Inhomogeneity of the Left Atrial Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiographic Assessment
2.2.1. Automatic Biplane Method for Left Atrial Strain [15]
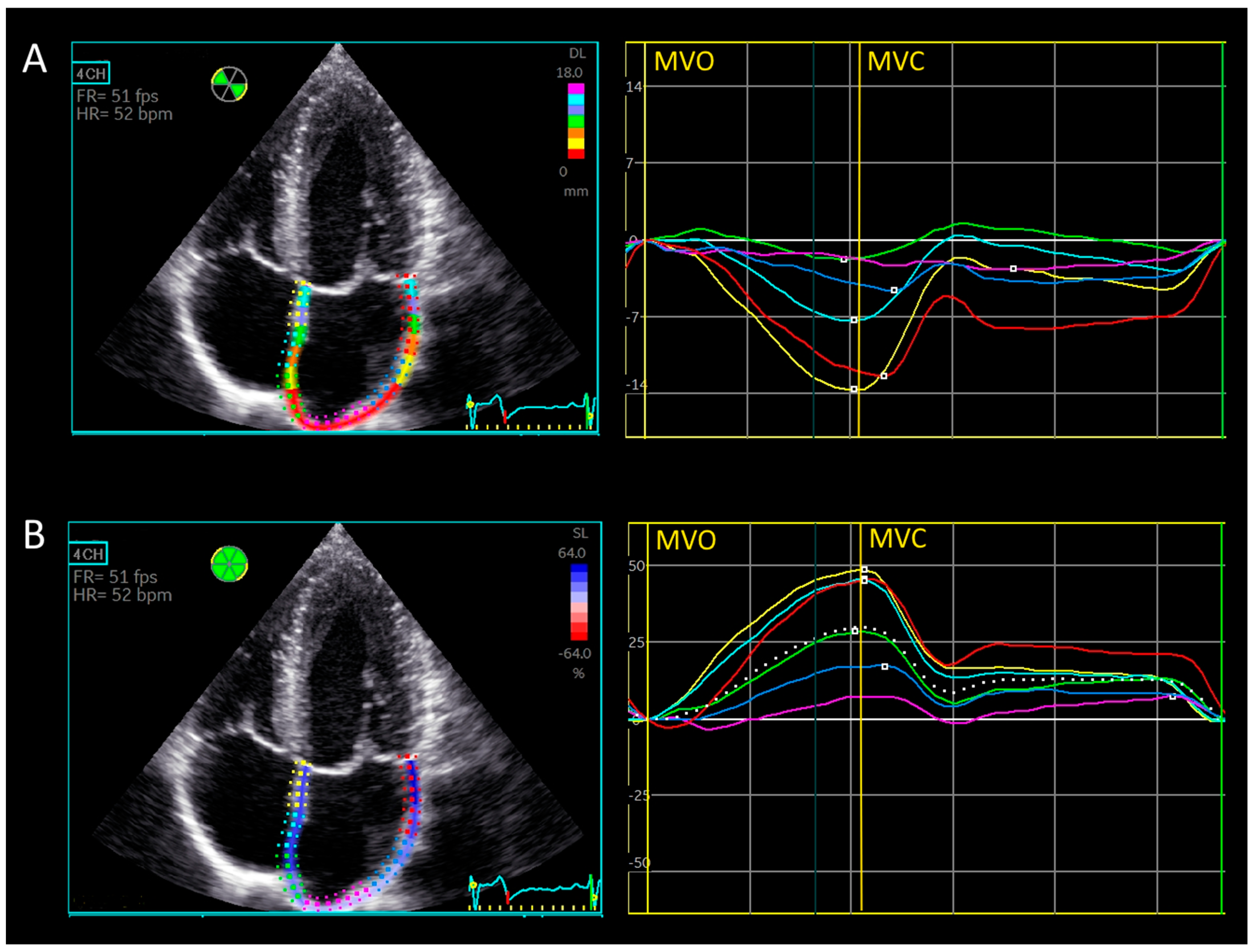
2.2.2. Manual Triplane Left Atrial Strain Assessment Using Ventricular-Dedicated 2D Strain Software
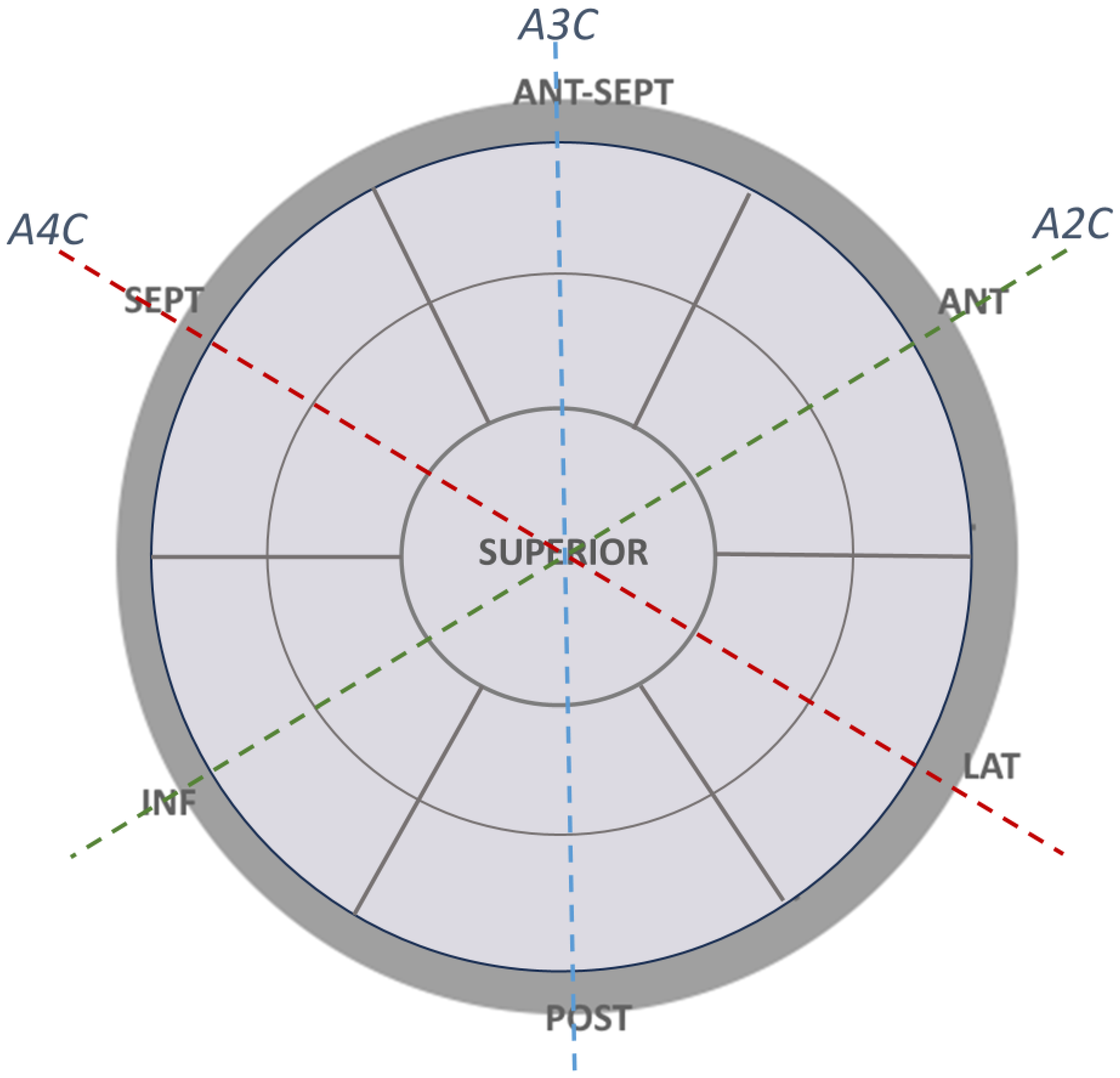
2.2.3. Left Atrial Walls and Segment Definitions for Regional Strain Calculation
2.2.4. Longitudinal Displacement
2.3. Statistical Methods
2.4. Ethical Approval
3. Results
3.1. Age, Anthropometric and Echocardiographic Data
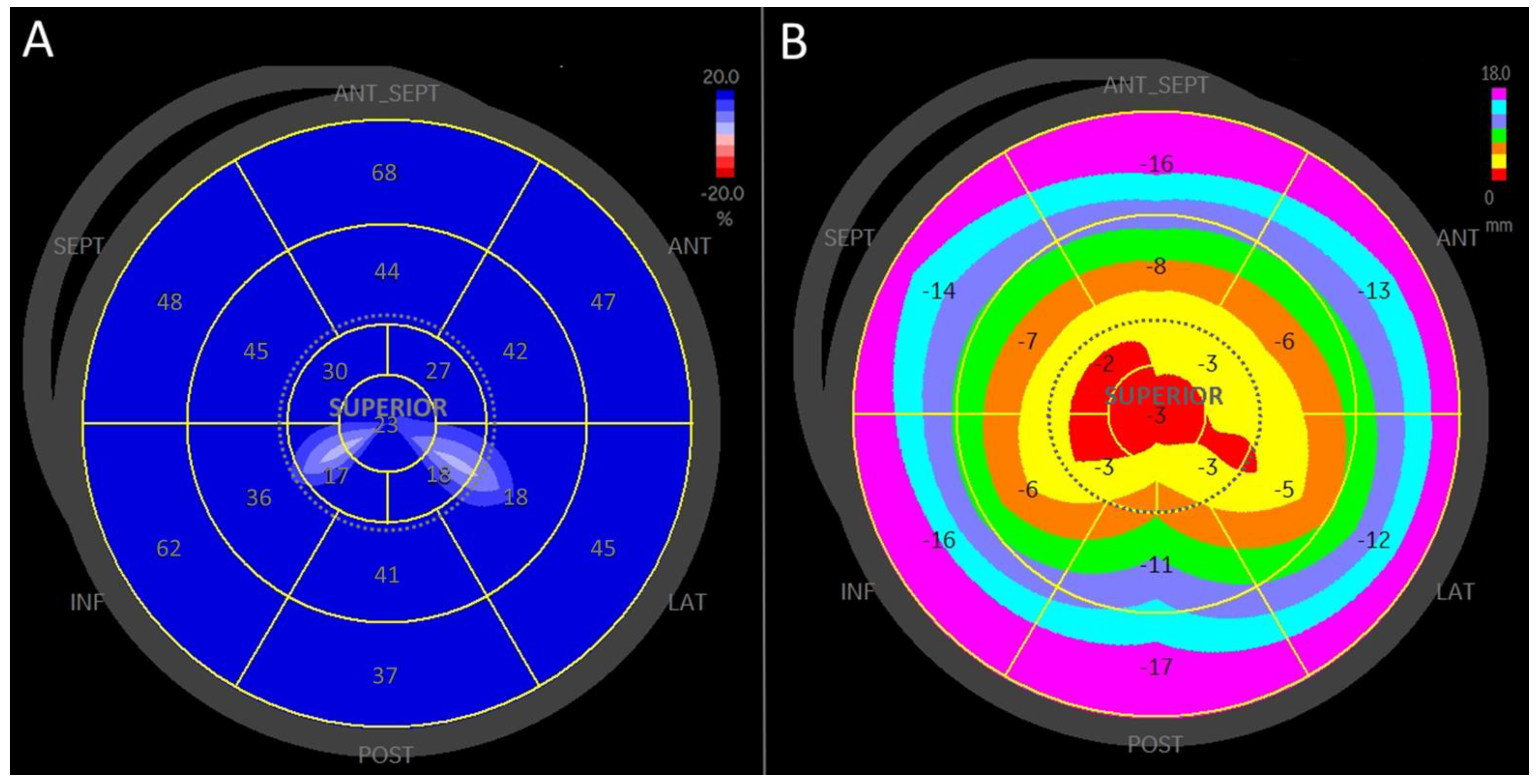
3.2. Strain Analysis
3.3. Longitudinal Displacement
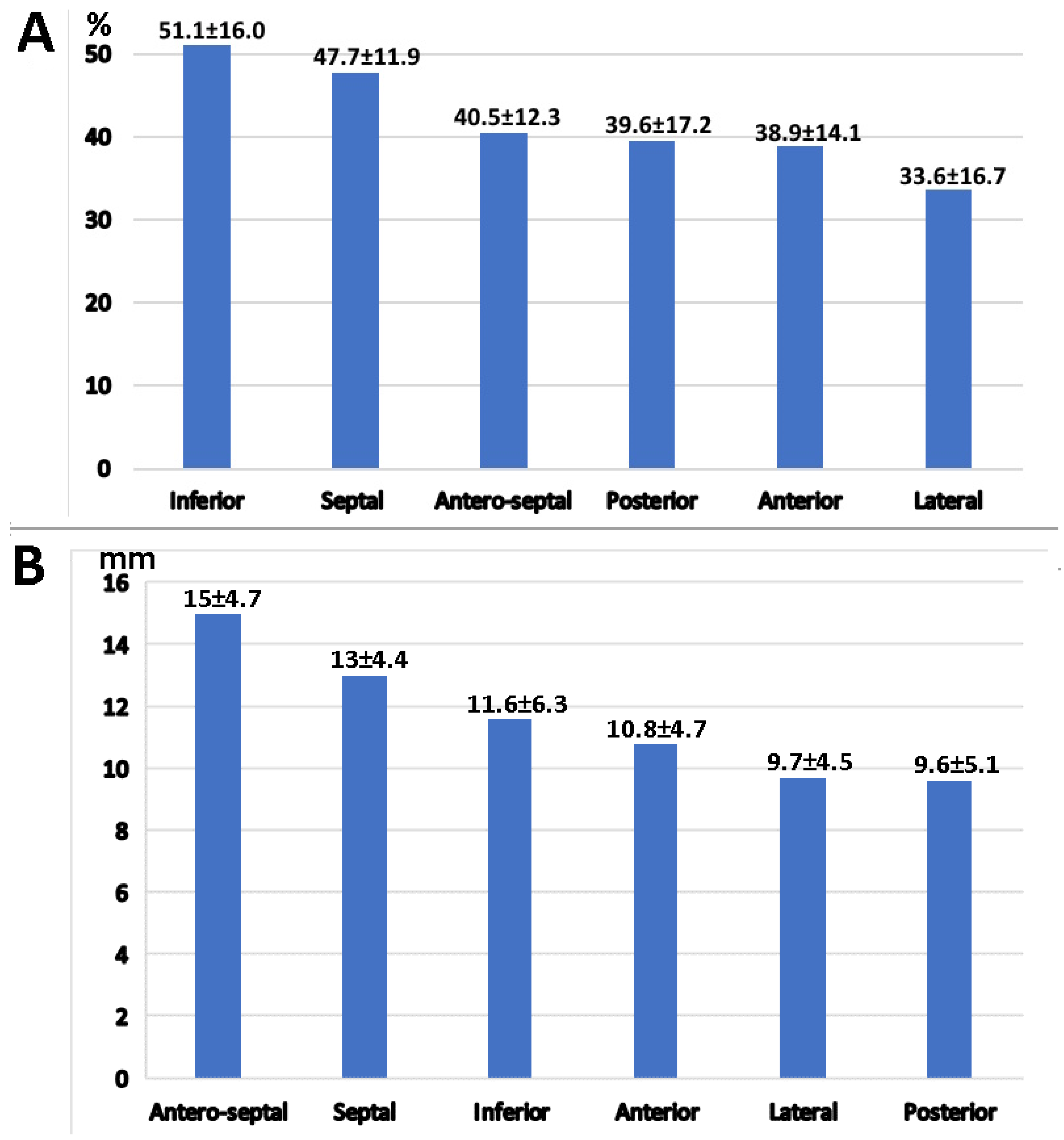
3.4. Left Atrial Wall Motion Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leitman, M.; Lysyansky, P.; Sidenko, S.; Shir, V.; Peleg, E.; Binenbaum, M.; Kaluski, E.; Krakover, R.; Vered, Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J. Am. Soc. Echocardiogr. 2004, 17, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Ferkh, A.; Clark, A.; Thomas, L. Left atrial phasic function: Physiology, clinical assessment and prognostic value. Heart 2023, 109, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600, Erratum in Eur. Heart J. Cardiovasc. Imaging 2018, 19, 830–833. https://doi.org/10.1093/ehjci/jey071. [Google Scholar] [CrossRef] [PubMed]
- Mălăescu, G.G.; Mirea, O.; Capotă, R.; Petrescu, A.M.; Duchenne, J.; Voigt, J.U. Left Atrial Strain Determinants During the Cardiac Phases. JACC Cardiovasc. Imaging 2022, 15, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, S.; Yang, H.; Wang, Y.; Nolan, M.; Negishi, T.; Negishi, K.; Marwick, T.H. Association of the Active and Passive Components of Left Atrial Deformation with Left Ventricular Function. J. Am. Soc. Echocardiogr. 