Effect of Daily Lactococcus cremoris spp. Consumption Immobilized on Oat Flakes on Blood and Urine Biomarkers: A Randomized Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Freeze-Dried L. cremoris Cells Immobilized on Oat Flakes
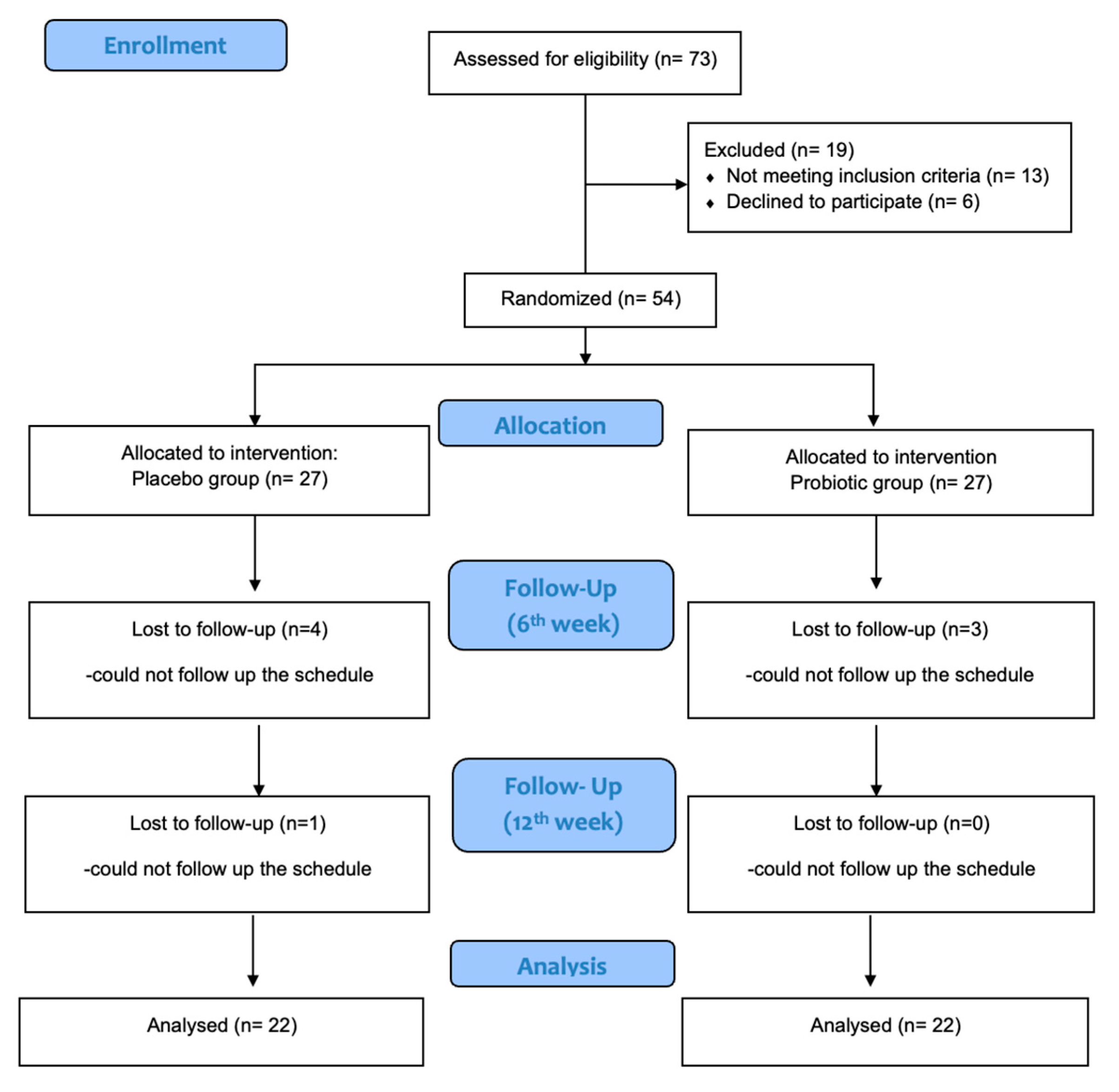
2.2. Study Design
2.3. Participants
2.4. Intervention
2.5. Anthropometric and Biochemical Measurements
2.6. Statistical Analysis
2.6.1. Sample Size
2.6.2. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Dietary Habits
3.3. Blood Biomarkers
3.3.1. Inflammatory and Immunological Biomarkers
3.3.2. Lipemia Biomarkers
3.3.3. Glycemia Biomarkers
3.3.4. Folate, VitB12, and VitD
3.3.5. Cortisol, Uric Acid, and Antioxidant Capacity
3.3.6. Urine Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to Select a Probiotic? A Review and Update of Methods and Criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Guarner, F. Probiotics and Human Health: A Clinical Perspective. Postgrad. Med. J. 2004, 80, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Lin, C.-S.; Chang, C.-J.; Lu, C.-C.; Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Young, J.D.; Lai, H.-C. Impact of the Gut Microbiota, Prebiotics, and Probiotics on Human Health and Disease. Biomed. J. 2014, 37, 259–268. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Cooney, J.C. Probiotic Bacteria Influence the Composition and Function of the Intestinal Microbiota. Interdiscip. Perspect. Infect. Dis. 2008, 2008, e175285. [Google Scholar] [CrossRef]
- Bajaj, B.; Claes, I.; Lebeer, S. Functional Mechanisms of Probiotics. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 321–327. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Pavlatou, C.; Prapa, I.; Stylianopoulou, E.; Mitropoulou, G.; Skavdis, G.; Kourkoutas, Y. Immobilized Plant-Based Probiotics as Functional Ingredients for Breakfast Cereals. Fermentation 2025, Submitted for Publication. [Google Scholar]
- Cimminiello, C.; Zambon, A.; Polo Friz, H. Hypercholesterolemia and cardiovascular risk: Advantages and limitations of current treatment options. G. Ital. Cardiol. (Rome) 2016, 17, 6S–13S. [Google Scholar] [CrossRef]
- Bhat, B.; Bajaj, B.K. Multifarious Cholesterol Lowering Potential of Lactic Acid Bacteria Equipped with Desired Probiotic Functional Attributes. 3 Biotech 2020, 10, 200. [Google Scholar] [CrossRef]
- Rigobelo, E. Probiotics; BoD—Books on Demand: Norderstedt, Germany, 2012; ISBN 978-953-51-0776-7. [Google Scholar]
