico, A.; De Pietro, G.; Giordano, A. Virtual Reality as a Distraction Intervention to Relieve Pain and Distress during Medical Procedures. Clin. J. Pain 2018, 34, 858–877. [Google Scholar] [CrossRef]
- Pouryousef, F.; Navidian, A.; Ghahdarijani, O.R.; Yaghoubinia, F. Comparing the Effect of Virtual Reality and Rhythmic Breathing on the Anxiety of the Patients Undergoing Coronary Angiography. Q. Horiz. Med. Sci. 2021, 27, 2–17. [Google Scholar]
- Turan, G.B.; Gür, F.; Özer, Z.; Tarkan, Ç. Effects of Virtual Reality on Pain, Anxiety, Patient Satisfaction in Coronary Angiography: A Randomized Trial. Pain Manag. Nurs. 2024, 25, e177–e185. [Google Scholar] [CrossRef]
- Lind, A.; Ahsan, M.; Totzeck, M.; Al-Rashid, F.; Haddad, A.; Dubler, S.; Brenner, T.; Skarabis, A.; El Gabry, M.; Rassaf, T.; et al. Virtual reality-assisted distraction during transcatheter aortic valve implantation under local anaesthesia: A randomised study. Int. J. Cardiol. 2023, 387, 131130. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.E.; Cabassut, V.; Peretout, P.; Marliere, S.; Vautrin, E.; Piliero, N.; Salvat, M.; Riou, L.; Vanzetto, G.; Vilotitch, A.; et al. Assessment of the Objective Effect of Virtual Reality for Preoperative Anxiety in Interventional Cardiology. Am. J. Cardiol. 2023, 205, 207–213. [Google Scholar] [CrossRef]
- Pitts, L.; Hellner, N.; Kofler, M.; Ryschka, M.; Unbehaun, A.; O’Brien, B.; Kempfert, J.; Hommel, M. The Influence of Audiovisual Distraction on Pain Reduction During Transcatheter Aortic Valve Implantation Under Monitored Anesthesia Care: A Prospective Randomized Trial. J. Cardiothorac. Vasc. Anesth. 2024, 38, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Keshvari, M.; Yeganeh, M.R.; Paryad, E.; Roushan, Z.A.; Pouralizadeh, M. The effect of virtual reality distraction on reducing patients’ anxiety before coronary angiography: A randomized clinical trial study. Egypt. Heart J. 2021, 73, 98. [Google Scholar] [CrossRef]
- Bruno, R.R.; Lin, Y.; Wolff, G.; Polzin, A.; Veulemans, V.; Klein, K.; Westenfeld, R.; Zeus, T.; Kelm, M.; Jung, C. Virtual reality-assisted conscious sedation during transcatheter aortic valve implantation: A randomised pilot study. EuroIntervention 2020, 16, e1014–e1020. [Google Scholar] [CrossRef] [PubMed]
- Verain, J.; Trouillet, C.; Moulin, F.; Christophe, C. Efficacy of virtual reality therapy versus pharmacological sedation for reducing pain and anxiety during coronary catheterisation procedures: A prospective randomised controlled trial. Health Sci. Rep. 2024, 7, e2151. [Google Scholar] [CrossRef]
- Gökçe, E.; Arslan, S. Effects of virtual reality and acupressure interventions on pain, anxiety, vital signs and comfort in catheter extraction processes for patients undergoing coronary angiography: A randomized controlled trial. Int. J. Nurs. Pract. 2023, 29, e13176. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2023. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Vist, G.E.; Falck-Ytter, Y.; Schünemann, H.J. Rating Quality of Evidence and Strength of Recommendations: What is “quality of evidence” and why is it important to clinicians? BMJ Br. Med. J. 2008, 336, 995. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. Rating Quality of Evidence and Strength of Recommendations: GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ Br. Med. J. 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- TSA–ctu.dk. 2021. Available online: http://ctu.dk/tsa/ (accessed on 17 December 2024).
