The Association Between Uterine Artery Pulsatility Index at Mid-Gestation and the Method of Conception: A Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Ethical Considerations and Reporting Standards
2.3. Study Population and Data Collection
2.4. Assisted Reproductive Technology Groups
2.5. Fetal Medicine Assessment
2.6. Statistical Analysis
3. Results
3.1. Cohort
3.2. Baseline Characteristics
3.3. Distribution of Uterine Artery Doppler Pulsatility Index
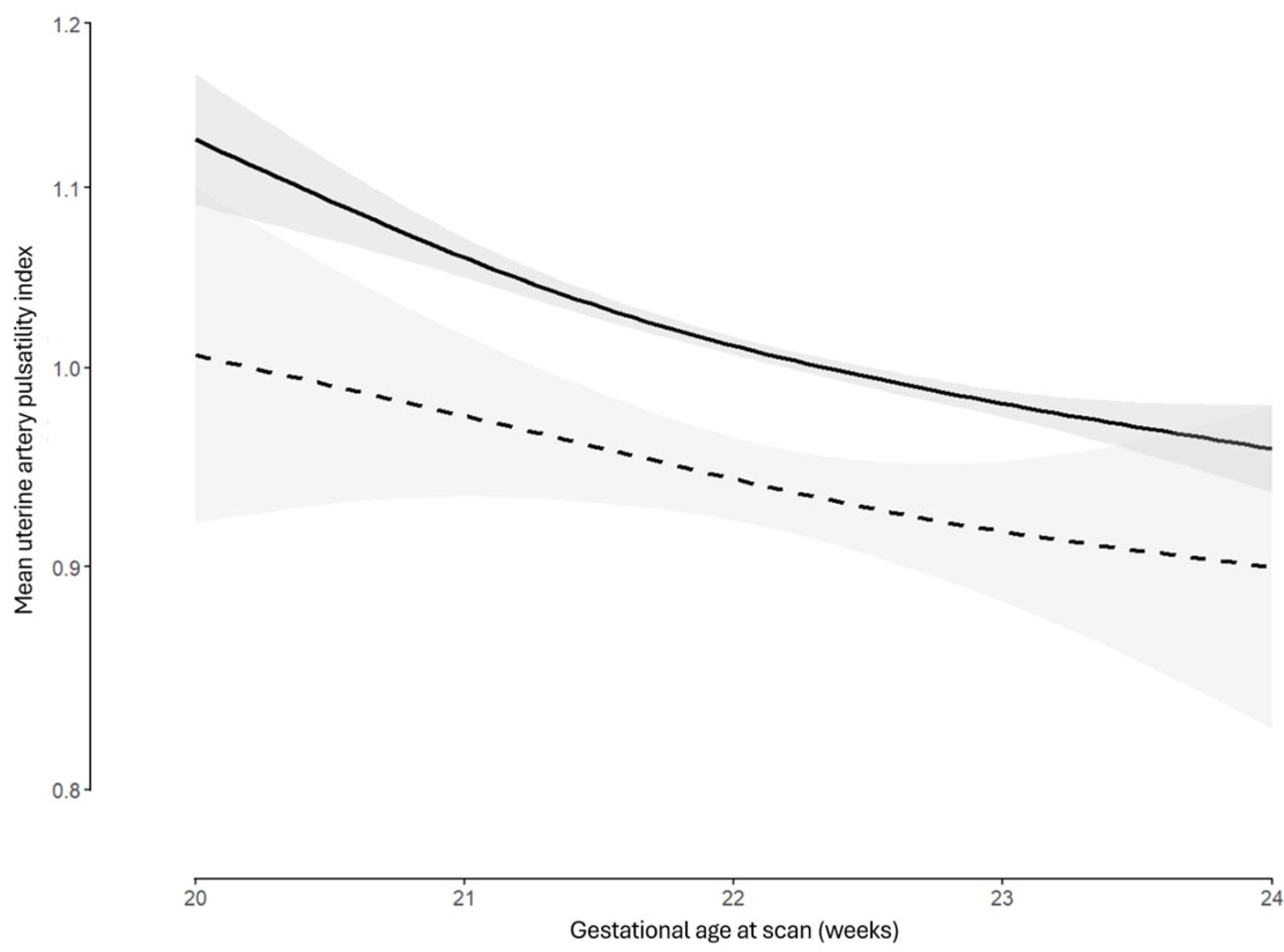
3.4. Multivariate Modelling
4. Discussion
4.1. Main Findings
4.2. Comparison with Previous Studies
4.3. The Role of Confounders
4.4. Interpretation of Findings
4.5. Clinical Implications
4.6. Strengths and Limitations
4.7. Future Research and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papageorghiou, A.T.; Yu, C.K.; Bindra, R.; Pandis, G.; Nicolaides, K.H. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2001, 18, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Albaiges, G.; Missfelder-Lobos, H.; Lees, C.; Parra, M.; Nicolaides, K.H. One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks’ gestation. Obstet. Gynecol. 2000, 96, 559–564. [Google Scholar] [CrossRef]
- Papastefanou, I.; Mesaric, V.; Gomes Castello, R.; Nicolaides, K.H.; Charakida, M. At mid-gestation, markers of placental function rather than maternal cardiac function are stronger determinants of birthweight. Am. J. Obstet. Gynecol. 2025; in press. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Papastefanou, I.; Gyokova, E.; Gungil, B.; Syngelaki, A.; Nicolaides, K.H. Prediction of adverse perinatal outcome at midgestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2023, 62, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Ashoor, G.