Effect of Freeze-Dried Porcine Platelet Lysate on Wound Healing in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objectives and Research Questions
- Can freeze-dried porcine PL improve the rate and quality of wound healing?
- Is the treatment associated with enhanced collagen formation?
- Is porcine PL a biocompatible and well-tolerated material for skin application in rats?
2.2. Platelet Lysate Preparation
2.3. Animal Study
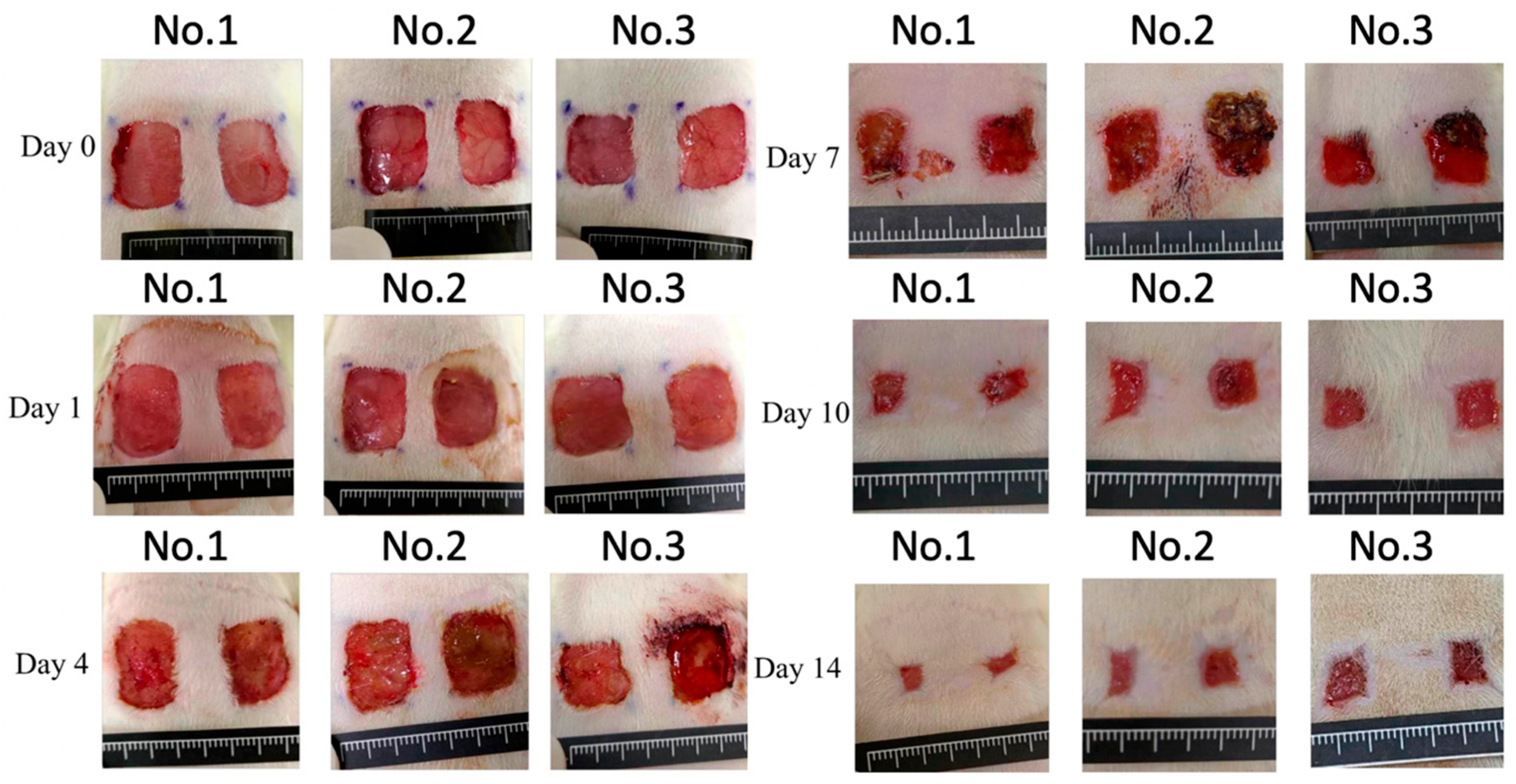
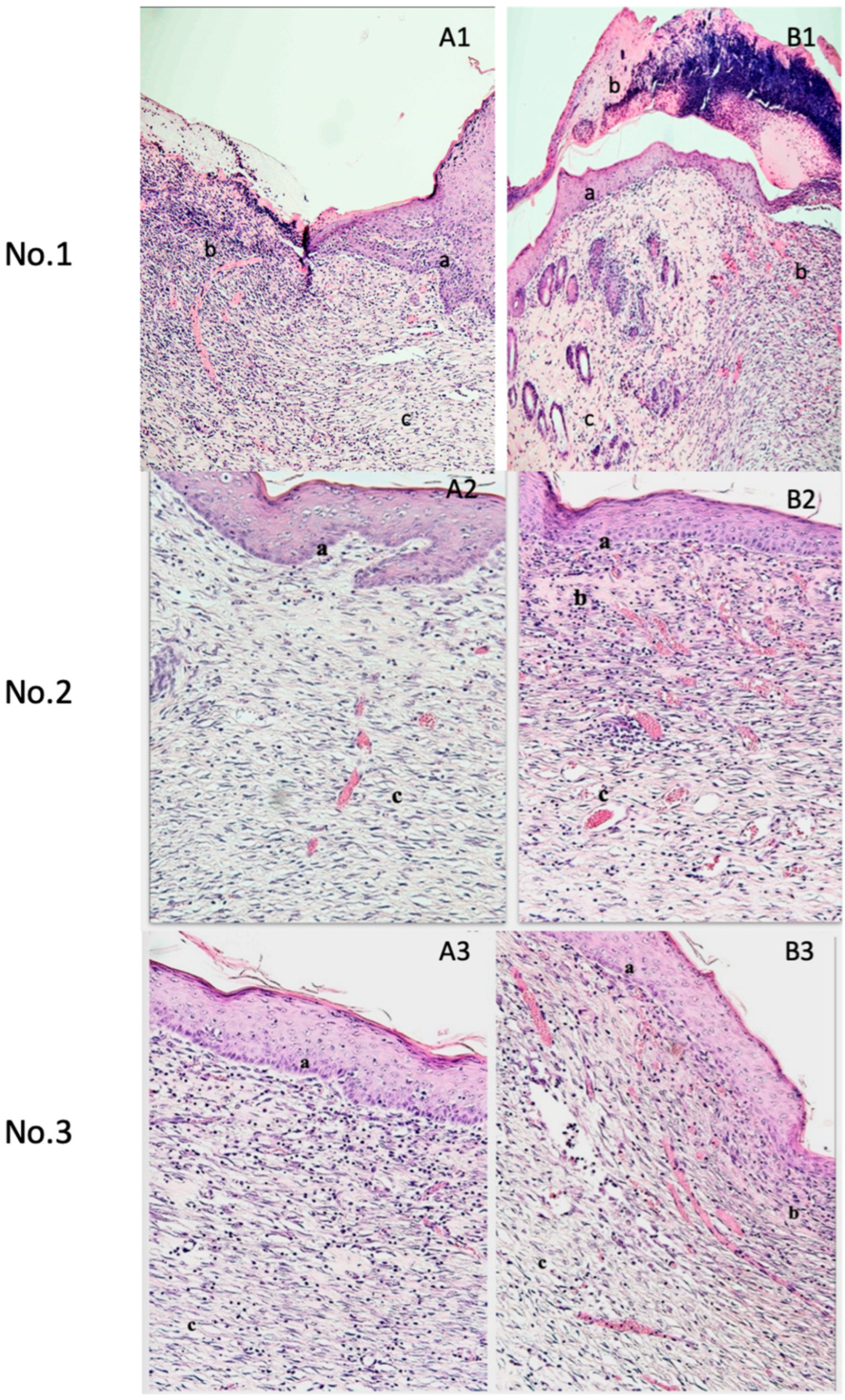
3. Results
4. Discussion
4.1. Perspectives for Clinical Practice
4.2. Therapeutic Mechanisms and Histological Effects of Porcine Platelet Lysate
4.3. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Theoret, C. Tissue Engineering in Wound Repair: The Three “R”s—Repair, Replace, Regenerate. Vet. Surg. 2009, 38, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Platelet Gel for Healing Cutaneous Chronic Wounds—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15062754/ (accessed on 2 May 2024).
- Teng, M.; Huang, Y.; Zhang, H. Application of Stems Cells in Wound Healing—An Update. Wound Repair Regen. 2014, 22, 151–160. [Google Scholar] [CrossRef]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Cutaneous Wound Healing—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/10471461/ (accessed on 2 May 2024).