- Alahmari, L.A. Dietary Fiber Influence on Overall Health, with an Emphasis on CVD, Diabetes, Obesity, Colon Cancer, and Inflammation. Front. Nutr. 2024, 11, 1510564. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-Dairy Probiotic Food Products: An Emerging Group of Functional Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. From Probiotics to Therapeutics: Another Step Forward? J. Clin. Investig. 2011, 121, 2149–2152. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Shafique, B.; Batool, M.; Kowalczewski, P.Ł.; Shehzad, Q.; Usman, M.; Manzoor, M.F.; Zahra, S.M.; Yaqub, S.; Aadil, R.M. Nutritional and Health Potential of Probiotics: A Review. Appl. Sci. 2021, 11, 11204. [Google Scholar] [CrossRef]
- Ranadheera, R.D.C.S.; Baines, S.K.; Adams, M.C. Importance of Food in Probiotic Efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Gibson, G.R. From Probiotics to Prebiotics and a Healthy Digestive System. J. Food Sci. 2004, 69, M141–M143. Available online: https://ift.onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2621.2004.tb10724.x?casa_token=YzW3rNy_DMoAAAAA:UDFodGkqNynYTBpVp6IR8OXq09o0-ZEg8QaW16YNnlrxWtRicBkb4--09EbIMLxArFHPtFH1-2Ji42s (accessed on 19 October 2023). [CrossRef]
- Axelsson, L.; Ahrné, S. Lactic Acid Bacteria. In Applied Microbial Systematics; Priest, F.G., Goodfellow, M., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 367–388. ISBN 978-0-7923-6518-1. [Google Scholar]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Ishida, T.; Yokota, A.; Umezawa, Y.; Toda, T.; Yamada, K. Identification and Characterization of Lactococcal and Acetobacter Strains Isolated from Traditional Caucasusian Fermented Milk. J. Nutr. Sci. Vitaminol. 2005, 51, 187–193. [Google Scholar] [CrossRef][Green Version]
- Watanabe, M.; Maruo, T.; Suzuki, T. Effects of Intake of Lactococcus cremoris subsp. cremoris FC on Constipation Symptoms and Immune System in Healthy Participants with Mild Constipation: A Double-Blind, Placebo-Controlled Study. Int. J. Food Sci. Nutr. 2023, 74, 695–706. [Google Scholar] [CrossRef]
- Ozaki, K.; Maruo, T.; Kosaka, H.; Mori, M.; Mori, H.; Yamori, Y.; Toda, T. The Effects of Fermented Milk Containing Lactococcus lactis subsp. cremoris FC on Defaecation in Healthy Young Japanese Women: A Double-Blind, Placebo-Controlled Study. Int. J. Food Sci. Nutr. 2018, 69, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Prapa, I.; Pavlatou, C.; Kompoura, V.; Nikolaou, A.; Stylianopoulou, E.; Skavdis, G.; Grigoriou, M.E.; Kourkoutas, Y. A Novel Wild-Type Lacticaseibacillus Paracasei Strain Suitable for the Production of Functional Yoghurt and Ayran Products. Fermentation 2025, 11, 37. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey III: Body Measurements (Anthropometry). Available online: https://stacks.cdc.gov/view/cdc/53134 (accessed on 1 April 2025).