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Relation of antepartum stillbirth to birthweight and gestational age: Prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2024, 131, 200–206. [Google Scholar] [CrossRef]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.H.Y.; Papastefanou, I.; Chen, Y.; Nguyen-Hoang, L.; Nguyen, D.A.; Dinh, L.T.; Pooh, R.K.; Shiozaki, A.; Zheng, M.; Hu, Y.; et al. Aspirin delays preterm birth in pregnancies at high risk for preterm pre-eclampsia: Evidence from randomized clinical trial in Asia. Ultrasound Obstet. Gynecol. 2025; early view. [Google Scholar] [CrossRef]
- Papastefanou, I.; Chen, Y.; Nguyen-Hoang, L.; Nguyen, D.A.; Dinh, L.T.; Pooh, R.K.; Shiozaki, A.; Zheng, M.; Hu, Y.; Wu, Y.; et al. Impact of Aspirin on Timing of Birth in Pregnancies With Clinical Manifestations of Placental Dysfunction: Evidence from a Multicentre Randomised Clinical Trial. BJOG, 2025; early view. [Google Scholar] [CrossRef]
- Adjahou, S.; Logdanidis, V.; Wright, A.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Assessment of risk for pre-eclampsia at mid-gestation to define subsequent care. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2025, 65, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Wright, D.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Personalized stratification of pregnancy care for small for gestational age neonates from biophysical markers at midgestation. Am. J. Obstet. Gynecol. 2023, 229, 57.e1–57.e14. [Google Scholar] [CrossRef] [PubMed]
- Ashoor, G.; Syngelaki, A.; Papastefanou, I.; Nicolaides, K.H.; Akolekar, R. Development and validation of model for prediction of placental dysfunction-related stillbirth from maternal factors, fetal weight and uterine artery Doppler at mid-gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2022, 59, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H.; Papastefanou, I.; Syngelaki, A.; Ashoor, G.; Akolekar, R. Predictive performance for placental dysfunction related stillbirth of the competing risks model for small-for-gestational-age fetuses. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1530–1537. [Google Scholar] [CrossRef]
