Cryptococcosis in Pediatric Renal Transplant Recipients: Comparative Insights from Adult Cases
Abstract
1. Introduction
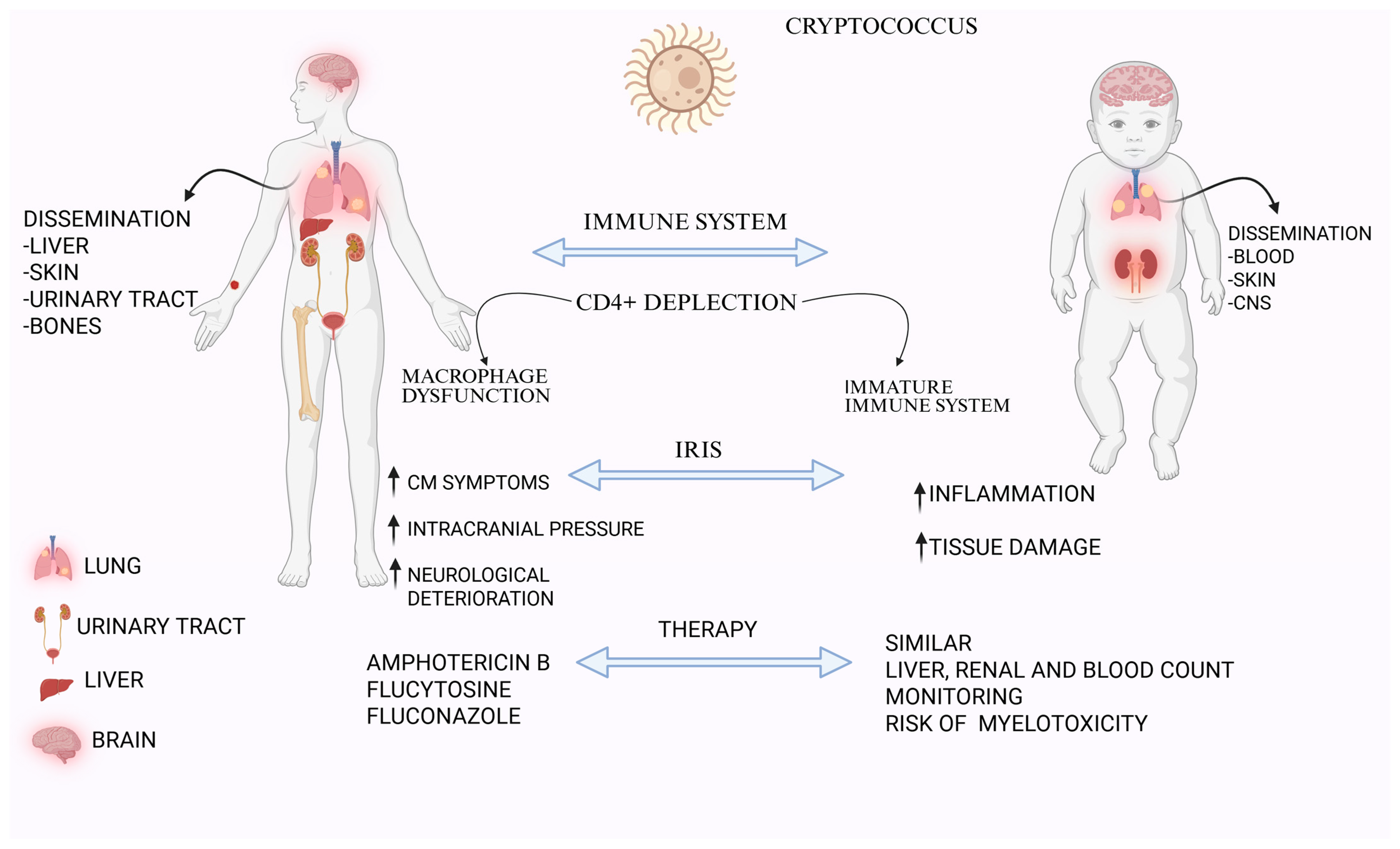
2. Clinical Manifestations: Adult vs. Pediatric Patients
3. Immune Response and Pathogenesis
4. Laboratory Diagnosis
5. Imaging and Physical Manifestations
5.1. Pulmonary Localization
5.2. Central Nervous System Localization
5.3. Renal Localization
5.4. Lymphatic Localization
5.5. Hepatobiliary Localization
5.6. Cutaneous Localization
6. Management
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meena, P.; Bhargava, V.; Singh, K.; Sethi, J.; Prabhakar, A.; Panda, S. Cryptococcosis in kidney transplant recipients: Current understanding and practices. World J. Nephrol. 2023, 12, 120–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Shoham, S.; Levitz, S.M. The immune response to fungal infections. Br. J. Haematol. 2005, 129, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Kozel, T.R.; Wilson, M.A.; Murphy, J.W. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect. Immun. 1991, 59, 3101–3110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, N.; Alexander, B.D.; Lortholary, O.; Dromer, F.; Gupta, K.L.; John, G.T.; del Busto, R.; Klintmalm, G.B.; Somani, J.; Lyon, G.M.; et al. Cryptococcal Collaborative Transplant Study Group. Cryptococcus neoformans in organ transplant recipients: Impact of calcineurin-inhibitor agents on mortality. J. Infect. Dis. 2007, 195, 756–764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, C.C.; Harrison, T.S.; Bicanic, T.A.; Chayakulkeeree, M.; Sorrell, T.C.; Warris, A.; Hagen, F.; Spec, A.; Oladele, R.; Govender, N.P.; et al. Global guideline for the diagnosis and management of cryptococcosis: An initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect. Dis. 2024, 24, e495–e512. [Google Scholar] [CrossRef]
- Saha, D.C.; Goldman, D.L.; Shao, X.; Casadevall, A.; Husain, S.; Limaye, A.P.; Lyon, M.; Somani, J.; Pursell, K.; Pruett, T.L.; et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin. Vaccine Immunol. 2007, 14, 1550–1554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez, C.E.; Shetty, D.; Lewis, L.L.; Mueller, B.U.; Pizzo, P.A.; Walsh, T.J. Cryptococcosis in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 1996, 15, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Ramdial, P.K.; Sing, Y.; Deonarain, J.; Bhimma, R.; Chotey, N.; Sewram, V. Pediatric renal cryptococcosis: Novel manifestations in the acquired immunodeficiency syndrome era. Int. J. Surg. Pathol. 