2017, 30, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Braunauer, K.; Düngen, H.D.; Belyavskiy, E.; Aravind-Kumar, R.; Frydas, A.; Kropf, M.; Huang, F.; Marquez, E.; Tadic, M.; Osmanoglou, E.; et al. Potential usefulness mitral with preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Azemi, T.; Rabdiya, V.M.; Ayirala, S.R.; McCullough, L.D.; Silverman, D.I. Left atrial strain is reduced in patients with atrial fibrillation, stroke or TIA, and low risk CHADS(2) scores. J. Am. Soc. Echocardiogr. 2012, 25, 1327–1332. [Google Scholar] [CrossRef]
- Pagola, J.; González-Alujas, T.; Flores, A.; Muchada, M.; Rodriguez-Luna, D.; Seró, L.; Rubiera, M.; Boned, S.; Ribó, M.; Alvarez-Sabin, J.; et al. Left atria strain is a surrogate marker for detection of atrial fibrillation in cryptogenic strokes. Stroke 2014, 45, e164–e166. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Pastore, M.C.; Benfari, G.; Bisleri, G.; Maccherini, M.; Lisi, G.; Cameli, P.; Lisi, M.; Dokollari, A.; Carrucola, C.; et al. Left atrial strain as a pre-operative prognostic marker for patients with severe mitral regurgitation. Int. J. Cardiol. 2021, 324, 139–145. [Google Scholar] [CrossRef]
- Nakajima, T.; Haruyama, A.; Fukuda, T.; Minami, K.; Hirose, S.; Yazawa, H.; Nakajima, T.; Hasegawa, T.; Kitagawa, Y.; Obi, S.; et al. Left atrial reservoir strain is a marker of atrial fibrotic remodeling in patients undergoing cardiovascular surgery: Analysis of gene expression. PLoS ONE 2024, 19, e0306323. [Google Scholar] [CrossRef] [PubMed]