- Papastefanou, I.; Menenez, M.; Szczepkowska, A.; Gungil, B.; Syngelaki, A.; Nicolaides, K.H. Comparison of competing-risks model with angiogenic factors in midgestation screening for preterm growth-related neonatal morbidity. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2024, 63, 613–618. [Google Scholar] [CrossRef]
- Tayyar, A.; Guerra, L.; Wright, A.; Wright, D.; Nicolaides, K.H. Uterine artery pulsatility index in the three trimesters of pregnancy: Effects of maternal characteristics and medical history. Ultrasound Obs. Gynecol. 2015, 45, 689–697. [Google Scholar] [CrossRef]
- La Verde, M.; Torella, M.; Ronsini, C.; Riemma, G.; Cobellis, L.; Marrapodi, M.M.; Capristo, C.; Rapisarda, A.M.C.; Morlando, M.; De Franciscis, P. The association between fetal Doppler and uterine artery blood volume flow in term pregnancies: A pilot study. Ultraschall Med. 2024, 45, 184–189. [Google Scholar] [CrossRef]
- Guedes-Martins, L.; Gaio, R.; Saraiva, J.; Cerdeira, S.; Matos, L.; Silva, E.; Macedo, F.; Almeida, H. Reference ranges for uterine artery pulsatility index during the menstrual cycle: A cross-sectional study. PLoS ONE 2015, 10, e0119103. [Google Scholar] [CrossRef]
- Litwinska, M.; Litwinska, E.; Lisnere, K.; Syngelaki, A.; Wright, A.; Nicolaides, K.H. Stratification of pregnancy care based on risk of pre-eclampsia derived from uterine artery Doppler at 19–24 weeks’ gestation. Ultrasound Obs. Gynecol. 2021, 58, 67–76. [Google Scholar] [CrossRef]