- Trotter, R.; Gallagher, R.; Donoghue, J. Anxiety in patients undergoing percutaneous coronary interventions. Heart Lung J. Acute Crit. Care 2011, 40, 185–192. [Google Scholar] [CrossRef]
- Tadesse, M.; Ahmed, S.; Regassa, T.; Girma, T.; Hailu, S.; Mohammed, A.; Mohammed, S. Effect of preoperative anxiety on postoperative pain on patients undergoing elective surgery: Prospective cohort study. Ann. Med. Surg. 2022, 73, 103190. [Google Scholar] [CrossRef]
- Liblik, K.; Théberge, E.; Gomes, Z.; Burbidge, E.; Menon, N.; Gobran, J.; Johri, A.M. Improving Wellbeing After Acute Coronary Syndrome. Curr. Probl. Cardiol. 2023, 48, 101201. [Google Scholar] [CrossRef]
- Caldwell, P.H.; Arthur, H.M.; Natarajan, M.; Anand, S.S. Fears and beliefs of patients regarding cardiac catheterization. Soc. Sci. Med. 2007, 65, 1038–1048. [Google Scholar] [CrossRef]
- Smith, V.; Warty, R.R.; Sursas, J.A.; Payne, O.; Nair, A.; Krishnan, S.; da Silva Costa, F.; Wallace, E.M.; Vollenhoven, B. The Effectiveness of Virtual Reality in Managing Acute Pain and Anxiety for Medical Inpatients: Systematic Review. J. Med. Internet Res. 2020, 22, e17980. [Google Scholar] [CrossRef]
- Seabrook, E.; Kelly, R.; Foley, F.; Theiler, S.; Thomas, N.; Wadley, G.; Nedeljkovic, M. Understanding how virtual reality can support mindfulness practice: Mixed methods study. J. Med. Internet Res. 2020, 22, e16106. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Chambers, G.T.; Meyer, W.J.; Arceneaux, L.L.; Russell, W.J.; Seibel, E.J.; Richards, T.L.; Sharar, S.R.; Patterson, D.R. Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Ann. Behav. Med. 2011, 41, 183–191. [Google Scholar] [CrossRef]
- Doyle, C.; Lennox, L.; Bell, D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013, 3, e001570. [Google Scholar] [CrossRef] [PubMed]
- Caes, L.; Orchard, A.; Christie, D. Connecting the mind–body split: Understanding the relationship between symptoms and emotional well-being in chronic pain and functional gastrointestinal disorders. Healthcare 2017, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Shoulders-Odom, B. Management of patients after percutaneous coronary interventions. Crit. Care Nurse 2008, 28, 26–40. [Google Scholar] [CrossRef]
- Menekli, T.; Yaprak, B.; Doğan, R. The Effect of Virtual Reality Distraction Intervention on Pain, Anxiety, and Vital Signs of Oncology Patients Undergoing Port Catheter Implantation: A Randomized Controlled Study. Pain Manag. Nurs. 2022, 23, 585–590. [Google Scholar] [CrossRef]