- Vianna-Pinton, R.; Moreno, C.A.; Baxter, C.M.; Lee, K.S.; Tsang, T.S.; Appleton, C.P. Two-dimensional speckle-tracking echocardiography of the left atrium: Feasibility and regional contraction and relaxation differences in normal subjects. J. Am. Soc. Echocardiogr. 2009, 22, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, C.; Herbots, L.; D’hooge, J.; Claus, P.; Marciniak, A.; Langeland, T.; Bijnens, B.; Rademakers, F.E.; Sutherland, G.R. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: A study in normal subjects. Eur. J. Echocardiogr. 2006, 7, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, A.; Ishizu, T.; Sato, M.; Igarashi, M.; Sato, K.; Yamamoto, M.; Machino-Ohtsuka, T.; Ieda, M. Left atrial regional strain assessed by novel dedicated three-dimensional speckle tracking echocardiography. J. Cardiol. 2021, 78, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Pastore, M.C.; Procopio, M.C.; Pica, A.; Vigna, M.; Benfari, G.; Diviggiano, E.E.; Martini, L.; Lunghetti, S.; Focardi, M.; et al. Unveiling the reliability of left atrial strain measurement: A dedicated speckle tracking software perspective in controls and cases. Eur. Heart J.—Imaging Methods Pract. 2024, 2, qyae061. [Google Scholar] [CrossRef]
- Sánchez-Quintana, D.; López-Mínguez, J.R.; Macías, Y.; Cabrera, J.A.; Saremi, F. Left atrial anatomy relevant to catheter ablation. Cardiol. Res. Pract. 2014, 2014, 289720, Erratum in: Cardiol. Res. Pract. 