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [CrossRef]
- A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 13 February 2024).
- Yeo, S.-K.; Ewe, J.-A.; Tham, C.S.-C.; Liong, M.-T. Carriers of Probiotic Microorganisms. In Probiotics: Biology, Genetics and Health Aspects; Liong, M.-T., Ed.; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2011; pp. 191–220. ISBN 978-3-642-20838-6. [Google Scholar]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional Foods and Nondairy Probiotic Food Development: Trends, Concepts, and Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-Dairy Probiotic Products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G.; Al-Kaabi, W.J.; Al-Manhel, A.J.A.; Niamah, A.K.; Altemimi, A.B.; Al-Wafi, H.; Cacciola, F. Effects of β-Glucan Extracted from Saccharomyces Cerevisiae on the Quality of Bio-Yoghurts: In Vitro and in Vivo Evaluation. Food Meas. 2022, 16, 3607–3617. [Google Scholar] [CrossRef]
- Rasika, D.M.D.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ranadheera, C.S. Probiotic Delivery through Non-Dairy Plant-Based Food Matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Mustafa, M.A.; Hassan, M.A.; Al-Asmari, F. Probiotic-Fermentation of Oat: Safety, Strategies for Improving Quality, Potential Food Applications and Biological Activities. Trends Food Sci. Technol. 2024, 151, 104640. [Google Scholar] [CrossRef]
- Nagpal, R.; Kaur, A. Synbiotic Effect of Various Prebiotics on In Vitro Activities of Probiotic Lactobacilli. Ecol. Food Nutr. 2011, 50, 63–68. [Google Scholar] [CrossRef]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-Cell Responses to Gut Microbiota Promote Experimental Autoimmune Encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4615–4622. [Google Scholar] [CrossRef]
- Lye, H.-S.; Kuan, C.-Y.; Ewe, J.-A.; Fung, W.-Y.; Liong, M.-T. The Improvement of Hypertension by Probiotics: Effects on Cholesterol, Diabetes, Renin, and Phytoestrogens. Int. J. Mol. Sci. 2009, 10, 3755–3775. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Soltani, S.; Ghorabi, S.; Keshtkar, A.; Daneshzad, E.; Nasri, F.; Mazloomi, S.M. Effect of Probiotic and Synbiotic Supplementation on Inflammatory Markers in Health and Disease Status: A Systematic Review and Meta-Analysis of Clinical Trials. Clin. Nutr. 2020, 39, 789–819. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, H.; Kumar, M.; Das, N.; Kumar, S.N.; Challa, H.R.; Nagpal, R. Effect of Probiotic Lactobacillus salivarius UBL S22 and Prebiotic Fructo-Oligosaccharide on Serum Lipids, Inflammatory Markers, Insulin Sensitivity, and Gut Bacteria in Healthy Young Volunteers: A Randomized Controlled Single-Blind Pilot Study. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; de Sousa, R.G.M.; Botelho, P.B.; Gomes, T.L.N.; Prada, P.O.; Mota, J.F. The Additional Effects of a Probiotic Mix on Abdominal Adiposity and Antioxidant Status: A Double-Blind, Randomized Trial. Obesity (Silver Spring) 2017, 25, 30–38. [Google Scholar] [CrossRef]
- Nihei, Y.; Suzuki, H.; Suzuki, Y. Current Understanding of IgA Antibodies in the Pathogenesis of IgA Nephropathy. Front. Immunol. 2023, 14, 1165394. [Google Scholar] [CrossRef]
- Marcotte, H.; Lavoie, M.C. Oral Microbial Ecology and the Role of Salivary Immunoglobulin A. Microbiol. Mol. Biol. Rev. 1998, 62, 71–109. [Google Scholar] [CrossRef]