- Gallagher, M.; Dowsett, R.; Ferrè, E.R. Vection in virtual reality modulates vestibular-evoked myogenic potentials. Eur. J. Neurosci. 2019, 50, 3557–3565. [Google Scholar] [CrossRef]
- Weech, S.; Kenny, S.; Barnett-Cowan, M. Presence and cybersickness in virtual reality are negatively related: A review. Front. Psychol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Juexuan, C.; Yuting, D.; Zhaoxiang, B.; Chi, Z.; Yaolong, C. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. Chin. J. Evid. Based Med. 2020, 20, 1439–1448. [Google Scholar] [CrossRef]
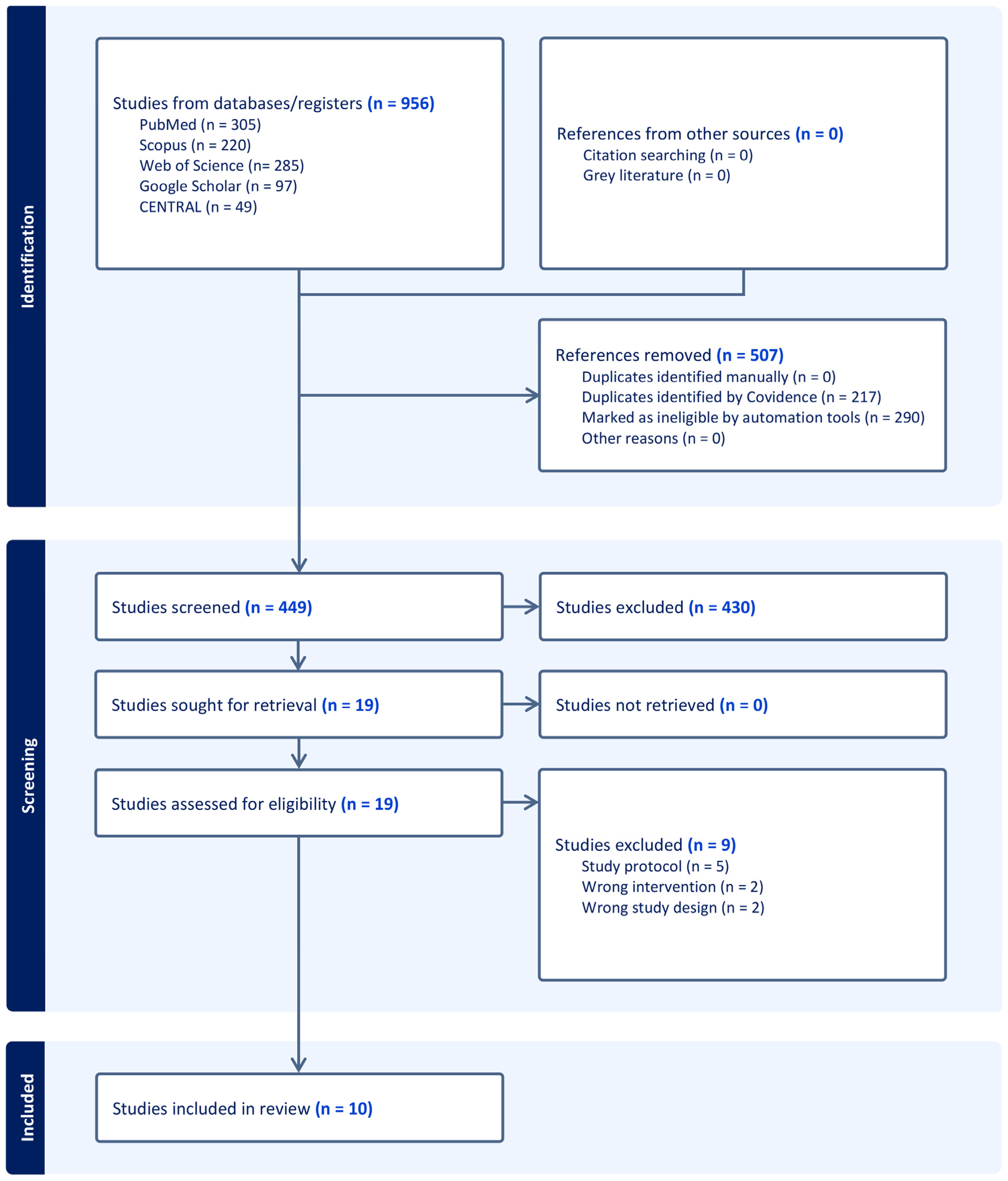
| Study ID | Study Design | Country | Sample Size | Procedure | Visual Content | VR Device | VR Duration | Distraction Timing | Control | Pain Assessment Tool | Anxiety Assessment Tool | Sedation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruno et al. 2020 [26] | Single-centre, open-label RCT | Germany | 32 | TAVR | Patients could choose one of the following videos: nature scenery, an aquarium, flying over a green landscape, diving underwater, or walking through a calm forest | MEDION® ERAZER® X1000 MR Glasses, 7.34 cm (2.89″) LC-Display (Medion AG, Essen, Germany) | 30.5 min (median) | Peri-procedure | Usual care | VAS-P (0–10) | VAS-A (0–10) | 1 mg lorazepam or 3.25 mg midazolam |
| Gökçe et al. 2023 [28] | Single-centre, single-blinded RCT | Turkey | 102 | CAG | Three different image types, including a coastal forest view, an undersea view, and an open-air museum tour, with nature sound effects and relaxing background music | Oculus Virtual Reality Glasses | 30 min | Peri-procedure | Usual care | VAS-P (0–10) | STAI | NA |
| Keshvari et al. 2021 [25] | Single-centre, open-label RCT | Iran | 80 | CAG | Natural scene that was filmed at various natural locations and landscapes such as the beach, mountains, waterfalls, and rivers with pleasant sounds | Remix video headset and a Huawei mobile phone | 5 min | Pre-procedure | Usual care | NA | STAI | NA |
| Larsson et al. 2023 [23] | Single-centre, open-label RCT | France | 156 | CAG | Five themes were proposed to the patient (Zen Garden, forest, mountain, beach, or diving) | The Healthy Mind company (Company, Ville, Pays, Paris, France) provided the study materials (2 headsets of VR and audio headphones) | About 20 min | Pre-procedure | Usual care | NA | VAS-A (0–10) | NA |
