A Comparative Analysis of Feeding Practices and Oral Immunity in Infants
Abstract
1. Introduction
2. Methodology
2.1. Study Design
2.2. Study Sample
Participant Flow and STROBE Compliance
2.2.1. Inclusion Criteria
- -
- Infants aged between 1 and 12 months.
- -
- Exclusively breastfed or formula-fed infants (for at least three months before enrollment).
- -
- Healthy infants with no known underlying medical conditions or congenital anomalies.
- -
- Parents/guardians who provided informed consent for participation.
2.2.2. Exclusion Criteria
- -
- Infants with known oral health issues, including oral infections, congenital defects affecting oral development, or diagnosed immunodeficiencies.
- -
- Infants receiving both breast milk and formula (mixed feeding).
- -
- Infants on antibiotic treatment within one month before sample collection.
- -
- Infants with special nutritional needs or metabolic disorders.
2.3. Participant Consent and Data Collection
2.3.1. Parental Questionnaire
- -
- A structured questionnaire was administered to parents or guardians to collect demographic data, feeding history, and general health information. Key variables included the following:
- -
- Demographic variables, including maternal and infant age, gender of the infant, and birth weight.
- -
- Feeding method (exclusive breastfeeding or formula feeding).
- -
- Duration of feeding type.
- -
- Oral hygiene practices (e.g., cleaning with a cloth and use of pacifiers).
- -
- Infant medical history, including any recent illnesses or medication use.
- -