- Walker, W.A. Role of Nutrients and Bacterial Colonization in the Development of Intestinal Host Defense. J. Pediatr. Gastroenterol. Nutr. 2000, 30 (Suppl. S2), S2–S7. [Google Scholar] [CrossRef]
- Guo, Y.-T.; Peng, Y.-C.; Yen, H.-Y.; Wu, J.-C.; Hou, W.-H. Effects of Probiotic Supplementation on Immune and Inflammatory Markers in Athletes: A Meta-Analysis of Randomized Clinical Trials. Medicina 2022, 58, 1188. [Google Scholar] [CrossRef]
- Gadelha, C.J.M.U.; Bezerra, A.N. Effects of Probiotics on the Lipid Profile: Systematic Review. J. Vasc. Bras. 2019, 18, e20180124. [Google Scholar] [CrossRef]
- Wang, L.; Guo, M.-J.; Gao, Q.; Yang, J.-F.; Yang, L.; Pang, X.-L.; Jiang, X.-J. The Effects of Probiotics on Total Cholesterol. Medicine (Baltimore) 2018, 97, e9679. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.B.; Ravenscroft, C.; Lamphiear, D.E.; Ostrander, L.D., Jr. Independence of Serum Lipid Levels and Dietary Habits: The Tecumseh Study. JAMA 1976, 236, 1948–1953. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary Lipids, Gut Microbiota and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Baars, A.; Oosting, A.; Lohuis, M.; Koehorst, M.; El Aidy, S.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex Differences in Lipid Metabolism Are Affected by Presence of the Gut Microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef]
- Kullisaar, T.; Zilmer, K.; Salum, T.; Rehema, A.; Zilmer, M. The Use of Probiotic L. fermentum ME-3 Containing Reg’Activ Cholesterol Supplement for 4 Weeks Has a Positive Influence on Blood Lipoprotein Profiles and Inflammatory Cytokines: An Open-Label Preliminary Study. Nutr. J. 2016, 15, 93. [Google Scholar] [CrossRef]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of Probiotic (VSL#3) and Omega-3 on Lipid Profile, Insulin Sensitivity, Inflammatory Markers, and Gut Colonization in Overweight Adults: A Randomized, Controlled Trial. Mediat. Inflamm. 2014, 2014, e348959. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuñé, J. Cholesterol-Lowering Efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in Hypercholesterolaemic Adults. Br. J. Nutr. 2013, 109, 1866–1872. [Google Scholar] [CrossRef]
- Angelino, D.; Martina, A.; Rosi, A.; Veronesi, L.; Antonini, M.; Mennella, I.; Vitaglione, P.; Grioni, S.; Brighenti, F.; Zavaroni, I.; et al. Glucose- and Lipid-Related Biomarkers Are Affected in Healthy Obese or Hyperglycemic Adults Consuming a Whole-Grain Pasta Enriched in Prebiotics and Probiotics: A 12-Week Randomized Controlled Trial. J. Nutr. 2019, 149, 1714–1723. [Google Scholar] [CrossRef]
- Rahayu, E.S.; Mariyatun, M.; Putri Manurung, N.E.; Hasan, P.N.; Therdtatha, P.; Mishima, R.; Komalasari, H.; Mahfuzah, N.A.; Pamungkaningtyas, F.H.; Yoga, W.K.; et al. Effect of Probiotic Lactobacillus plantarum Dad-13 Powder Consumption on the Gut Microbiota and Intestinal Health of Overweight Adults. World J. Gastroenterol. 2021, 27, 107–128. [Google Scholar] [CrossRef]
- Ruan, Y.; Sun, J.; He, J.; Chen, F.; Chen, R.; Chen, H. Effect of Probiotics on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLoS ONE 2015, 10, e0132121. [Google Scholar] [CrossRef]