| Lind et al. 2023 [22] | Single-centre, open-label RCT | Germany | 117 | TAVR | Different categories, e.g., nature and relaxation, travel, documentations, Hollywood movies, classical concerts | Happy Med video glasses (Happy Med GmbH, Vienna, Austria) | NA | Peri-procedure | Usual care | VAS-P (0–10) | STAI | No sedation |
| Pitts et al. 2024 [24] | Single-centre, open-label RCT | Germany | 90 | TAVR | NA | Happy Med video glasses (Happy Med GmbH, Vienna, Austria) | NA | Peri-procedure | Usual care | NRS (0–100) | STAI | 10 mg of propofol 1% were given if patients could not be adequately sedated through titration of the remifentanil dose to a maximum of 0.08 mg/kg/min |
| Pouryousef et al. 2021 [20] | Single-centre, single-blinded RCT | Iran | 60 | CAG | Calming images | NA | 5 min | Pre-procedure | Usual care | NA | STAI | NA |
| Squara et al. 2024 [18] | Single-centre, open-label RCT | France | 61 | ICD implantation | Static landscapes: river delta, rural India, Spitzberg, mountains in summer, or mountains in winter. Every 5 min, the video recording evolved to another static point-of-view of the chosen landscape | Deepsen (Lyon, France) | NA | Peri-procedure | Usual care | NRS (0–10) | NRS (0–10) | Intravenous paracetamol (1 g) 60 min before the procedure |
| Turan et al. 2024 [21] | Single-centre, open-label RCT | Turkey | 70 | CAG | The licenced product “Secret Garden” | An android mobile phone placed in the Cardboard Super Flex Binoculars Glasses | 30–45 min | Peri-procedure | Usual care | VAS-P (0–10) | Anxiety Assessment Scale (AAS) | NA |
| Verain et al. 2024 [27] | Single-centre, open-label RCT | France | 122 | CAG, coronary angioplasty, or peripheral angioplasty | NA | Deepsen (Lyon, France) | NA | Peri-procedure | Sedation | VAS-P (0–10) | STAI | Midazolam and fentanyl |
| Study ID | Number of Patients in Each Group | Age (Years) | Gender (Male) | BMI | Comorbidities | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VR | Control | VR | Control | VR | Control | VR | Control | Heart Failure | COPD | CAD | DM | HTN | Smoking | |||||||
| VR | Control | VR | Control | VR | Control | VR | Control | VR | Control | VR | Control | |||||||||
| Bruno et al. 2020 [26] | 16 | 16 | 82 (78.3–87) | 83 (78.3–86.8) | 11 (68.8) | 9 (56.3) | NA | NA | 9 (56.3) | 9 (56.3) | 6 (37.5) | 4 (25) | 11 (68.8) | 14 (87.5) | 6 (37.5) | 4 (25) | NA | NA | NA | NA |
| Gökçe et al. 2023 [28] | 51 | 51 | 59.4 ± 12.1 | 58.2 ± 12.0 | 25 (49) | 31 (60.8) | 28.5 ± 5.9 | 27.3 ± 4.5 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 14 (27.5) | 13 (25.5) |