- Environmental exposure to pollutants.
- -
- Teething and pain management tools (teething process, beginning time of the first teething, pain during teething, and pain management tools).
2.3.2. Estimation of Salivary IL-17 [17]
Saliva Sample Collection
Salivary IL-17 Estimation by ELISA
2.3.3. Oral Sample Collection and Microbiological Processing
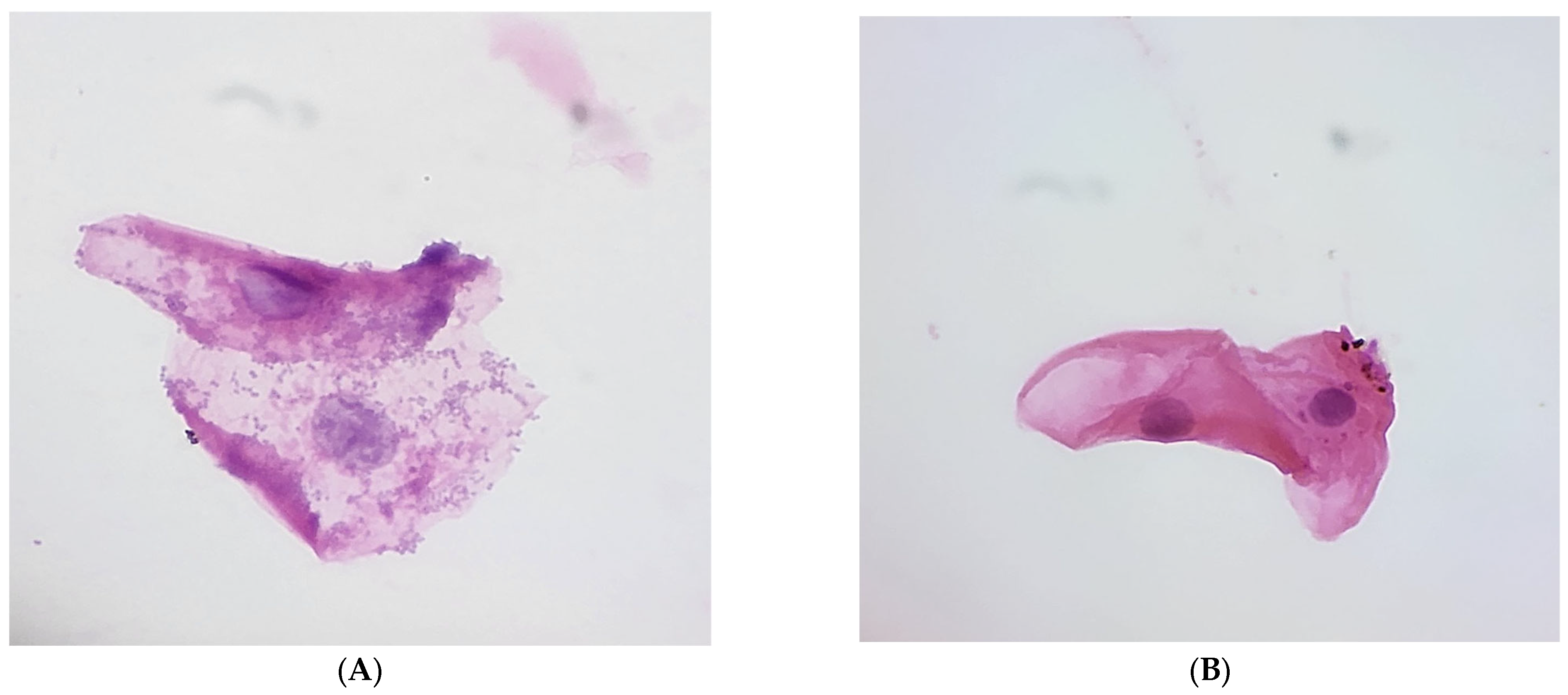
2.3.4. Epithelial Cell Sample Collection and Cytological Analysis
2.4. Ethical Considerations
2.5. Statistical Data Analysis
2.6. Reliability and Validity
3. Results
3.1. Salivary IL-17 Levels
3.2. Bacterial Culture Analysis Results
3.3. Epithelial Cell Analysis and Feeding Practices
3.4. Nuclear and Cytoplasmic Features
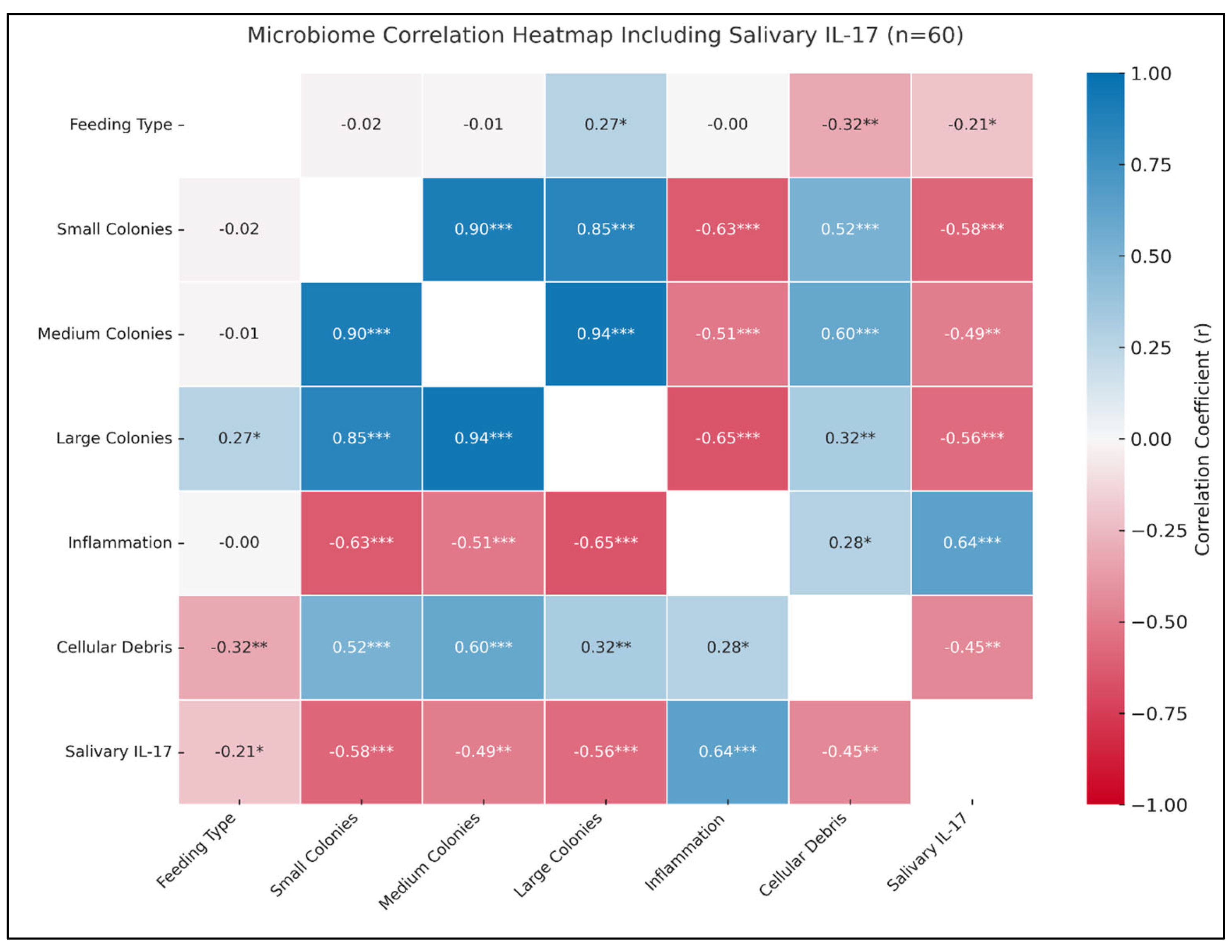
3.5. Normal Flora Coverage