- Nikbakht, E.; Khalesi, S.; Singh, I.; Williams, L.T.; West, N.P.; Colson, N. Effect of Probiotics and Synbiotics on Blood Glucose: A Systematic Review and Meta-Analysis of Controlled Trials. Eur. J. Nutr. 2018, 57, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Jafari, P.; Ebrahimi, M.T.; Amini, M. The Effects of Probiotics and Synbiotic Supplementation on Glucose and Insulin Metabolism in Adults with Prediabetes: A Double-Blind Randomized Clinical Trial. Acta Diabetol. 2018, 55, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and Clinical Aspects of Cortisol as a Biochemical Marker of Chronic Stress. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.A.; Mangos, G.J.; Kelly, J.J. Cushing, Cortisol, and Cardiovascular Disease. Hypertension 2000, 36, 912–916. [Google Scholar] [CrossRef]
- Whitworth, J.A.; Williamson, P.M.; Mangos, G.; Kelly, J.J. Cardiovascular Consequences of Cortisol Excess. Vasc. Health Risk Manag. 2005, 1, 291–299. [Google Scholar] [CrossRef]
- Lalitsuradej, E.; Sirilun, S.; Sittiprapaporn, P.; Sivamaruthi, B.S.; Pintha, K.; Tantipaiboonwong, P.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Chaiyasut, C. The Effects of Synbiotics Administration on Stress-Related Parameters in Thai Subjects—A Preliminary Study. Foods 2022, 11, 759. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Djafarian, K. Effect of Prebiotic and Probiotic Supplementation on Circulating Pro-Inflammatory Cytokines and Urinary Cortisol Levels in Patients with Major Depressive Disorder: A Double-Blind, Placebo-Controlled Randomized Clinical Trial. J. Funct. Foods 2019, 52, 596–602. [Google Scholar] [CrossRef]
- Nishihira, J.; Kagami-Katsuyama, H.; Tanaka, A.; Nishimura, M.; Kobayashi, T.; Kawasaki, Y. Elevation of Natural Killer Cell Activity and Alleviation of Mental Stress by the Consumption of Yogurt Containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a Double-Blind, Placebo-Controlled Clinical Trial. J. Funct. Foods 2014, 11, 261–268. [Google Scholar] [CrossRef]
- Wu, A.H.; Gladden, J.D.; Ahmed, M.; Ahmed, A.; Filippatos, G. Relation of Serum Uric Acid to Cardiovascular Disease. Int. J. Cardiol. 2016, 213, 4–7. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, Z.; Lu, Y. The Potential of Probiotics in the Amelioration of Hyperuricemia. Food Funct. 2022, 13, 2394–2414. [Google Scholar] [CrossRef]
- Rezazadeh, L.; Alipour, B.; Jafarabadi, M.A.; Behrooz, M.; Gargari, B.P. Daily Consumption Effects of Probiotic Yogurt Containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 on Oxidative Stress in Metabolic Syndrome Patients. Clin. Nutr. ESPEN 2021, 41, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Taniguchi, A.; Tsuboi, H.; Kano, H.; Asami, Y. Hypouricaemic Effects of Yoghurt Containing Lactobacillus gasseri PA-3 in Patients with Hyperuricaemia and/or Gout: A Randomised, Double-Blind, Placebo-Controlled Study. Mod. Rheumatol. 2019, 29, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant Properties of Potentially Probiotic Bacteria: In Vitro and in Vivo Activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef]
- Harasym, J.; Oledzki, R. Effect of Fruit and Vegetable Antioxidants on Total Antioxidant Capacity of Blood Plasma. Nutrition 2014, 30, 511–517. [Google Scholar] [CrossRef]
- Kleniewska, P.; Hoffmann, A.; Pniewska, E.; Pawliczak, R. The Influence of Probiotic Lactobacillus casei in Combination with Prebiotic Inulin on the Antioxidant Capacity of Human Plasma. Oxidative Med. Cell. Longev. 2016, 2016, e1340903. [Google Scholar] [CrossRef]
- LeMone, P. Vitamins and Minerals. J. Obstet. Gynecol. Neonatal Nurs. 1999, 28, 520–533. [Google Scholar] [CrossRef]