2011, 19, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Balaji, A.B.; An, Q.; Smith, J.C.; Newcomb, M.E.; Mustanski, B.; Prachand, N.G.; Brady, K.A.; Braunstein, S.; Paz-Bailey, G.; National HIV Behavioral Surveillance for Young Men Who Have Sex with Men (NHBS-YMSM) Study Group. High Human Immunodeficiency Virus Incidence and Prevalence and Associated Factors Among Adolescent Sexual Minority Males—3 Cities, 2015. Clin. Infect. Dis. 2017, 66, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Gaga, M.; Loverdos, K.; Fotiadis, A.; Kontogianni, C.; Iliopoulou, M. Lung nodules: A comprehensive review on current approach and management. Ann. Thorac. Med. 2019, 14, 226–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gushiken, A.C.; Saharia, K.K.; Baddley, J.W. Cryptococcosis. Infect. Dis. Clin. N. Am. 2021, 35, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.N.; Tilak, R.; Yadav, J.; Bansal, M. Cutaneous Cryptococcus: Marker for disseminated infection. BMJ Case Rep. 2015, 2015, bcr2015210898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, Y.; Liu, X.; de Hoog, G.S.; Li, R. Disseminated Cryptococcosis Presenting as Cellulitis Diagnosed by Laser Capture Microdissection: A Case Report and Literature Review. Mycopathologia 2021, 186, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Nakatudde, I.; Kasirye, P.; Kiguli, S.; Musoke, P. It is not always Tuberculosis! A case of pulmonary cryptococcosis in an immunocompetent child in Uganda. Afr. Health Sci. 2021, 21, 990–994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suárez-Rivera, M.; Abadeer, R.A.; Kott, M.M.; Braun, M.C. Cryptococcosis associated with crescentic glomerulonephritis. Pediatr. Nephrol. 2008, 23, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.; Cervi, M.C.; Martinez, R. Vertical transmission of Cryptococcus neoformans from a mother coinfected with human immunodeficiency virus: Case report. Rev. Soc. Bras. Med. Trop. 2006, 39, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Taner, S.; Bulut, I.K.; Ekici, B.; Basaran, C.; Polat, F.; Kabasakal, C. Cryptococcal Pneumonia in a Pediatric Patient with Renal Transplantation. Transplant. Case Rep. 2020, 1, 1–3. [Google Scholar] [CrossRef]
- Bundy, L.M.; Rajnik, M.; Noor, A. Neonatal Meningitis. [Updated 6 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532264/ (accessed on 25 March 2025).