3.6. Questionnaire Responses
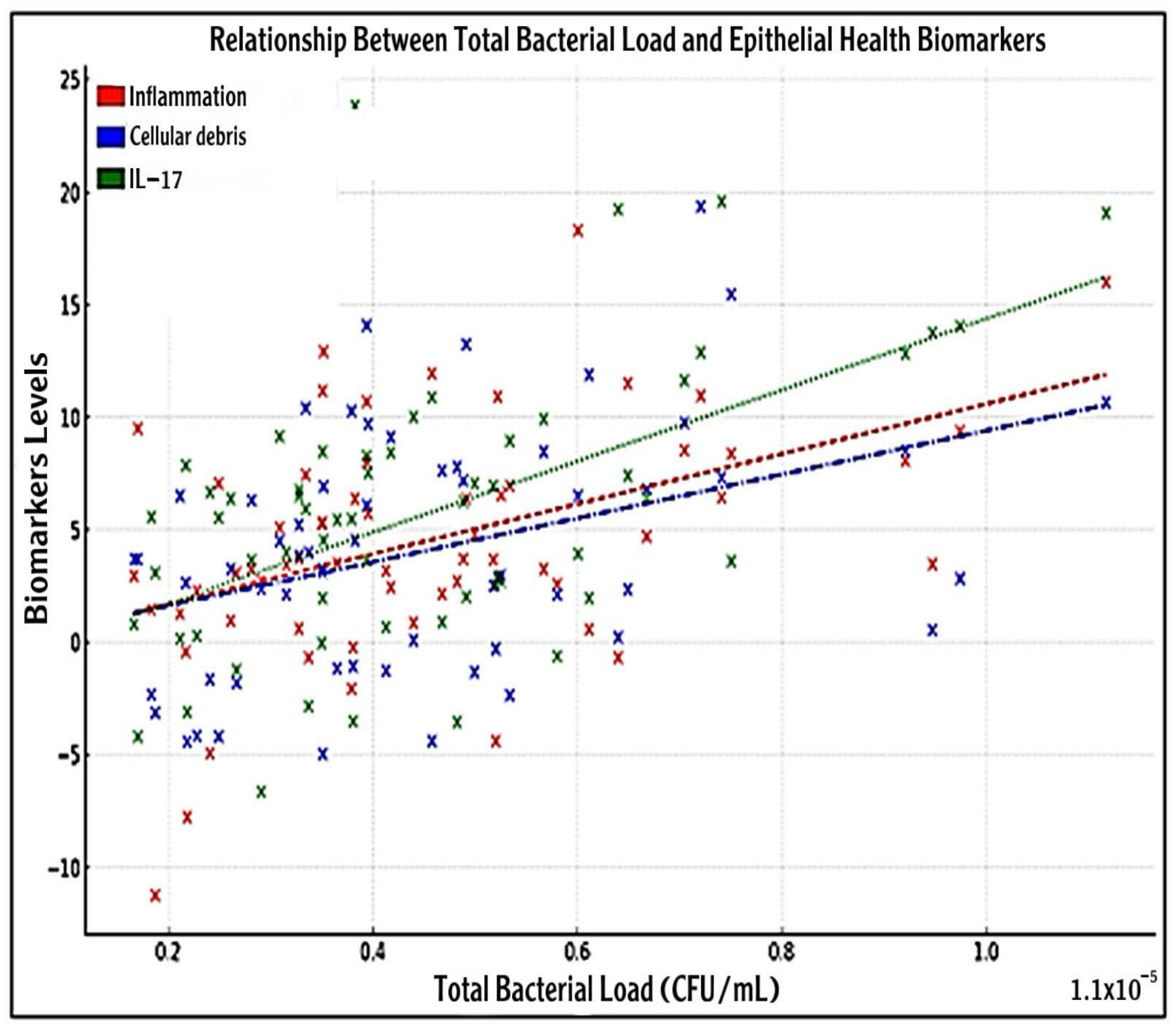
3.7. Association Between Bacterial Load with IL-17 and Epithelial Health Biomarkers
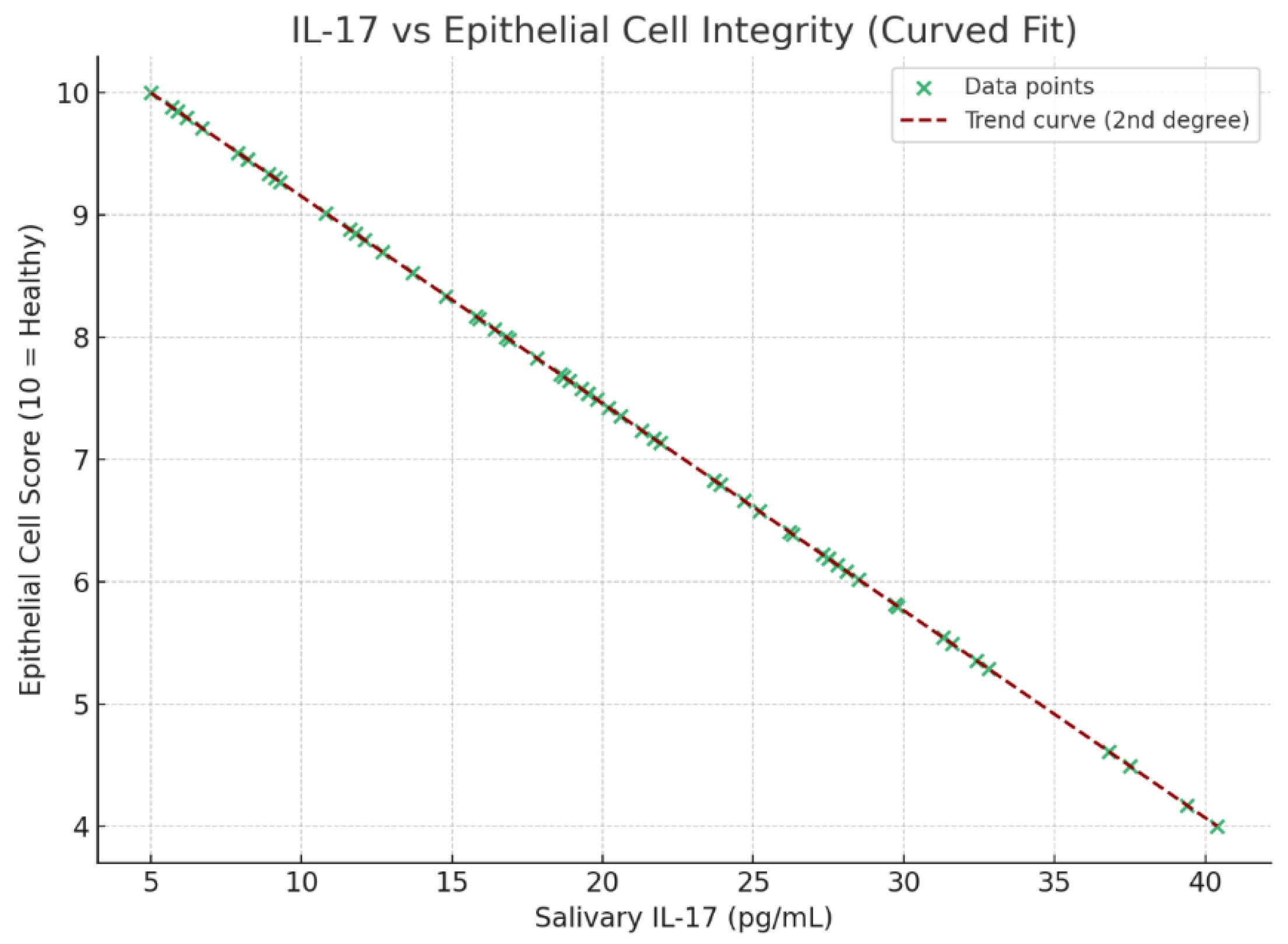
3.8. Correlation of IL-17 with Epithelial Integrity, Oral Hygiene, Environmental Exposure, and Teething Discomfort
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and Their Potential to Modulate the Microbiome and Immunity. Cell. Mol. Immunol. 2021, 18, 889–904. [Google Scholar] [CrossRef] [PubMed]
- American Society for Microbiology. Feeding Mode of Newborns Could Influence Oral Bacteria Linked to Obesity and Allergies. 2022. Available online: https://asm.org/Press-Releases/2022/Feeding-Mode-of-Newborns-Could-Influence-Oral-Bact (accessed on 18 January 2022).