- Gangadharan, D.; Nampoothiri, K.M. Folate Production Using Lactococcus lactis ssp cremoris with Implications for Fortification of Skim Milk and Fruit Juices. LWT—Food Sci. Technol. 2011, 44, 1859–1864. [Google Scholar] [CrossRef]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef]
- Rong, N.; Selhub, J.; Goldin, B.R.; Rosenberg, I.H. Bacterially Synthesized Folate in Rat Large Intestine Is Incorporated into Host Tissue Folyl Polyglutamates. J. Nutr. 1991, 121, 1955–1959. [Google Scholar] [CrossRef]
- Clarke, R.; Grimley Evans, J.; Schneede, J.; Nexo, E.; Bates, C.; Fletcher, A.; Prentice, A.; Johnston, C.; Ueland, P.M.; Refsum, H.; et al. Vitamin B12 and Folate Deficiency in Later Life. Age Ageing 2004, 33, 34–41. [Google Scholar] [CrossRef]
- Gu, Q.; Li, P.; Gu, Q.; Li, P. Biosynthesis of Vitamins by Probiotic Bacteria. In Probiotics and Prebiotics in Human Nutrition and Health; IntechOpen: London, UK, 2016; ISBN 978-953-51-2476-4. [Google Scholar]
- Barkhidarian, B.; Roldos, L.; Iskandar, M.M.; Saedisomeolia, A.; Kubow, S. Probiotic Supplementation and Micronutrient Status in Healthy Subjects: A Systematic Review of Clinical Trials. Nutrients 2021, 13, 3001. [Google Scholar] [CrossRef]
- Mokhtari, Z.; Karbaschian, Z.; Pazouki, A.; Kabir, A.; Hedayati, M.; Mirmiran, P.; Hekmatdoost, A. The Effects of Probiotic Supplements on Blood Markers of Endotoxin and Lipid Peroxidation in Patients Undergoing Gastric Bypass Surgery; a Randomized, Double-Blind, Placebo-Controlled, Clinical Trial with 13 Months Follow-Up. Obes. Surg. 2019, 29, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Woodard, G.A.; Encarnacion, B.; Downey, J.R.; Peraza, J.; Chong, K.; Hernandez-Boussard, T.; Morton, J.M. Probiotics Improve Outcomes After Roux-En-Y Gastric Bypass Surgery: A Prospective Randomized Trial. J. Gastrointest. Surg. 2009, 13, 1198–1204. [Google Scholar] [CrossRef]
- Valentini, L.; Pinto, A.; Bourdel-Marchasson, I.; Ostan, R.; Brigidi, P.; Turroni, S.; Hrelia, S.; Hrelia, P.; Bereswill, S.; Fischer, A.; et al. Impact of Personalized Diet and Probiotic Supplementation on Inflammation, Nutritional Parameters and Intestinal Microbiota—The “RISTOMED Project”: Randomized Controlled Trial in Healthy Older People. Clin. Nutr. 2015, 34, 593–602. [Google Scholar] [CrossRef]
- Prentice, A. Vitamin D Deficiency: A Global Perspective. Nutr. Rev. 2008, 66, S153–S164. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.a.S.T.; Santos, D.B. Obesity and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral Supplementation with Probiotic L. reuteri NCIMB 30242 Increases Mean Circulating 25-Hydroxyvitamin D: A Post Hoc Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef]
- Musso, C.G. Magnesium Metabolism in Health and Disease. Int. Urol. Nephrol. 2009, 41, 357–362. [Google Scholar] [CrossRef]
- Pérez-Conesa, D.; López, G.; Abellán, P.; Ros, G. Bioavailability of Calcium, Magnesium and Phosphorus in Rats Fed Probiotic, Prebiotic and Synbiotic Powder Follow-up Infant Formulas and Their Effect on Physiological and Nutritional Parameters. J. Sci. Food Agric. 2006, 86, 2327–2336. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Adolphi, B.; Rochat, F.; Barclay, D.V.; de Vrese, M.; Açil, Y.; Schrezenmeir, J. Effects of Probiotics, Prebiotics, and Synbiotics on Mineral Metabolism in Ovariectomized Rats—Impact of Bacterial Mass, Intestinal Absorptive Area and Reduction of Bone Turn-Over. NFS J. 2016, 3, 41–50. [Google Scholar] [CrossRef]
| Variables | Probiotic Group (n = 24) | Control Group (n = 22) | p-Value a |
|---|---|---|---|