- Poloni, J.A.; Rotta, L.N.; Voegeli, C.F.; Pasqualotto, A.C. Cryptococcus within a urinary cast. Kidney Int. 2013, 84, 218. [Google Scholar] [CrossRef] [PubMed]
- Alsaad, K.; Oudah, N.; Al Ameer, A.; Fakeeh, K.; Al Jomaih, A.; Al Sayyari, A. Glomerulonephritis with crescents in children: Etiology and predictors of renal outcome. ISRN Pediatr. 2011, 2011, 507298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Hellman, R.N.; Hinrichs, J.; Sicard, G.; Hoover, R.; Golden, P.; Hoffsten, P. Cryptococcal pyelonephritis and disseminated cryptococcosis in a renal transplant recipient. Arch. Intern. Med. 1981, 141, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.E., Jr.; Stacy, W.K.; Toone, E.C.; Prout, G.R.; Madge, G.E.; Shadomy, H.J.; Shadomy, S.; Utz, J.P. Cryptococcal pyelonephritis. N. Engl. J. Med. 1968, 279, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Hill-Edgar, A.A.; Nasr, S.H.; Borczuk, A.C.; D’Agati, V.D.; Radhakrishnan, J.; Markowitz, G.S. A rare infectious cause of renal allograft dysfunction. Am. J. Kidney Dis. 2002, 40, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, S.; Pirofski, L.A. Host immunity to Cryptococcus neoformans. Future Microbiol. 2015, 10, 565–581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunkelberger, J.R.; Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pawar, S.; Dutta, O.; Wang, K.; Rivera, A.; Xue, C. Macrophage Mediated Immunomodulation During Cryptococcus Pulmonary Infection. Front. Cell. Infect. Microbiol. 2022, 12, 859049. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campuzano, A.; Wormley, F.L. Innate Immunity against Cryptococcus, from Recognition to Elimination. J. Fungi 2018, 4, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neal, L.M.; Xing, E.; Xu, J.; Kolbe, J.L.; Osterholzer, J.J.; Segal, B.M.; Williamson, P.R.; Olszewski, M.A.; Dromer, F. CD4+ T Cells Orchestrate Lethal Immune Pathology despite Fungal Clearance during Cryptococcus neoformans Meningoencephalitis. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uicker, W.C.; McCracken, J.P.; Buchanan, K.L. Role of CD4+ T cells in a protective immune response against Cryptococcus neoformans in the central nervous system. Med. Mycol. 2006, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Severo, C.B.; Xavier, M.O.; Gazzoni, A.F.; Severo, L.C. Cryptococcosis in children. Paediatr. Respir. Rev. 2009, 10, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Manfroi, B.; Cuc, B.T.; Sokal, A.; Vandenberghe, A.; Temmam, S.; Attia, M.; El Behi, M.; Camaglia, F.; Nguyen, N.T.; Pohar, J.; et al. Preschool-age children maintain a distinct memory CD4+ T cell and memory B cell response after SARS-CoV-2 infection. Sci. Transl. Med. 2024, 16, eadl1997. [Google Scholar] [CrossRef]
- Al-Huthaifi, A.M.; Radman, B.A.; Al-Alawi, A.A.; Mahmood, F.; Liu, T.-B. Mechanisms and Virulence Factors of Cryptococcus neoformans Dissemination to the Central Nervous System. J. Fungi 2024, 10, 586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wager, C.M.L.; Hole, C.R.; Wozniak, K.L.; Wormley, F.L. Cryptococcus and Phagocytes: Complex Interactions that Influence Disease Outcome. Front. Microbiol. 