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Sweeney, E.L.; Al-Shehri, S.S.; Cowley, D.M.; Liley, H.G.; Bansal, N.; Charles, B.G.; Shaw, P.N.; Duley, J.A.; Knox, C.L. The effect of breastmilk and saliva combinations on the in vitro growth of oral pathogenic and commensal microorganisms. Sci. Rep. 2018, 8, 15112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Saraithong, P.; Zhang, L.; Dills, A.; Paster, B.J.; Xiao, J.; Wu, T.T.; Jones, Z. Dynamics of oral microbiome acquisition in healthy infants: A pilot study. Front. Oral Health 2023, 4, 1152601. [Google Scholar] [CrossRef]
- Stinson, L.F. Establishment of the early-life microbiome: A DOHaD perspective. J. Dev. Orig. Health Dis. 2020, 11, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.A.; Adams, G.G.; Blum, J.; Byrne, S.J.; Carpenter, L.; Gussy, M.G.; Calache, H.; Catmull, D.V.; Reynolds, E.C.; Dashper, S.G. Breastmilk influences development and composition of the oral microbiome. J. Oral Microbiol. 2022, 14, 2096287. [Google Scholar] [CrossRef]
- Holdsworth, E.A.; Williams, J.E.; Pace, R.M.; Lane, A.A.; Gartstein, M.; McGuire, M.A.; McGuire, M.K.; Meehan, C.L. Breastfeeding patterns are associated with human milk microbiome composition: The Mother-Infant Microbiomes, Behavior, and Ecology Study (MIMBES). PLoS ONE 2023, 18, e0287839. [Google Scholar] [CrossRef]
- Binns, C.; Lee, M.; Low, W.Y. The Long-Term Public Health Benefits of Breastfeeding. Asia Pac. J. Public Health 2016, 28, 7–14. [Google Scholar] [CrossRef]
- Arishi, R.A.; Lai, C.T.; Geddes, D.T.; Stinson, L.F. Impact of breastfeeding and other early-life factors on the development of the oral microbiome. Front. Microbiol. 2023, 14, 1236601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sankhla, B.; Sharma, A.; Shetty, R.S.; Bolla, S.C.; Gantha, N.S.; Reddy, P. Exfoliative cytology of buccal squames: A quantitative cytomorphometric analysis of patients with diabetes. J. Int. Soc. Prev. Community Dent. 2014, 4, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Dharini, S.; Ramani, P.; Ramalingam, K. Cytomorphometric Analysis of Buccal Exfoliated Cells in Geriatric and Pediatric Age Groups—A Cross-Sectional Study. Cureus 2023, 15, e39082. [Google Scholar] [CrossRef]
- Song, X.; He, X.; Li, X.; Qian, Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol. Immunol. 2016, 13, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chung, H.; Troy, E.; Kasper, D.L. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe 2010, 7, 140–150. [Google Scholar] [CrossRef]
- Pisarska, M.M.; Dunne, M.R.; O’Shea, D.; Hogan, A.E. Interleukin-17 producing mucosal-associated invariant T cells-emerging players in chronic inflammatory diseases? Eur. J. Immunol. 2020, 50, 1098–1108. [Google Scholar] [CrossRef]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and Th17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef]
- Al-Shehri, S.S.; Sweeney, E.L.; Cowley, D.M.; Liley, H.G.; Ranasinghe, P.D.; Charles, B.G.; Shaw, P.N.; Vagenas, D.; Duley, J.A.; Knox, C.L. Deep sequencing of the 16S ribosomal RNA of the neonatal oral microbiome: A comparison of breast-fed and formula-fed infants. Sci. Rep. 2016, 6, 38309. [Google Scholar] [CrossRef]
- Lawrence, S.M.; Ruoss, J.L.; Wynn, J.L. IL-17 in Neonatal Health and Disease. Am. J. Reprod. Immunol. 2018, 79, e12800. [Google Scholar] [CrossRef] [PubMed]
- Rinderknecht, C.; Filippi, C.; Ritz, N.; Fritschi, N.; Simmen, U.; Filippi, A.; Diesch-Furlanetto, T. Associations between Salivary Cytokines and Oral Health, Age, and Sex in Healthy Children. Sci. Rep. 2022, 12, 15991. [Google Scholar] [CrossRef]
- Chen, J.; Liao, M.Y.; Gao, X.L.; Zhong, Q.; Tang, T.T.; Yu, X.; Liao, Y.H.; Cheng, X. IL-17A Induces Pro-Inflammatory Cytokines Production in Macrophages via MAPKinases, NF-κB and AP-1. Cell Physiol. Biochem. 2013, 32, 1265–1274. [Google Scholar] [CrossRef]