- Baki Yıldırım, S.; Ayaydın Yılmaz, K.; Gulerman, C. The Effect of Active and Passive Maternal Smoking During Pregnancy on the Uterine Artery Blood Flow and Obstetric Outcomes: A Prospective Study. Cureus 2023, 15, e35270. [Google Scholar] [CrossRef]
- Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I.A.; Goossens, V. ART in Europe, 2018: Results generated from European registries by ESHRE. Hum. Reprod. Open 2022, 2022, hoac022. [Google Scholar] [CrossRef]
- Gui, J.; Ling, Z.; Hou, X.; Fan, Y.; Xie, K.; Shen, R. In vitro fertilization is associated with the onset and progression of preeclampsia. Placenta 2020, 89, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Schieve, L.A.; Meikle, S.F.; Ferre, C.; Peterson, H.B.; Jeng, G.; Wilcox, L.S. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N. Engl. J. Med. 2002, 346, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Wisborg, K.; Ingerslev, H.J.; Henriksen, T.B. IVF and stillbirth: A prospective follow-up study. Hum. Reprod. 2010, 25, 1312–1316. [Google Scholar] [CrossRef]
- Niu, Z.H.; Ouyang, Y.S.; Zhang, Y.X.; Xu, Z.H.; Yang, M.; Lu, J.; Wu, X.N.; Zhang, P.P.; Dai, Q.; Lv, K.; et al. The multiple reference range of mean uterine artery pulsatility index for natural and in vitro fertilization singletons during 11–14 gestational weeks. Quant. Imaging Med. Surg. 2023, 13, 8587–8598. [Google Scholar] [CrossRef]
- Donno, V.; Prats, P.; Rodriguez, I.; Polyzos, N.P. First-trimester uterine artery pulsatility index and preeclampsia risk in pregnancies after artificial frozen embryo transfer: Analysis of over 27,000 pregnancies. Am. J. Obstet. Gynecol. 2024, 232, 464-e1. [Google Scholar] [CrossRef]
- Prefumo, F.; Fratelli, N.; Soares, S.C.; Thilaganathan, B. Uterine artery Doppler velocimetry at 11-14 weeks in singleton pregnancies conceived by assisted reproductive technology. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2007, 29, 141–145. [Google Scholar] [CrossRef]