| Keshvari et al. 2021 [25] | 40 | 40 | 4.002 ± 52.08 | 4.120 ± 50.95 | 32 (80) | 25 (62.5) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Larsson et al. 2023 [23] | 76 | 80 | 62.5 ± 10.9 | 62.6 ± 9.5 | 56(73.7) | 55(68.7) | 27.3 ± 5.3 | 27.8 ± 5.2 | NA | NA | NA | NA | NA | NA | 16 (21) | 22 (27.5) | 37 (48.7) | 34 (42.5) | 14(18.4) | 15(18.7) |
| Lind et al. 2023 [22] | 59 | 58 | 81.1 ± 5.7 | 81.2 ± 5.5 | 30 (50.8) | 29 (50.0) | 27.3 ± 4.3 | 26.3 ± 4.3 | 48 (81.4) | 42 (72.4) | NA | NA | 41 (69.5) | 34 (58.6) | 16 (29.1) | 17 (32.1) | NA | NA | NA | NA |
| Pitts et al. 2024 [24] | 45 | 45 | 79 (76–83) | 81 (76–84) | 32 (77) | 19 (42) | 28.3 (24.9–30.6) | 28.1 (23.8–30.1) | NA | NA | 7 (16) | 5 (11) | 34 (76) | 31(69) | 12 (27) | 15 (33) | 39 (87) | 44 (98) | NA | NA |
| Pouryousef et al. 2021 [20] | 30 | 30 | 49.96 ± 8.10 | 51.36 ± 8.11 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 12 (40) | 13 (43.3) | NA | NA | NA | NA |
| Squara et al. 2024 [18] | 30 | 31 | 78.3 ± 8.4 | 77.5 ± 8.0 | 21 (70) | 17 (54.5) | NA | NA | NA | NA | NA | NA | NA | NA | 13 (43.3) | 11 (35.5) | 23 (76.7) | 21 (67.8) | NA | NA |
| Turan et al. 2024 [21] | 35 | 35 | 70.00 ± 9.82 | 65.20 ± 13.57 | 16 (45.7) | 18 (51.4) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Verain et al. 2024 [27] | 63 | 59 | 68.5 ± 10.0 | 68.8 ± 9.5 | 50 (79.4) | 42 (71.2) | 28.2 ± 5.4 | 29.0 ± 5.0 | NA | NA | NA | NA | NA | NA | 22 (34.9) | 20 (33.9) | 38 (60.3) | 43 (72.9) | NA | NA |
| Certainty Assessment | Summary of Findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (Studies) Follow-Up | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty of Evidence | Study Event Rates (%) | Relative Effect (95% CI) | Anticipated Absolute Effects | ||
| With [Control] | With [VR] | Risk with [Control] | Risk Difference with [VR] | ||||||||
| Peri-procedural Anxiety | |||||||||||
| 783 (9 RCTs) | serious a | very serious b | not serious | serious c | none | ⨁◯◯◯ Very low a,b,c | 397 | 386 | - | - | SMD 0.7 SD lower (1.15 lower to 0.26 lower) |
| Peri-procedural Pain | |||||||||||
| 504 (6 RCTs) | serious a | very serious b | not serious | serious c | none | ⨁◯◯◯ Very low a,b,c | 250 | 254 | - | - | SMD 0.64 SD lower (1.45 lower to 0.16 higher) |
| Systolic Blood Pressure | |||||||||||
| 374 (4 RCTs) | serious a | very serious b | not serious | serious c | none | ⨁◯◯◯ Very low a,b,c | 185 | 189 | - | - | SMD 0.31 SD lower (1.23 lower to 0.61 higher) |
| Diastolic Blood Pressure | |||||||||||