- Wilharm, A.; Tabib, Y.; Nassar, M.; Reinhardt, A.; Mizraji, G.; Sandrock, I.; Heyman, O.; Barros-Martins, J.; Aizenbud, Y.; Khalaileh, A.; et al. Mutual Interplay between IL–17–Producing γδT Cells and Microbiota Orchestrates Oral Mucosal Homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Yamada, H.; Hara, H.; Kishihara, K.; Yoshikai, Y. Resident Vδ1+ γδ T Cells Control Early Infiltration of Neutrophils after Escherichia coli Infection via IL-17 Production. J. Immunol. 2007, 178, 4466–4472. [Google Scholar] [CrossRef]
- Holgerson, P.L.; Vestman, N.R.; Claesson, R.; Twetman, S.; Larson, A.; Ekelund, H.; Johansson, I. Oral Microbial Profile Discriminates Breast-Fed from Formula-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 127–136. [Google Scholar] [CrossRef]
- Belstrøm, D.; Damgaard, C.; Könönen, E.; Gürsoy, M.; Holmstrup, P.; Gürsoy, U.K. Salivary Cytokine Levels in Early Gingival Inflammation. J. Oral Microbiol. 2017, 9, 1364101. [Google Scholar] [CrossRef]
- Tu, Y.; Xu, X.; Zhou, X.D. Development and Influencing Factors of Oral Microbiota in Early Life. Sichuan Da Xue Xue Bao Yi Xue Ban 2022, 53, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Wilharm, A.; Binz, C.; Sandrock, I.; Rampoldi, F.; Lienenklaus, S.; Blank, E.; Winkel, A.; Demera, A.; Hovav, A.H.; Stiesch, M.; et al. Interleukin-17 Is Disease Promoting in Early Stages and Protective in Late Stages of Experimental Periodontitis. PLoS ONE 2022, 17, e0265486. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The Gut Microbiota-Immune-Brain Axis: Therapeutic Implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Gaffen, S.L. Structure and Signalling in the IL-17 Receptor Family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.Y.; Dissanayeke, S.; Pampura, A.; Boner, A.L.; Geddes, D.T.; Boyle, R.J.; et al. Immune Components in Human Milk Are Associated with Early Infant Immunological Health Outcomes: A Prospective Three-Country Analysis. Nutrients 2017, 9, 532. [Google Scholar] [CrossRef]
- Dzidic, M.; Mira, A.; Artacho, A.; Abrahamsson, T.R.; Jenmalm, M.C.; Collado, M.C. Allergy Development Is Associated with Consumption of Breastmilk with a Reduced Microbial Richness in the First Month of Life. Pediatr. Allergy Immunol. 2020, 31, 250–257. [Google Scholar] [CrossRef]
- de Oliveira, I.R.; de Araujo, A.N.; Bao, S.N.; Giugliano, L.G. Binding of Lactoferrin and Free Secretory Component to Enterotoxigenic Escherichia coli. FEMS Microbiol. Lett. 2001, 203, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Levy, S.M.; Warren, J.J.; Broffitt, B.; Wefel, J.S.; Tiberia, M.J.; Dawson, D.V. Infant Breast-Feeding and Childhood Caries: A Nine-Year Study. Pediatr. Dent. 2014, 36, 342–347. [Google Scholar] [PubMed] [PubMed Central]
- Li, H.; Xiao, B.; Zhang, Y.; Xiao, S.; Luo, J.; Huang, W. Impact of Maternal Intrapartum Antibiotics on the Initial Oral Microbiome of Neonates. Pediatr. Neonatol. 2019, 60, 654–661. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides: Every Baby Needs a Sugar Mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Gopalakrishna, K.P.; Hand, T.W. Influence of Maternal Milk on the Neonatal Intestinal Microbiome. Nutrients 2020, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Boudry, G.; Charton, E.; Le Huërou-Luron, I.; Ferret-Bernard, S.; Le Gall, S.; Even, S.; Blat, S. The Relationship between Breast Milk Components and the Infant Gut Microbiota. Front. Nutr. 2021, 8, 629740. [Google Scholar] [CrossRef]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human Milk Oligosaccharides: Shaping the Infant Gut Microbiota and Supporting Health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, T.; Chen, W.; Huo, Y.; Mou, X.; Zhao, W. Microbial Regulation of Offspring Diseases Mediated by Maternal-Associated Microbial Metabolites. Front. Microbiol. 2022, 13, 955297. [Google Scholar] [CrossRef]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C. Tight Junction Proteins Occludin and ZO-1 as Regulators of Epithelial Proliferation and Survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Geuking, M.B.; Slack, E.; Hapfelmeier, S.; McCoy, K.D. The Habitat, Double Life, Citizenship, and Forgetfulness of IgA. Immunol. Rev. 2012, 245, 132–146. [Google Scholar] [CrossRef]