2016, 7, 105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Temfack, E.; Rim, J.J.B.; Spijker, R.; Loyse, A.; Chiller, T.; Pappas, P.G.; Perfect, J.; Sorell, T.C.; Harrison, T.S.; Cohen, J.F.; et al. Cryptococcal Antigen in Serum and Cerebrospinal Fluid for Detecting Cryptococcal Meningitis in Adults Living with Human Immunodeficiency Virus: Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies. Clin. Infect. Dis. 2020, 72, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.H.; Adil, M.S.; Lin, X.; Chastain, D.B.; Henao-Martínez, A.F.; Franco-Paredes, C.; Somanath, P.R. Elevated Intracranial Pressure in Cryptococcal Meningoencephalitis: Examining Old, New, and Promising Drug Therapies. Pathogens 2022, 11, 783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veatch, A.; Dikman, S.H. Images in clinical medicine. Human immunodeficiency virus nephropathy and intraglomerular Cryptococcus neoformans. N. Engl. J. Med. 1998, 339, 887. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Lu, J.; Chen, T.; Xu, R. Comparison of the clinical manifestations and chest CT findings of pulmonary cryptococcosis in immunocompetent and immunocompromised patients: A systematic review and meta-analysis. BMC Pulm. Med. 2022, 22, 415. [Google Scholar] [CrossRef]
- Varotto, A.; Orsatti, G.; Crimì, F.; Cecchin, D.; Toffolutti, T.; Zucchetta, P.; Stramare, R. Radiological Assessment of Paediatric Fungal Infections: A Pictorial Review with Focus on PET/MRI. In Vivo 2019, 33, 1727–1735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fox, D.L.; Müller, N.L. Pulmonary cryptococcosis in immunocompetent patients: CT findings in 12 patients. AJR Am. J. Roentgenol. 2005, 185, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Lindell, R.M.; Hartman, T.E.; Nadrous, H.F.; Ryu, J.H. Pulmonary cryptococcosis: CT findings in immunocompetent patients. Radiology 2005, 236, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Wainwright, H.; Harrison, T.S.; Rebe, K.; Meintjes, G. Pulmonary cryptococcosis misdiagnosed as smear-negative pulmonary tuberculosis with fatal consequences. Int. J. Infect. Dis. 2010, 14, e310–e312. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Kim, J.E.; La, K.S.; Yoo, Y.; Lee, K.H.; Park, S.H.; Choung, J.T.; Kim, C.W. Isolated pulmonary cryptococcosis in an immunocompetent boy. Korean J. Pediatr. 2010, 53, 971–974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, L.-W.; Jiao, A.-X.; Wu, X.-R.; Zhao, S.-Y.; Ma, Y.; Liu, G.; Yin, J.; Xu, B.-P.; Shen, K.-L. Clinical characteristics of disseminated cryptococcosis in previously healthy children in China. BMC Infect. Dis. 2017, 17, 359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qu, J.; Zhang, X.; Lu, Y.; Liu, X.; Lv, X. Clinical analysis in immunocompetent and immunocompromised patients with pulmonary cryptococcosis in western China. Sci. Rep. 2020, 10, 9387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.-N.; Chen, J.-H.; Lu, Z.-W. Pulmonary cryptococcosis in immunocompetent children presenting with chest pain: Three case reports. World J. Clin. Cases 2025, 13, 100672. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, Y.; Ying, Q.; Ye, Q. Cryptococcosis with pulmonary cavitation in an immunocompetent child: A case report and literature review. BMC Infect. Dis. 2024, 24, 162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rojo-Martin, M.D.; de Toro Peinado, I.; Ruiz Mesa, J.D.; Borrás, B.P. Cryptococcal infection in renal transplant: Two case reports and a literature review. Rev. Esp. Quimioter. 