2020, 2020, 3490543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cameli, M.; Lisi, M.; Righini, F.M.; Mondillo, S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc. Ultrasound. 2012, 10, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ancona, R.; Comenale Pinto, S.; Caso, P.; D’Andrea, A.; Di Salvo, G.; Arenga, F.; Coppola, M.G.; Sellitto, V.; Macrino, M.; Calabrò, R. Left atrium by echocardiography in clinical practice: From conventional methods to new echocardiographic techniques. Sci. World J. 2014, 2014, 451042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Neill, T.; Kang, P.; Hagendorff, A.; Tayal, B. The Clinical Applications of Left Atrial Strain: A Comprehensive Review. Medicina 2024, 60, 693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos, A.B.; Kraigher-Krainer, E.; Gupta, D.K.; Claggett, B.; Zile, M.R.; Pieske, B.; Voors, A.A.; Lefkowitz, M.; Bransford, T.; Shi, V.; et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 1096–1103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olsen, F.J.; Diederichsen, S.Z.; Jørgensen, P.G.; Jensen, M.T.; Dahl, A.; Landler, N.E.; Graff, C.; Brandes, A.; Krieger, D.; Haugan, K.; et al. Left Atrial Strain Predicts Subclinical Atrial Fibrillation Detected by Long-term Continuous Monitoring in Elderly High-Risk Individuals. Circ. Cardiovasc. Imaging 2024, 17, e016197. [Google Scholar] [CrossRef] [PubMed]
- Deferm, S.; Martens, P.; Verbrugge, F.H.; Bertrand, P.B.; Dauw, J.; Verhaert, D.; Dupont, M.; Vandervoort, P.M.; Mullens, W. LA Mechanics in Decompensated Heart Failure: Insights from Strain Echocardiography with Invasive Hemodynamics. JACC Cardiovasc. Imaging 2020, 13, 1107–1115, Erratum in JACC Cardiovasc. Imaging 2020, 13, 1862. https://doi.org/10.1016/j.jcmg.2020.07.001. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Addetia, K.; Maffessanti, F.; Mor-Avi, V.; Lang, R.M. LA Strain for Categorization of LV Diastolic Dysfunction. JACC Cardiovasc. Imaging. 2017, 10, 735–743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagueh, S.F.; Khan, S.U. Left Atrial Strain for Assessment of Left Ventricular Diastolic Function: Focus on Populations with Normal LVEF. JACC Cardiovasc. Imaging 2023, 16, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Fournet, M.; Chabanne, C.; Lelong, B.; Leguerrier, A.; Flecher, E.; Mabo, P.; Donal, E. Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: A 2D speckle-tracking echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Stassen, J.; Namazi, F.; van der Bijl, P.; van Wijngaarden, S.E.; Kamperidis, V.; Marsan, N.A.; Delgado, V.; Bax, J.J. Left Atrial Reservoir Function and Outcomes in Secondary Mitral Regurgitation. J. Am. Soc. Echocardiogr. 2022, 35, 477–485.e3. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.P.; Pandit, J.A.; Jensen, P.N.; Wiggins, K.L.; Patel, R.B.; Freed, B.H.; Bertoni, A.G.; Shah, S.J.; Heckbert, S.R.; Floyd, J.S. Left Atrial Strain and the Risk of Atrial Arrhythmias from Extended Ambulatory Cardiac Monitoring: MESA. J. Am. Heart Assoc. 2022, 11, e026875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.A.; Chugh, S.S.; de Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial cardiomyopathy revisited-evolution of a concept: A clinical consensus statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS). Europace 2024, 26, euae204. [Google Scholar] [CrossRef]