- Ko, S.H.; Nauta, A.; Wong, V.; Glotzbach, J.; Gurtner, G.C.; Longaker, M.T. The Role of Stem Cells in Cutaneous Wound Healing: What Do We Really Know? Plast. Reconstr. Surg. 2011, 127 (Suppl. S1), 10S–20S. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, L.; Yang, F.; Tong, W.; Jia, R.; Zou, Y.; Yin, L.; Li, L.; He, C.; Liang, X.; et al. Tannic Acid Accelerates Cutaneous Wound Healing in Rats Via Activation of the ERK 1/2 Signaling Pathways. Adv. Wound Care 2019, 8, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Caramella, C.; Del Fante, C.; Perotti, C.; Miele, D.; Vigani, B.; Ferrari, F. Bioactive Medications for the Delivery of Platelet Derivatives to Skin Wounds. Curr. Drug Deliv. 2019, 16, 472–483. [Google Scholar] [CrossRef]
- O’Connell, S.M.; Impeduglia, T.; Hessler, K.; Wang, X.-J.; Carroll, R.J.; Dardik, H. Autologous Platelet-Rich Fibrin Matrix as Cell Therapy in the Healing of Chronic Lower-Extremity Ulcers. Wound Repair Regen. 2008, 16, 749–756. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Chu, Y.-C.; Hsiao, J.-T.; Shu, Y.-T.; Hsieh, M.-F.; Lee, H.-M. Porcine Platelet Lysate Intra-Articular Knee Joint Injections for the Treatment of Rabbit Cartilage Lesions and Osteoarthritis. J. Med. Biol. Eng. 2023, 43, 102–111. [Google Scholar] [CrossRef]
- Ng, S.-L.; Azhar, N.A.; Budin, S.B.; Ibrahim, N.; Abdul Ghani, N.A.; Abd Ghafar, N.; Law, J.-X. Effects of Platelet Lysate Gels Derived from Different Blood Sources on Oral Mucosal Wound Healing: An In Vitro Study. Gels 2023, 9, 343. [Google Scholar] [CrossRef]
- Niemann, M.; Ort, M.; Lauterbach, L.; Streitz, M.; Wilhelm, A.; Grütz, G.; Fleckenstein, F.N.; Graef, F.; Blankenstein, A.; Reinke, S.; et al. Individual Immune Cell and Cytokine Profiles Determine Platelet-Rich Plasma Composition. Arthritis Res. Ther. 2023, 25, 6. [Google Scholar] [CrossRef]
- Alsousou, J.; Ali, A.; Willett, K.; Harrison, P. The Role of Platelet-Rich Plasma in Tissue Regeneration. Platelets 2013, 24, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Alsousou, J.; Andia, I.; Burnouf, T.; Dohan Ehrenfest, D.; Everts, P.; Langer, H.; Magalon, J.; Marck, R.; Gresele, P. The Use of Platelets in Regenerative Medicine and Proposal for a New Classification System: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Aldén, A.; Gonzalez, L.; Persson, A.; Christensson, K.; Holmqvist, O.; Ohlson, S. Porcine Platelet Lysate as a Supplement for Animal Cell Culture. Cytotechnology 2007, 55, 3–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Del Fante, C.; Perotti, C.; Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Scudeller, L.; Caramella, C.M. Platelet Lysate Mucohadesive Formulation to Treat Oral Mucositis in Graft versus Host Disease Patients: A New Therapeutic Approach. AAPS PharmSciTech 2011, 12, 893–899. [Google Scholar] [CrossRef]
- Shanskii, Y.D.; Sergeeva, N.S.; Sviridova, I.K.; Kirakozov, M.S.; Kirsanova, V.A.; Akhmedova, S.A.; Antokhin, A.I.; Chissov, V.I. Human Platelet Lysate as a Promising Growth-Stimulating Additive for Culturing of Stem Cells and Other Cell Types. Bull. Exp. Biol. Med. 2013, 156, 146–151. [Google Scholar] [CrossRef]
- Bernardini, C.; Romagnoli, N.; Casalini, I.; Turba, M.E.; Spadari, A.; Forni, M.; Gentilini, F. Freeze-Drying Protocols and Methods of Maintaining the in-Vitro Biological Activity of Horse Platelet Lysate. Int. J. Vet. Sci. Med. 2024, 12, 71–80. [Google Scholar] [CrossRef]