2021, 34, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.L.; Bagai, S.; Ramachandran, R.; Kumar, V.; Rathi, M.; Kohli, H.S.; Sharma, A.; Chakrabarti, A. Fungal infection in post-renal transplant patient: Single-center experience. Indian J. Pathol. Microbiol. 2020, 63, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Gassiep, I.; McDougall, D.; Douglas, J.; Francis, R.; Playford, E.G. Cryptococcal infections in solid organ transplant recipients over a 15-year period at a state transplant center. Transpl. Infect. Dis. 2017, 19, e12639. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.; Carmo, R.; Ferreira, I.; Bustorff, M.; Sampaio, S.; Pestana, M. Cryptococcosis in Renal Transplant Recipients: A Single-Center Experience. Transplant. Proc. 2016, 48, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Chen, Y.C.; Chang, S.C.; Luh, K.T.; Hsieh, W.C. Cryptococcal meningitis in non-HIV-infected patients. QJM 2000, 93, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Li, X.; Li, H. Imaging characterization of cryptococcal meningoencephalitis. Radiol. Infect. Dis. 2016, 3, 187–191. [Google Scholar] [CrossRef]
- Yang, C.-W.R.; Mason, M.; Parizel, P.M.; Warne, R. Magnetic resonance imaging patterns of paediatric brain infections: A pictorial review based on the Western Australian experience. Insights Imaging 2022, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, R.A.; Mays, M.; Isada, C.; Ahmed, M. MRI findings in cryptococcal meningitis of the non-HIV population. Neurologist 2015, 19, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Liliang, P.C.; Liang, C.L.; Chang, W.N.; Lu, K.; Lu, C. Use of ventriculoperitoneal shunts to treat uncontrollable intracranial hypertension in patients who have cryptococcal meningitis without hydrocephalus. Clin. Infect. Dis. 2002, 34, e64–e68. [Google Scholar] [CrossRef]
- Tan, Z.-R.; Long, X.-Y.; Li, G.-L.; Zhou, J.-X.; Long, L. Spectrum of neuroimaging findings in cryptococcal meningitis in immunocompetent patients in China—A series of 18 cases. J. Neurol. Sci. 2016, 368, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Dekker, G.; Andronikou, S.; van Toorn, R.; Scheepers, S.; Brandt, A.; Ackermann, C. MRI findings in children with tuberculous meningitis: A comparison of HIV-infected and non-infected patients. Child’s Nerv. Syst. 2011, 27, 1943–1949. [Google Scholar] [CrossRef]
- Daher, E.D.; Nasserala, J.C.; Silva Junior, G.B.; Oliveira, A.R.; Medeiros Neto, J.U.; Sousa, A.Q. Fatal Disseminated Cryptococcosis with Renal Involvement in an Hiv-Infected Patient. Rev. Inst. Med. Trop. São Paulo 2015, 57, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sri-Ganeshan, M.; Smit, D.V.; Mitra, B. Ultrasound for acute pyelonephritis: A systematic review and meta-analysis. Intern. Med. J. 2024, 54, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.L.; Hu, H.J.; Dai, N.; Cai, X.J. Extrahepatic biliary cryptococcosis: A case report. J. Dig. Dis. 2011, 12, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Zeng, X.-B.; Shi, S.-L.; Wang, M.; Li, D.M. Cryptococcosis manifesting as isolated biliary infection: Case report and brief review of literature. Clin. Res. Hepatol. Gastroenterol. 2018, 42, e56–e59. [Google Scholar] [CrossRef] [PubMed]