- Hohendanner, F.; Romero, I.; Blaschke, F.; Heinzel, F.R.; Pieske, B.; Boldt, L.H.; Parwani, A.S. Extent and magnitude of low-voltage areas assessed by ultra-high-density electroanatomical mapping correlate with left atrial function. Int. J. Cardiol. 2018, 272, 108–112. [Google Scholar] [CrossRef]
- Sillett, C.; Razeghi, O.; Lee, A.W.C.; Solis Lemus, J.A.; Roney, C.; Mannina, C.; de Vere, F.; Ananthan, K.; Ennis, D.B.; Haberland, U.; et al. A three-dimensional left atrial motion estimation from retrospective gated computed tomography: Application in heart failure patients with atrial fibrillation. Front. Cardiovasc. Med. 2024, 11, 1359715. [Google Scholar] [CrossRef]
- Sun, B.J.; Park, J.H. Echocardiographic Measurement of Left Atrial Strain—A Key Requirement in Clinical Practice. Circ. J. 2021, 86, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Nakao, S.; Goda, A.; Yuba, M.; Naito, Y.; Shimizu, M.; Otsuka, M.; Tsujino, T.; Ohyanagi, M.; Masuyama, T. Value of the measurements of left atrial geometry in patients with normalized or restrictive mitral flow velocity pattern. J. Echocardiogr. 2005, 3, 109–117. [Google Scholar] [CrossRef]
- Gan, G.C.H.; Ferkh, A.; Boyd, A.; Thomas, L. Left atrial function: Evaluation by strain analysis. Cardiovasc. Diagn. Ther. 2018, 8, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Caputo, M.; Mondillo, S.; Ballo, P.; Palmerini, E.; Lisi, M.; Marino, E.; Galderisi, M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound. 2009, 7, 6. [Google Scholar] [CrossRef]