- Rizzo, G.; Aiello, E.; Pietrolucci, M.E.; Arduini, D. Placental volume and uterine artery Doppler evaluation at 11 + 0 to 13 + 6 weeks’ gestation in pregnancies conceived with in-vitro fertilization: Comparison between autologous and donor oocyte recipients. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016, 47, 726–731. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Farina, A.; Miglio, R.; Zamagni, G.; Girardelli, S.; Vanni, V.S.; Morano, D.; Spinillo, S.; Sartor, F.; Candiani, M. Prospective longitudinal cohort study of uterine arteries Doppler in singleton pregnancies obtained by IVF/ICSI with oocyte donation or natural conception. Hum. Reprod. 2020, 35, 2428–2438. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef]
- Bhide, A.; Acharya, G.; Baschat, A.; Bilardo, C.M.; Brezinka, C.; Cafici, D.; Ebbing, C.; Hernandez-Andrade, E.; Kalache, K.; Kingdom, J.; et al. ISUOG Practice Guidelines (updated): Use of Doppler velocimetry in obstetrics. Ultrasound Obstet. Gynecol. 2021, 58, 331–339. [Google Scholar] [CrossRef]
- Robinson, H.P.; Fleming, J.E. A critical evaluation of sonar “crown-rump length” measurements. Br. J. Obstet. Gynaecol. 1975, 82, 702–710. [Google Scholar] [CrossRef]
- Carbone, I.F.; Cruz, J.J.; Sarquis, R.; Akolekar, R.; Nicolaides, K.H. Assisted conception and placental perfusion assessed by uterine artery Doppler at 11–13 weeks’ gestation. Hum. Reprod. 2011, 26, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, H.J.; Shin, J.E.; Lee, Y.; Shin, J.C.; Park, T.C.; Park, I.Y. The predictive value of the uterine artery pulsatility index during the early third trimester for the occurrence of adverse pregnancy outcomes depending on the maternal obesity. Obes. Res. Clin. Pract. 