- Sovkova, V.; Vocetkova, K.; Rampichova, M.; Mickova, A.; Buzgo, M.; Lukasova, V.; Dankova, J.; Filova, E.; Necas, A.; Amler, E. Platelet Lysate as a Serum Replacement for Skin Cell Culture on Biomimetic PCL Nanofibers. Platelets 2018, 29, 395–405. [Google Scholar] [CrossRef]
- Barsotti, M.C.; Losi, P.; Briganti, E.; Sanguinetti, E.; Magera, A.; Al Kayal, T.; Feriani, R.; Di Stefano, R.; Soldani, G. Effect of Platelet Lysate on Human Cells Involved in Different Phases of Wound Healing. PLoS ONE 2013, 8, e84753. [Google Scholar] [CrossRef]
- Jafar, H.; Hasan, M.; Al-Hattab, D.; Saleh, M.; Ameereh, L.A.; Khraisha, S.; Younes, N.; Awidi, A. Platelet Lysate Promotes the Healing of Long-Standing Diabetic Foot Ulcers: A Report of Two Cases and in Vitro Study. Heliyon 2020, 6, e03929. [Google Scholar] [CrossRef]
- Jonsdottir-Buch, S.M.; Lieder, R.; Sigurjonsson, O.E. Platelet Lysates Produced from Expired Platelet Concentrates Support Growth and Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2013, 8, e68984. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. BMC Vet. Res. 2020, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar]
- Galiano, R.D.; Michaels, V.J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and Reproducible Murine Model of Excisional Wound Healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-6:2016(En); Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. International Organization for Standardization: Geneva, Switzerland, 2016.
- Cangelosi, G.; Mancin, S.; Bei, D.; Clementi, E.; Pantanetti, P.; Caggianelli, G.; Petrelli, F. Multidisciplinary Management and Autologous Skin Grafting in a Patient with Severe Burns: A Case Study. Medicina 2024, 60, 1201. [Google Scholar] [CrossRef]
- Foubert, P.; Liu, M.; Anderson, S.; Rajoria, R.; Gutierrez, D.; Zafra, D.; Tenenhaus, M.; Fraser, J.K. Preclinical Assessment of Safety and Efficacy of Intravenous Delivery of Autologous Adipose-Derived Regenerative Cells (ADRCs) in the Treatment of Severe Thermal Burns Using a Porcine Model. Burns 2018, 44, 1531–1542. [Google Scholar] [CrossRef]
- Sánchez, M.; Anitua, E.; Andia, I. Poor Standardization in Platelet-Rich Therapies Hampers Advancement. Arthroscopy 2010, 26, 725–726, author reply 726. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; da Cruz Pacheco, Í.; Corrêa do Amaral, R.J.F.; Granjeiro, J.M.; Borojevic, R. Platelet-Rich Plasma Preparation for Regenerative Medicine: Optimization and Quantification of Cytokines and Growth Factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.C.; Schallmoser, K. Human Platelet Lysate: Replacing Fetal Bovine Serum as a Gold Standard for Human Cell Propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Chicharro-Alcántara, D.; Rubio-Zaragoza, M.; Damiá-Giménez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Peláez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. J. Funct. Biomater. 2018, 9, 10. [Google Scholar] [CrossRef]
- Merchán, W.H.; Gómez, L.A.; Chasoy, M.E.; Alfonso-Rodríguez, C.A.; Muñoz, A.L. Platelet-Rich Plasma, a Powerful Tool in Dermatology. J. Tissue Eng. Regen. Med. 2019, 13, 892–901. [Google Scholar] [CrossRef]