- Di Salvo, G.; Pacileo, G.; Castaldi, B.; Gala, S.; Morelli, C.; D’Andrea, A.; Limongelli, G.; Del Gaizo, F.; Merlino, E.; Russo, M.G.; et al. Two-dimensional strain and atrial function: A study on patients after percutaneous closure of atrial septal defect. Eur. J. Echocardiogr. 2009, 10, 256–259. [Google Scholar] [CrossRef]

| Variable | Value |
|---|---|
| Age, years | 24 ± 4.3 |
| Height, cm | 179.7 ± 8.8 |
| Weight, kg | 74.2 ± 6.2 |
| BSA m2 | 1.93 ± 0.13 |
| LAVi, mL/m2 | 33.3 ± 9.3 |
| LVEDD, cm | 5.03 ± 0.37 |
| LVESD, cm | 3.04 ± 0.38 |
| IVS, cm | 0.95 ± 0.09 |
| PW, cm | 0.95 ± 0.1 |
| LVMi, g/m2 | 84.5 ± 15.9 |
| EF, % | 58.7 ± 2.6 |
| E/A ratio | 1.9 ± 0.6 |
| Edec, msec | 161.8 ± 40.1 |
| E/E’ ratio | 5.03 ± 0.89 |
| Segment | Strain, % | p-Value | Displacement, mm | p-Value |
|---|---|---|---|---|
| Basal | 46.1 ± 16.9 | NA | 15.2 ± 4.0 | NA |
| Mid-atrium | 37.7 ± 13.7 | 1.6 × 10−5 | 8 ± 4.0 | <10−35 † |
| Superior | 27.5 ± 7.6 | <10−7 ‡ | 2.6 ± 1.0 | <10−9 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitman, M.; Tyomkin, V. Inhomogeneity of the Left Atrial Strain. Medicina 2025, 61, 944. https://doi.org/10.3390/medicina61060944
Leitman M, Tyomkin V. Inhomogeneity of the Left Atrial Strain. Medicina. 2025; 61(6):944. https://doi.org/10.3390/medicina61060944
Chicago/Turabian StyleLeitman, Marina, and Vladimir Tyomkin. 2025. "Inhomogeneity of the Left Atrial Strain" Medicina 61, no. 6: 944. https://doi.org/10.3390/medicina61060944
APA StyleLeitman, M., & Tyomkin, V. (2025). Inhomogeneity of the Left Atrial Strain. Medicina, 61(6), 944. https://doi.org/10.3390/medicina61060944