- Siggers, R.H.; Siggers, J.; Thymann, T.; Burrin, D.G.; Tappenden, K.A.; Bjornvad, C.R.; Jensen, S.K.; Sangild, P.T. Early Administration of Probiotics Prevents Epithelial Structural Damage in Formula-Fed Neonatal Pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G1141–G1149. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Host Interactions of Probiotic Bacterial Surface Molecules: Comparison with Commensals and Pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef]
- Karched, M.; Alyahya, A.; Khalaf, M.E.; Bhardwaj, R.G.; Al-Sane, M.; Qudeimat, M.A. Comparative Analysis of Salivary Cytine Profiles and Oral Microbial Composition in Caries-Active and Caries-Free Children. J. Dent. 2025, 154, 105611. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Kong, J.; Jia, P.; Wei, C.; Wang, Y.; Pan, Z.; Huang, W.; Li, L.; Chen, H.; Xiang, C. Analysis of Oral Microbiota in Children with Dental Caries by PCR-DGGE and Barcoded Pyrosequencing. Microb. Ecol. 2010, 60, 677–690. [Google Scholar] [CrossRef]
- Andersson, Y.; Hammarström, M.-L.; Lönnerdal, B.; Graverholt, G.; Fält, H.; Hernell, O. Formula Feeding Skews Immune Cell Composition toward Adaptive Immunity Compared to Breastfeeding. J. Immunol. 2009, 183, 4322–4328. [Google Scholar] [CrossRef]
- van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; De Leonardis, N.; Sardano, R.; Pezzolla, C.; De Ruvo, E.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; et al. The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 2023, 16, 1313. [Google Scholar] [CrossRef]
- Zhou, L.; Lopes, J.E.; Chong, M.M.W.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-β-Induced Foxp3 Inhibits TH17 Cell Differentiation by Antagonizing RORγt Function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef]
- Negi, S.; Hashimoto-Hill, S.; Alenghat, T. Neonatal Microbiota–Epithelial Interactions That Impact Infection. Front. Microbiol. 2022, 13, 955051. [Google Scholar] [CrossRef] [PubMed]
- Memarpour, M.; Soltanimehr, E.; Eskandarian, T. Signs and Symptoms Associated with Primary Tooth Eruption: A Clinical Trial on Children. BMC Oral Health 2015, 15, 88. [Google Scholar] [CrossRef]
- Stockinger, B.; Omenetti, S. The Dichotomous Nature of T Helper 17 Cells. Nat. Rev. Immunol. 2017, 17, 535–544. [Google Scholar] [CrossRef]
- Shapira, J.; Berenstein-Ajzman, G.; Engelhard, D.; Cahan, S.; Kalickman, I.; Barak, V. Cytine Levels in Gingival Crevicular Fluid of Erupting Primary Teeth Correlated with Systemic Disturbances Accompanying Teething. Pediatr. Dent. 2003, 25, 441–448. [Google Scholar] [PubMed]
- Taki, M.; Mizuno, K.; Murase, M.; Nishida, Y.; Itabashi, K.; Mukai, Y. Maturational Changes in the Feeding Behaviour of Infants—A Comparison between Breast-Feeding and Bottle-Feeding. Acta Paediatr. 2010, 99, 61–67. [Google Scholar] [CrossRef]
- Conesa, C.; Bellés, A.; Grasa, L.; Sánchez, L. The Role of Lactoferrin in Intestinal Health. Pharmaceutics 2023, 15, 1569. [Google Scholar] [CrossRef]
- Mira, A.; Simon-Soro, A.; Curtis, M.A. Role of Microbial Communities in the Pathogenesis of Periodontal Diseases and Caries. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S23–S38. [Google Scholar] [CrossRef] [PubMed]