- Li, T.; Ma, Y.; Wang, M.; Wang, T.; Wei, J.; Ren, R.; He, M.; Wang, G.; Boey, J.; Armstrong, D.G.; et al. Platelet-Rich Plasma Plays an Antibacterial, Anti-Inflammatory and Cell Proliferation-Promoting Role in an in Vitro Model for Diabetic Infected Wounds. Infect. Drug Resist. 2019, 12, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, F.; Cattani, C.; Dimartino, V.; Mirisola, C.; Cavani, A. Platelet Derivatives and the Immunomodulation of Wound Healing. Int. J. Mol. Sci. 2022, 23, 8370. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.L.; et al. Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics 2020, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Zielins, E.R.; Atashroo, D.A.; Maan, Z.N.; Duscher, D.; Walmsley, G.G.; Hu, M.; Senarath-Yapa, K.; McArdle, A.; Tevlin, R.; Wearda, T.; et al. Wound Healing: An Update. Regen. Med. 2014, 9, 817–830. [Google Scholar] [CrossRef]
- Pallua, N.; Wolter, T.; Markowicz, M. Platelet-Rich Plasma in Burns. Burns J. Int. Soc. Burn Inj. 2010, 36, 4–8. [Google Scholar] [CrossRef]
- Villela, D.L.; Santos, V.L.C.G. Evidence on the Use of Platelet-Rich Plasma for Diabetic Ulcer: A Systematic Review. Growth Factors 2010, 28, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, L.; Medici, D.; Serra, M.; Panizza, R.; Rivara, G.; Orecchia, S.; Libener, R.; Cattana, E.; Levis, A.; Betta, P.G.; et al. The Use of Autologous Platelet Gel to Treat Difficult-to-Heal Wounds: A Pilot Study. Transfusion 2004, 44, 1013–1018. [Google Scholar] [CrossRef]
- Lee, H.-W.; Reddy, M.S.; Geurs, N.; Palcanis, K.G.; Lemons, J.E.; Rahemtulla, F.G.; Ho, K.-J.; Chen, D.-T.; Davis, C.R.; Feldman, D.S. Efficacy of Platelet-Rich Plasma on Wound Healing in Rabbits. J. Periodontol. 2008, 79, 691–696. [Google Scholar] [CrossRef]
- Young, A.; McNaught, C.-E. The Physiology of Wound Healing. Surg. Oxf. 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Shah, J.M.Y.; Omar, E.; Pai, D.R.; Sood, S. Cellular Events and Biomarkers of Wound Healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.E.; Nixon, J.C.; Colvin, R.B.; Antoniades, H.N. Role of Platelet-Derived Growth Factor in Wound Healing: Synergistic Effects with Other Growth Factors. Proc. Natl. Acad. Sci. USA 1987, 84, 7696–7700. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Stolzenburg-Veeser, L.; Golubnitschaja, O. Mini-Encyclopaedia of the Wound Healing—Opportunities for Integrating Multi-Omic Approaches into Medical Practice. J. Proteom. 2018, 188, 71–84. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ferreira, A.M.; Oberyszyn, T.M.; Bergdall, V.K.; Dipietro, L.A. Regulation of Scar Formation by Vascular Endothelial Growth Factor. Lab. Investig. J. Tech. Methods Pathol. 2008, 88, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, L.; Borzini, P.; Gope, R. Platelet-Derived Factors Involved in Tissue Repair-from Signal to Function. Transfus. Med. Rev. 2010, 24, 218–234. [Google Scholar] [CrossRef]
- Comparison of Wound Healing Effects of Different Micro-Patterned Hydrogels on the Skin of Secondary Intention Rat Model. Available online: https://www.mdpi.com/2310-2861/11/4/239 (accessed on 7 June 2025).
- Tsuchiya, M.; Kushibiki, T.; Yamashiro, T.; Mayumi, Y.; Ishihara, M.; Azuma, R. Continuous Negative-pressure Wound Therapy Improves the Survival Rate of Skin Grafts and Shortens the Time Required for Skin Graft Survival. Skin Res. Technol. 2024, 30, e13865. [Google Scholar] [CrossRef]
- Sindhi, K.; Pingili, R.B.; Beldar, V.; Bhattacharya, S.; Rahaman, J.; Mukherjee, D. The Role of Biomaterials-Based Scaffolds in Advancing Skin Tissue Construct. J. Tissue Viability 2025, 34, 100858. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirayama, F.; Wakamoto, S.; Fujihara, M.; Murahashi, H.; Sato, N.; Ikebuchi, K.; Sawada, K.; Koike, T.; Kuwabara, M.; et al. Bone Marrow Stromal Cells Prepared Using AB Serum and bFGF for Hematopoietic Stem Cells Expansion. Transfusion 2002, 42, 921–927. [Google Scholar] [CrossRef]