- Das, C.J.; Pangtey, G.S.; Hari, S.; Hari, P.; Das, A.K. Biliary cryptococcosis in a child: MR imaging findings. Pediatr. Radiol. 2006, 36, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.A.; Putynkowska, A.; Barańska-Rybak, W.; Czarnacka, K.; Dębska-Ślizień, M. Cutaneous cryptococcosis: An underlying immunosuppression? Clinical manifestations, pathogenesis, diagnostic examinations and treatment. Adv. Dermatol. Allergol. 2020, 37, 154–158. [Google Scholar] [CrossRef]
- Salyer, W.R.; Salyer, D.C. Involvement of the kidney and prostate in cryptococcosis. J. Urol. 1973, 109, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Spivack, A.P.; Nadel, J.A.; Eisenberg, G.M. Cryptococcus renal infection: Report of a case. Ann. Intern. Med. 1957, 47, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, J.B. Two cases of cryptococcosis. J. Clin. Pathol. 1966, 19, 424–428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raghavan, R.; Date, A.; Bhaktaviziam, A. Fungal and nocardial infections of the kidney. Histopathology 1987, 11, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, V.R.; Mtitimila, E.; Hart, C.A.; Broadhead, R.L. Cryptococcal meningitis in African children. Ann. Trop. Paediatr. 1997, 17, 165–167. [Google Scholar] [CrossRef] [PubMed]
| Feature | Adult KT Recipients | Pediatric KT Recipients |
|---|---|---|
| Primary Clinical Presentation | Meningitis, fever, headache, altered mental status | Pulmonary involvement, disseminated disease |
| Prevalence of Pulmonary Involvement | Often asymptomatic or mild | More common, nodules and interstitial infiltrates on imaging |
| Renal Involvement | Rare, includes pyelonephritis and cortical involvement | Rare, includes crescentic glomerulonephritis |
| Risk Factors | Chronic immunosuppression, history of rejection therapy | Severe immunosuppression, environmental exposure |
| Main Features | Atypical Features |
|---|---|
| Multiple nodules | Cavitation |
| Segmental/lobar consolidation | Pleural effusion |
| Reticular interstitial thickening | Lymphoadenopathy |
| Miliary disease | |
| Ground glass/Halo Sign |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gembillo, G.; Terzo, C.; Silipigni, S.; Soraci, L.; Rullo, E.V.; Russotto, Y.; Casuscelli, C.; Gambuzza, M.E.; Princiotto, M.; Cicero, L.L.; et al. Cryptococcosis in Pediatric Renal Transplant Recipients: Comparative Insights from Adult Cases. Medicina 2025, 61, 1108. https://doi.org/10.3390/medicina61061108
Gembillo G, Terzo C, Silipigni S, Soraci L, Rullo EV, Russotto Y, Casuscelli C, Gambuzza ME, Princiotto M, Cicero LL, et al. Cryptococcosis in Pediatric Renal Transplant Recipients: Comparative Insights from Adult Cases. Medicina. 2025; 61(6):1108. https://doi.org/10.3390/medicina61061108
Chicago/Turabian StyleGembillo, Guido, Chiara Terzo, Salvatore Silipigni, Luca Soraci, Emmanuele Venanzi Rullo, Ylenia Russotto, Chiara Casuscelli, Maria Elsa Gambuzza, Maria Princiotto, Lorenzo Lo Cicero, and et al. 2025. "Cryptococcosis in Pediatric Renal Transplant Recipients: Comparative Insights from Adult Cases" Medicina 61, no. 6: 1108. https://doi.org/10.3390/medicina61061108
APA StyleGembillo, G., Terzo, C., Silipigni, S., Soraci, L., Rullo, E. V., Russotto, Y., Casuscelli, C., Gambuzza, M. E., Princiotto, M., Cicero, L. L., Peritore, L., Sessa, C., & Santoro, D. (2025). Cryptococcosis in Pediatric Renal Transplant Recipients: Comparative Insights from Adult Cases. Medicina, 61(6), 1108. https://doi.org/10.3390/medicina61061108