| Female (%) | 70.8 | 68.2 | p = 0.847 |
| Age (years) | 36.6 (13.9) | 30.3 (10.2) | p = 0.301 |
| Height (m) | 1.68 (0.1) | 1.66 (0.1) | p = 0.436 |
| Body mass index (kg/m2) | 26.9 (5.5) | 23.3 (3.5) | p = 0.021 |
| Waist-to-hip ratio | 0.82 (0.1) | 0.80 (0.1) | p = 0.300 |
| Smoking (%) | 25.0 | 18.2 | p = 0.580 |
| Physical activity (%) | p = 0.919 | ||
| High | 29.2 | 40.9 | |
| Regular | 25.0 | 13.6 | |
| Cholesterol (mg/dL) | 171.0 (24.4) | 172.1 (25.8) | p = 0.657 |
| Glucose (mg/dL) | 79.8 (11.5) | 79.6 (9.7) | p = 0.586 |
| Probiotic Group (n = 24) | Placebo Group (n = 22) | p b | |||||
|---|---|---|---|---|---|---|---|
| Total Cholesterol | Mean (SD) | Δ from Baseline | p a | Mean (SD) | Change | p a | |
| 1st week | 171.0 (24.4) | 172.1 (25.8) | 0.673 | ||||
| 6th week | 190.2 (38.2) | 19.2 (44.0) | 0.039 | 176.1 (40.0) | 4.0 (30.4) | 0.548 | |
| 12th week | 175.8 (28.9) | 4.8 (28.3) | 0.040 | 179.3 (49.7) | 7.1 (33.2) | 0.325 | |
| LDL | |||||||
| 1st week | 86.2 (21.8) | 86.9 (23.4) | 0.468 | ||||
| 6th week | 91.3 (35.9) | 5.2 (28.0) | 0.373 | 80.5 (29.5) | −6.4 (18.0) | 0.108 | |
| 12th week | 91.7 (25.8) | 5.8 (14.0) | 0.064 | 92.3 (34.8) | 5.4 (17.7) | 0.168 | |
| HDL | |||||||
| 1st week | 53.5 (13.2) | 54.1 (9.8) | 0.313 | ||||
| 6th week | 51.6 (16.1) | −1.9 (14.9) | 0.544 | 53.6 (13.2) | −0.5 (13.0) | 0.858 | |
| 12th week | 54.63 (12.0) | 1.1 (9.3) | 0.560 | 58.5 (16.0) | 4.4 (11.4) | 0.086 | |
| TRGL | |||||||
| 1st week | 73.7 (30.4) | 83.5 (64.5) | 0.719 | ||||
| 6th week | 77.1 (37.1) | 3.4 (28.4) | 0.530 | 79.7 (52.9) | −3.9 (36.5) | 0.615 | |
| 12th week | 82.4 (34.6) | 8.7 (19.1) | 0.04 | 80.0 (43.9) | −3.5 (29.6) | 0.685 | |
| GLU | |||||||
| 1st week | 79.8 (11.5) | 79.6 (9.7) | 0.660 | ||||
| 6th week | 90.3 (19.4) | 10.5 (21.5) | 0.025 | 86.3 (16.3) | 6.7 (12.4) | 0.019 | |
| 12th week | 93.1 (10.5) | 13.3 (10.4) | <0.001 | 93.9 (11.2) | 14.3 (10.6) | <0.001 | |
| INS | |||||||
| 1st week | 9.5 (3.4) | 7.7 (3.4) | 0.028 | ||||
| 6th week | 7.8 (2.9) | −1.7 (1.9) | <0.001 | 8.2 (4.9) | 0.5 (3.7) | 0.528 | |
| 12th week | 11.2 (5.8) | +1.7 (3.7) | 0.042 | 7.8 (3.0) | 0.1 (2.4) | 0.908 | |
| UA | |||||||
| 1st week | 4.5 (1.4) | 4.5 (1.1) | 0.888 | ||||
| 6th week | 4.0 (1.8) | −0.5 (0.9) | 0.008 | 4.1 (1.4) | −0.4 (0.9) | 0.025 | |
| 12th week | 4.6 (1.2) | 0.1 (0.6) | 0.495 | 4.7 (1.2) | 0.2 (1.0) | 0.341 | |
| Cortisol | |||||||
| 1st week | 165.7 (64.8) | 154.6 (50.1) | 0.814 | ||||
| 6th week | 131.0 (44.9) | −34.8 (50.6) | 0.003 | 139.8 (52.6) | 14.8 (36.4) | 0.070 | |
| 12th week | 137.5 (48.4) | −28.2 (38.4) | 0.002 | 132.7 (61.3) | 22.0 (58.1) | 0.091 | |
| hs-CRP | |||||||
| 1st week | 20.2 (21.7) | 9.1 (0.0) | 0.002 | ||||
| 6th week | 21.6 (33.0) | 1.4 (32.0) | 0.179 | 7.9 (11.0) | −1.2 (10.2) | 0.067 | |
| 12th week | 16.8 (20.8) | −3.4 (16.3) | 0.376 | 10.3 (12.0) | 1.2 (12.5) | 0.615 | |
| IgA | |||||||
| 1st week | 2265.0 (842.3) | 2188.5 (1179.0) | 0.923 | ||||
| 6th week | 2278.2 (752.9) | 13.2 (608.1) | 0.998 | 2515.4 (1304.1) | 327.9 (1266.7) | 0.263 | |
| 12th week | 2178.8 (716.2) | −86.2 (377.1) | 0.280 | 2213.8 (952.1) | 25.4 (780.4) | 0.880 | |
| IL-6 | |||||||
| 1st week | 4.3 (2.6) | 3.0 (1.8) | 0.035 | ||||
| 6th week | 2.5 (2.0) | −1.8 (2.6) | 0.002 | 1.5 (0.9) | −1.6 (2.2) | 0.004 | |
| 12th week | 2.9 (1.9) | −1.3 (2.9) | 0.03 | 2.9 (2.5) | −0.2 (3.4) | 0.816 | |
| Folate | |||||||