- Bieback, K.; Hecker, A.; Kocaömer, A.; Lannert, H.; Schallmoser, K.; Strunk, D.; Klüter, H. Human Alternatives to Fetal Bovine Serum for the Expansion of Mesenchymal Stromal Cells from Bone Marrow. Stem Cells Dayt. Ohio 2009, 27, 2331–2341. [Google Scholar] [CrossRef]
- Shahdadfar, A.; Frønsdal, K.; Haug, T.; Reinholt, F.P.; Brinchmann, J.E. In Vitro Expansion of Human Mesenchymal Stem Cells: Choice of Serum Is a Determinant of Cell Proliferation, Differentiation, Gene Expression, and Transcriptome Stability. Stem Cells Dayt. Ohio 2005, 23, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Stute, N.; Holtz, K.; Bubenheim, M.; Lange, C.; Blake, F.; Zander, A.R. Autologous Serum for Isolation and Expansion of Human Mesenchymal Stem Cells for Clinical Use. Exp. Hematol. 2004, 32, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Doucet, C.; Ernou, I.; Zhang, Y.; Llense, J.-R.; Begot, L.; Holy, X.; Lataillade, J.-J. Platelet Lysates Promote Mesenchymal Stem Cell Expansion: A Safety Substitute for Animal Serum in Cell-Based Therapy Applications. J. Cell. Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Muraglia, A.; Ottonello, C.; Spanò, R.; Dozin, B.; Strada, P.; Grandizio, M.; Cancedda, R.; Mastrogiacomo, M. Biological Activity of a Standardized Freeze-Dried Platelet Derivative to Be Used as Cell Culture Medium Supplement. Platelets 2014, 25, 211–220. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chuang, E.-Y.; Tu, Y.-K.; Hsu, C.-L.; Cheng, N.-C. Human Platelet Lysate-Cultured Adipose-Derived Stem Cell Sheets Promote Angiogenesis and Accelerate Wound Healing via CCL5 Modulation. Stem Cell Res. Ther. 2024, 15, 163. [Google Scholar] [CrossRef]
- Zuniga, K.; Isaac, A.; Christy, S.; Wrice, N.; Mangum, L.; Natesan, S.; Burnett, L.; Christy, R.; Kowalczewski, C. Characterization of a Human Platelet Lysate-Loaded Keratin Hydrogel for Wound Healing Applications In Vitro. Int. J. Mol. Sci. 2022, 23, 4100. [Google Scholar] [CrossRef]
| Animal No. | Gender | Body Weight (g) | ||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | ||
| 1 | Male | 275.3 | 306.3 | 313.2 |
| 2 | 272.1 | 295.9 | 313.9 | |
| 3 | 271.2 | 309.4 | 321.3 | |
| Group | Day 1 | Day 4 | Day 7 | Day 10 | Day 14 |
|---|---|---|---|---|---|
| Control | 100 ± 0% | 81.2 ± 11.8% | 36.6 ± 6.5% | 13.8 ± 3.7% | 10.7 ± 3.9% |
| Experiment | 100 ± 0% | 81.1 ± 6.9% | 41.2 ± 6.7% | 15.7 ± 3.8% | 11.5 ± 4.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, W.M.-T.; Hsieh, Y.-H.; Chu, Y.-C.; Hsiao, J.-T.; Shu, Y.-T.; Lee, H.-M.; Hsieh, M.-F. Effect of Freeze-Dried Porcine Platelet Lysate on Wound Healing in Rats. Medicina 2025, 61, 1098. https://doi.org/10.3390/medicina61061098
Low WM-T, Hsieh Y-H, Chu Y-C, Hsiao J-T, Shu Y-T, Lee H-M, Hsieh M-F. Effect of Freeze-Dried Porcine Platelet Lysate on Wound Healing in Rats. Medicina. 2025; 61(6):1098. https://doi.org/10.3390/medicina61061098
Chicago/Turabian StyleLow, Winson Min-Teng, Yi-Ho Hsieh, Yi-Chieh Chu, Jui-Ting Hsiao, Yi-Ting Shu, Hung-Maan Lee, and Ming-Fa Hsieh. 2025. "Effect of Freeze-Dried Porcine Platelet Lysate on Wound Healing in Rats" Medicina 61, no. 6: 1098. https://doi.org/10.3390/medicina61061098
APA StyleLow, W. M.-T., Hsieh, Y.-H., Chu, Y.-C., Hsiao, J.-T., Shu, Y.-T., Lee, H.-M., & Hsieh, M.-F. (2025). Effect of Freeze-Dried Porcine Platelet Lysate on Wound Healing in Rats. Medicina, 61(6), 1098. https://doi.org/10.3390/medicina61061098