- Koukou, Z.; Theodoridou, A.; Taousani, E.; Antonakou, A.; Panteris, E.; Papadopoulou, S.-S.; Skordou, A.; Sifakis, S. Effectiveness of Non-Pharmacological Methods, Such as Breastfeeding, to Mitigate Pain in NICU Infants. Children 2022, 9, 1568. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, C.; Sun, Y.; Dong, L.; Si, Y.; Yang, J.; Zhu, P.; Yang, F. Symbiotic Relationship between Prevotella denticola and Streptococcus mutans Enhances Caries-Associated Virulence of Plaque Biofilms. Arch. Oral Biol. 2023, 145, 105714. [Google Scholar] [CrossRef]
- Mahilkar, S.; Malagi, S.K.; Soni, A.; Abraham, D.V.; Johnson, L.; Pattanshetti, K.S. IL-17, A Possible Salivary Biomarker for Preterm Birth in Females with Periodontitis. J. Obstet. Gynaecol. India 2021, 71, 262–267. [Google Scholar] [CrossRef]
- Pereira, T.S.; da Silva, C.A.; Quirino, E.C.S.; Xavier Junior, G.F.; Takeshita, E.M.; Oliveira, L.B.; De Luca Canto, G.; Massignan, C. Parental Beliefs in and Attitudes toward Teething Signs and Symptoms: A Systematic Review. Int. J. Paediatr. Dent. 2023, 33, 577–584. [Google Scholar] [CrossRef]
- Owais, A.I.; Zawaideh, F.; Bataineh, O. Challenging Parents’ Myths Regarding Their Children’s Teething. Int. J. Dent. Hyg. 2010, 8, 28–34. [Google Scholar] [CrossRef]


| Variable | Group 1 (Breastfed, n = 30) | Group 2 (Formula-fed, n = 30) | Total (n = 60) | Statistical Test |
|---|---|---|---|---|
| Mothers’ Age (years) | 26.32 ± 3.76 | 28.40 ± 3.64 | 28.5 ± 6.2 | t = − 2.67, p = 0.281 |
| Infants’ Age (months) | 4.2 ± 2.47 | 4.51 ± 2.74 | 4.2 ± 5.31 | t = − 0.145, p = 0.886 |
| Salivary IL-17 (pg/mL) | 13.26 ±5.52 | 27.14 ± 6.37 | - | t = − 9.02, p < 0.001. |
| Staphylococcal Infection | χ2 = 4.226, p = 0.035# #OR = 0.333 (95% CI: 0.116–0.956) | |||
| -Positive Cases (%) | 10 (33.3%) | 18 (60%) | 28 (46.6%) | |
| -Negative Cases (%) | 20 (66.6%) | 12 (40%) | 32 (53.3%) | |
| Oral Bacterial Load (CFU/mL) | 334.35± 25.4 | 2057.000 ± 828.13 | — | t = −2.063, p = 0.043 |
| Oral Hygiene Practices (n, % cleaning mouth regularly). | 6 (20%) | 14 (46.6%) | 20 (33.33%) | χ2 = 4.8, p = 0.028# #OR = 0.288 (95% CI: 0.01–0.89) |
| Environmental Exposure (n, % exposed to pollutants). | 5 (16.6%) | 6 (20%) | 11(18.3%) | χ2 = 0.8, p = 0. 371# #OR = 0.583 (95% CI: 0.178–1.913) |
| Teething Discomfort (n, % reporting pain). | 12 (40%) | 22 (73%) | 34(56.6%) | χ2 = 6.787, p = 0.009# #OR = 0.242 (95% CI: 0.081–0.721) |
| Main Nuclear Area | Main Cytoplasmic Area | Main Nuclear and Cytoplasmic Ratio# #(N/C) | Percentage of Normal Flora | |
|---|---|---|---|---|
| Breastfed sample | Normal size | Normal amount | Normal change | 100% covering epithelial cells |
| Formula-Fed sample | Normal size | Normal amount | Normal change | 10–20% covering epithelial cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackawy, A.M.H.; Alturky, F.S.; Mohammed, A.H.; Alharbi, B.F.; Huq, M.; Wasti, A.Z.; Ahmed, M.A.A.; Alharbi, H.O.A. A Comparative Analysis of Feeding Practices and Oral Immunity in Infants. Medicina 2025, 61, 1114. https://doi.org/10.3390/medicina61061114
Mackawy AMH, Alturky FS, Mohammed AH, Alharbi BF, Huq M, Wasti AZ, Ahmed MAA, Alharbi HOA. A Comparative Analysis of Feeding Practices and Oral Immunity in Infants. Medicina. 2025; 61(6):1114. https://doi.org/10.3390/medicina61061114
Chicago/Turabian StyleMackawy, Amal Mohamad Husein, Fay Saleh Alturky, Amal Hussain Mohammed, Basmah F. Alharbi, Mohsina Huq, Afshan Zeeshan Wasti, Mawahib Alhag Ali Ahmed, and Hajed Obaid Abdullah Alharbi. 2025. "A Comparative Analysis of Feeding Practices and Oral Immunity in Infants" Medicina 61, no. 6: 1114. https://doi.org/10.3390/medicina61061114
APA StyleMackawy, A. M. H., Alturky, F. S., Mohammed, A. H., Alharbi, B. F., Huq, M., Wasti, A. Z., Ahmed, M. A. A., & Alharbi, H. O. A. (2025). A Comparative Analysis of Feeding Practices and Oral Immunity in Infants. Medicina, 61(6), 1114. https://doi.org/10.3390/medicina61061114