| 374 (4 RCTs) | serious a | very serious c | not serious | serious c | none | ⨁◯◯◯ Very low a,c | 185 | 189 | - | - | SMD 0.25 SD lower (1.07 lower to 0.56 higher) |
| Heart Rate | |||||||||||
| 374 (4 RCTs) | serious a | very serious c | not serious | serious c | none | ⨁◯◯◯ Very low a,c | 185 | 189 | - | - | SMD 0.44 SD lower (0.93 lower to 0.05 higher) |
| Respiratory Rate | |||||||||||
| 252 (3 RCTs) | serious a | very serious b | not serious | serious c | none | ⨁◯◯◯ Very low a,b,c | 126 | 126 | - | - | SMD 0.93 SD lower (2.18 lower to 0.31 higher) |
| Procedure Duration | |||||||||||
| 346 (3 RCTs) | serious a | not serious | not serious | not serious | none | ⨁⨁⨁◯ Moderate a | 180 | 166 | - | - | SMD 0.07 SD higher (0.14 lower to 0.28 higher) |
| Delirium | |||||||||||
| 361 (4 RCTs) | serious a | not serious | not serious | serious c | none | ⨁⨁◯◯ Low a,c | 7/178 (3.9%) | 7/183 (3.8%) | RR 0.98 (0.37 to 2.63) | 7/178 (3.9%) | 1 fewer per 1000 (from 25 fewer to 64 more) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albazee, E.; Alrajehi, A.; Alsahli, F.M.; Alqemlas, A.; Aldhaen, A.; Alkandari, A.; Alkandari, H.; Alkanderi, W. Virtual Reality Distraction for Reducing Pain and Anxiety During Percutaneous Cardiovascular Interventions: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Medicina 2025, 61, 957. https://doi.org/10.3390/medicina61060957
Albazee E, Alrajehi A, Alsahli FM, Alqemlas A, Aldhaen A, Alkandari A, Alkandari H, Alkanderi W. Virtual Reality Distraction for Reducing Pain and Anxiety During Percutaneous Cardiovascular Interventions: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Medicina. 2025; 61(6):957. https://doi.org/10.3390/medicina61060957
Chicago/Turabian StyleAlbazee, Ebraheem, Abdullhadi Alrajehi, Fahad M. Alsahli, Abdillatef Alqemlas, Ahmad Aldhaen, Abdullah Alkandari, Hamad Alkandari, and Waleed Alkanderi. 2025. "Virtual Reality Distraction for Reducing Pain and Anxiety During Percutaneous Cardiovascular Interventions: A Systematic Review and Meta-Analysis with Trial Sequential Analysis" Medicina 61, no. 6: 957. https://doi.org/10.3390/medicina61060957
APA StyleAlbazee, E., Alrajehi, A., Alsahli, F. M., Alqemlas, A., Aldhaen, A., Alkandari, A., Alkandari, H., & Alkanderi, W. (2025). Virtual Reality Distraction for Reducing Pain and Anxiety During Percutaneous Cardiovascular Interventions: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Medicina, 61(6), 957. https://doi.org/10.3390/medicina61060957