2015, 9, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Yapan, P.; Tachawatcharapunya, S.; Surasereewong, S.; Thongkloung, P.; Pooliam, J.; Poon, L.C.; Wataganara, T. Uterine artery Doppler indices throughout gestation in women with and without previous Cesarean deliveries: A prospective longitudinal case-control study. Sci. Rep. 2022, 12, 20913. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Chiantera, V.; Perino, A.; Greco, M.E.; Laganà, A.S.; Marinelli, E.; Basile, G.; Zaami, S. Neonatal Outcomes and Long-Term Follow-Up of Children Born from Frozen Embryo, a Narrative Review of Latest Research Findings. Medicina 2022, 58, 1218. [Google Scholar] [CrossRef]
- Chang, K.; Lubo, Z. Review article: Steroid hormones and uterine vascular adaptation to pregnancy. Reprod. Sci. 2008, 15, 336–348. [Google Scholar] [CrossRef]
- Inversetti, A.; Mandia, L.; Candiani, M.; Cetin, I.; Larcher, A.; Savasi, V.; Papaleo, E.; Cavoretto, P. Uterine artery Doppler pulsatility index at 11-38 weeks in ICSI pregnancies with egg donation. J. Perinat. Med. 2018, 46, 21–27. [Google Scholar] [CrossRef]
- Dallagiovanna, C.; Benaglia, L.; Reschini, M.; Di Gesaro, L.; Li Piani, L.; Persico, N.; Vigano, P.; Somigliana, E. Impact of Endometrial Preparation on the Maternal and Fetal Cardiovascular Variables of the First Trimester Combined Screening Test. J. Clin. Med. 2023, 12, 6854. [Google Scholar] [CrossRef]
- Chih, H.J.; Elias, F.T.S.; Gaudet, L.; Velez, M.P. Assisted reproductive technology and hypertensive disorders of pregnancy: Systematic review and meta-analyses. BMC Pregnancy Childbirth 2021, 21, 449. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, I.; Szczepkowska, A.; Tezhaeva, M.; De Pauli, M.; Charakida, M.; Nicolaides, K.H. Maternal cardiovascular function in midgestation is related to placental angiogenesis. Ultrasound Obs. Gynecol. 