| 1st week | 8.3 (3.7) | 7.7 (3.7) | 0.944 | ||||
| 6th week | 7.2 (3.3) | −1.1 (2.1) | 0.012 | 7.7 (5.7) | −0.1 (1.9) | 0.860 | |
| 12th week | 5.9 (3.6) | −2.2 (2.2) | <0.001 | 5.5 (3.8) | −2.2 (3.2) | 0.004 | |
| VitB12 | |||||||
| 1st week | 468.7 (115.5) | 488.8 (187.5) | 0.762 | ||||
| 6th week | 502.3 (103.3) | 33.6 (83.4) | 0.061 | 511.6 (169.2) | 22.8 (120.4) | 0.385 | |
| 12th week | 522.7 (114.4) | 54.0 (118.7) | 0.036 | 506.8 (121.3) | 18.0 (93.6) | 0.378 | |
| VitD | |||||||
| 1st week | 23.5 (9.1) | 23.3 (8.2) | 0.802 | ||||
| 6th week | 22.3 (8.5) | −1.2 (3.0) | 0.057 | 24.0 (8.3) | 0.7 (4.1) | 0.449 | |
| 12th week | 25.9 (6.2) | 2.4 (7.7) | 0.138 | 25.1 (5.2) | 1.7 (6.7) | 0.239 | |
| TAC | |||||||
| 1st week | 0.8 (0.2) | 0.8 (0.2) | |||||
| 6th week | 0.9 (0.2) | 0.04 (0.1) | 0.137 | 0.8 (0.2) | 0.03 (0.1) | 0.241 | 0.391 |
| 12th week | 0.9 (0.2) | 0.06 (0.1) | 0.026 | 0.9 (0.2) | 0.05 (0.1) | 0.085 | |
| Probiotic Group (n = 24) | Placebo Group (n = 22) | p b | |||||
|---|---|---|---|---|---|---|---|
| Urine Magnesium | Mean (SD) | Δ from Baseline | p a | Mean (SD) | Change | p a | |
| 1st week | 11.0 (5.7) | 9.8 (7.7) | 0.585 | ||||
| 6th week | 7.9 (3.9) | −3.05 (5.6) | 0.013 | 8.2 (7.4) | −1.7 (9.7) | 0.433 | |
| 12th week | 10.4 (6.6) | −0.6 (8.2) | 0.713 | 9.0 (6.2) | −0.9 (6.4) | 0.529 | |
| Urine Phosphorus | |||||||
| 1st week | 103.7 (54.0) | 98.8 (44.8) | 0.933 | ||||
| 6th week | 105.0 (45.4) | 1.3 (50.3) | 0.902 | 91.0 (41.3) | −7.7 (54.8) | 0.516 | |
| 12th week | 106.1 (66.3) | 2.4 (50.6) | 0.817 | 120.9 (59.3) | 22.1 (73.8) | 0.174 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousdouni, P.; Kandyliari, A.; Kargadouri, A.; Potsaki, P.; Papagianni, O.I.; Stylianou, M.-E.; Stathopoulou, N.; Andrianopoulou, P.; Kapsokefalou, M.; Bountziouka, V.; et al. Effect of Daily Lactococcus cremoris spp. Consumption Immobilized on Oat Flakes on Blood and Urine Biomarkers: A Randomized Placebo-Controlled Clinical Trial. Medicina 2025, 61, 956. https://doi.org/10.3390/medicina61060956
Bousdouni P, Kandyliari A, Kargadouri A, Potsaki P, Papagianni OI, Stylianou M-E, Stathopoulou N, Andrianopoulou P, Kapsokefalou M, Bountziouka V, et al. Effect of Daily Lactococcus cremoris spp. Consumption Immobilized on Oat Flakes on Blood and Urine Biomarkers: A Randomized Placebo-Controlled Clinical Trial. Medicina. 2025; 61(6):956. https://doi.org/10.3390/medicina61060956
Chicago/Turabian StyleBousdouni, Panoraia, Aikaterini Kandyliari, Anastasia Kargadouri, Panagiota Potsaki, Olga I. Papagianni, Maria-Eleni Stylianou, Nikoletta Stathopoulou, Panagiota Andrianopoulou, Maria Kapsokefalou, Vasiliki Bountziouka, and et al. 2025. "Effect of Daily Lactococcus cremoris spp. Consumption Immobilized on Oat Flakes on Blood and Urine Biomarkers: A Randomized Placebo-Controlled Clinical Trial" Medicina 61, no. 6: 956. https://doi.org/10.3390/medicina61060956
APA StyleBousdouni, P., Kandyliari, A., Kargadouri, A., Potsaki, P., Papagianni, O. I., Stylianou, M.-E., Stathopoulou, N., Andrianopoulou, P., Kapsokefalou, M., Bountziouka, V., Kolomvotsou, A., Prapa, I., Mitropoulou, G., Pavlatou, C., Tzakos, A. G., Panas, P., Kourkoutas, Y., & Koutelidakis, A. E. (2025). Effect of Daily Lactococcus cremoris spp. Consumption Immobilized on Oat Flakes on Blood and Urine Biomarkers: A Randomized Placebo-Controlled Clinical Trial. Medicina, 61(6), 956. https://doi.org/10.3390/medicina61060956