2024, 64, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Choux, C.; Ginod, P.; Barberet, J.; Rousseau, T.; Bruno, C.; Sagot, P.; Astruc, K.; Fauque, P. Placental volume and other first-trimester outcomes: Are there differences between fresh embryo transfer, frozen-thawed embryo transfer and natural conception? Reprod. Biomed. Online 2019, 38, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G.; Basile, G.; Cucinella, G.; Greco, M.E.; Perino, A.; Chiantera, V.; Marinelli, S. Fresh vs. frozen embryo transfer in assisted reproductive techniques: A single center retrospective cohort study and ethical-legal implications. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6809–6823. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Laganà, A.S.; Perino, A.; Andrisani, A.; Chiantera, V.; Cucinella, G.; Gitas, G.; Barra, F.; Riemma, G. Assisted Reproductive Techniques and Risk of Congenital Heart Diseases in Children: A Systematic Review and Meta-analysis. Reprod. Sci. 2023, 30, 2896–2906. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Perino, A.; Chiantera, V.; D’Anna, R.; Laganà, A.S.; Buzzaccarini, G. Impact of assisted reproduction techniques on the neuro-psycho-motor outcome of newborns: A critical appraisal. J. Obs. Gynaecol. 2022, 42, 2583–2587. [Google Scholar] [CrossRef]
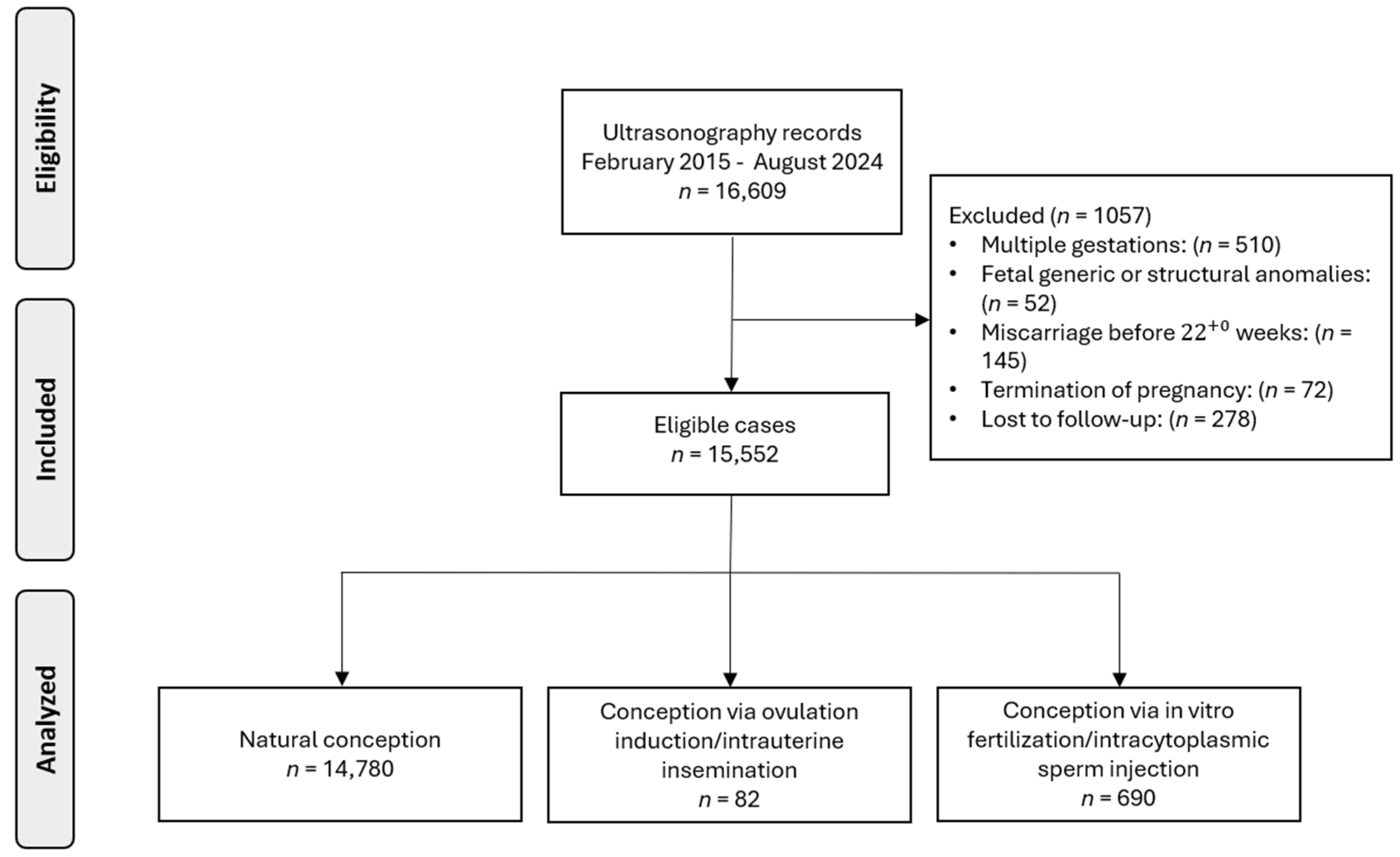
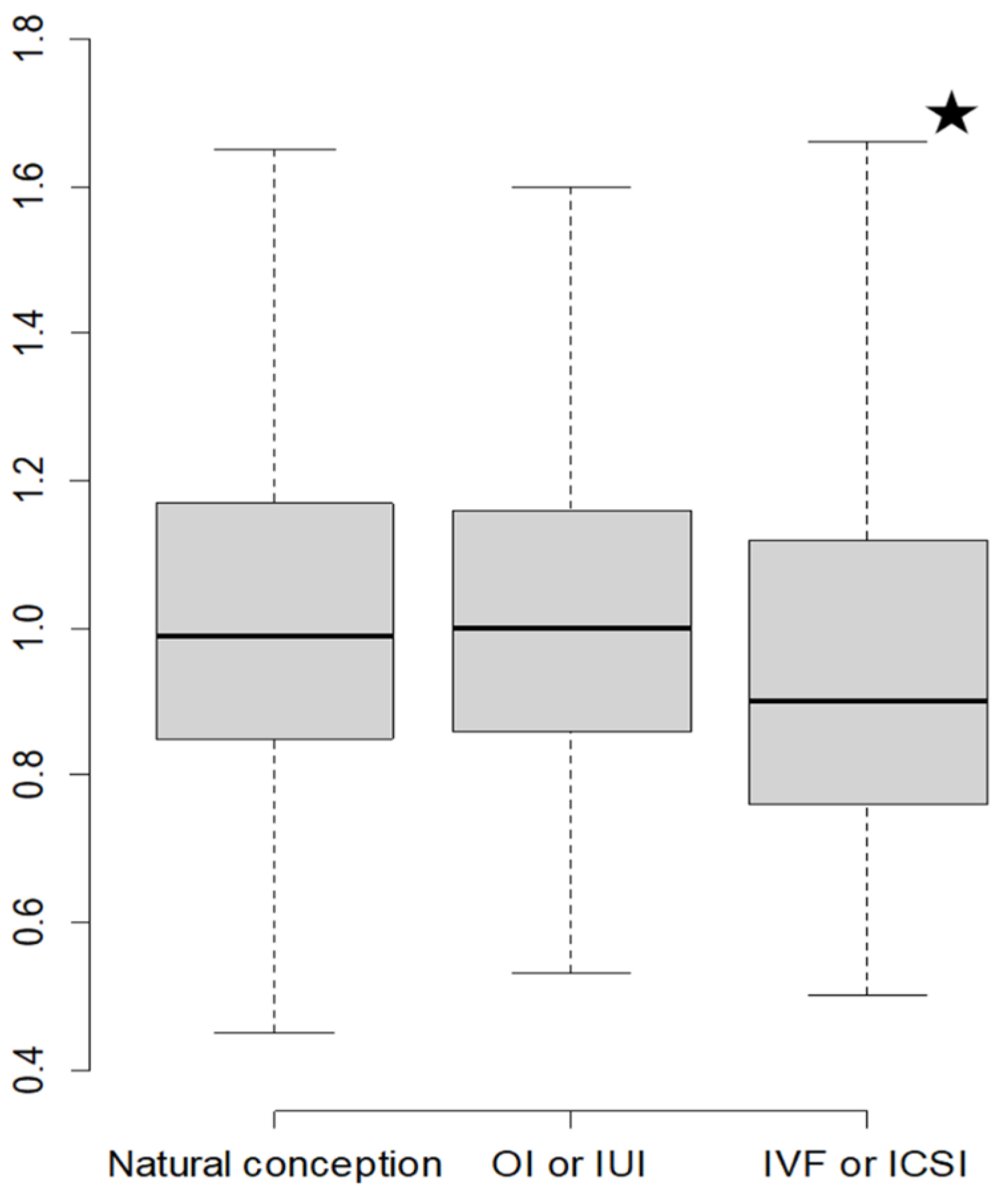
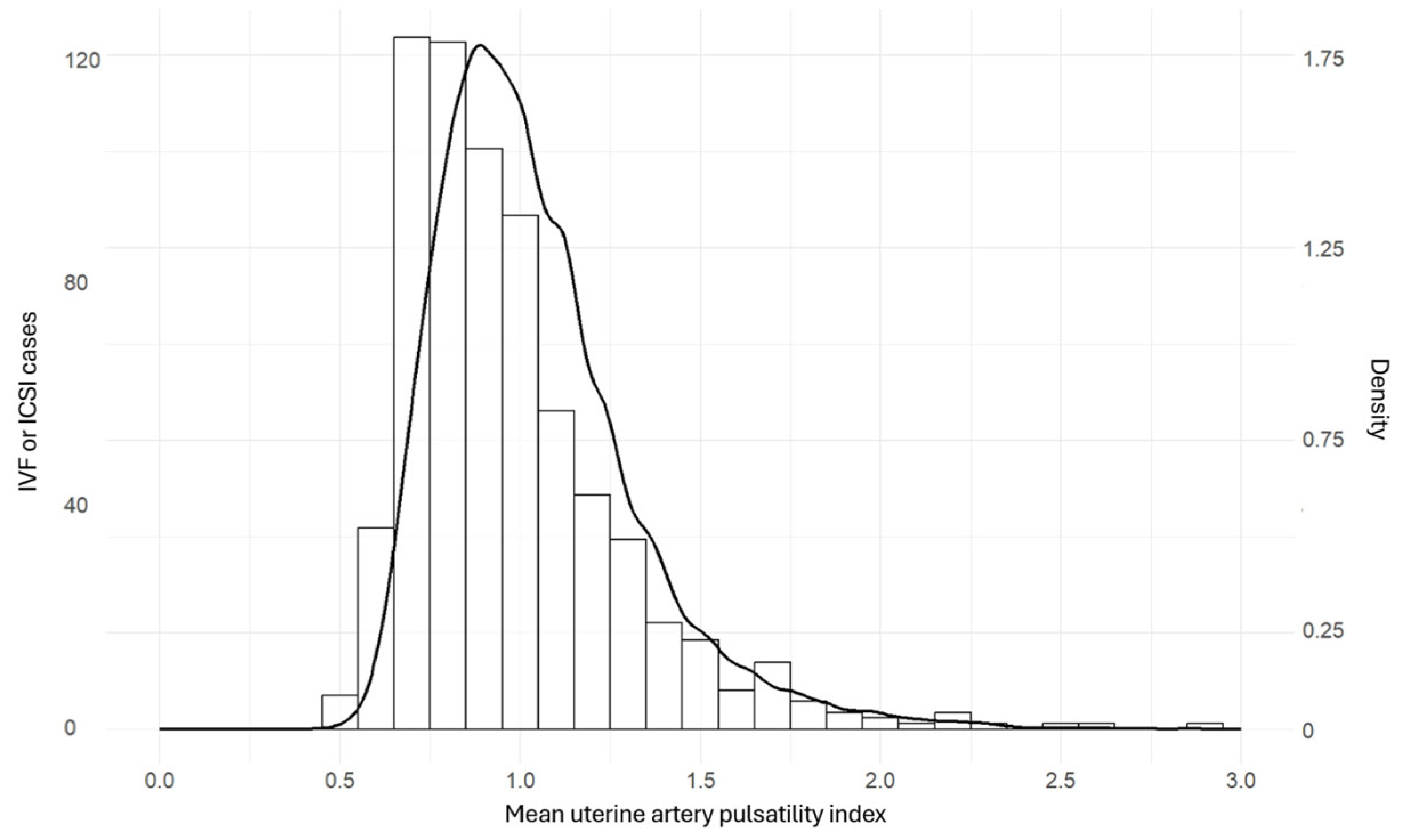
| Spontaneous Conception | OI/IUI | IVF/ICSI | p-Value | |
|---|---|---|---|---|
| N | 14,780 | 82 | 690 | |
| Maternal age mean (SD) * | 31.03 (5.14) | 33.58 (4.78) | 37.20 (5.37) | <0.001 |
| Maternal weight, median [IQR] † | 64.00 [57.00, 73.15] | 63.00 [57.12, 72.00] | 64.00 [58.00, 73.00] | 0.239 |
| Height, median [IQR] † | 165.00 [160.00, 170.00] | 167.00 [162.00, 170.00] | 165.00 [162.00, 170.00] | 0.015 |
| BMI, median [IQR] † | 23.10 [20.80, 26.60] | 23.00 [20.90, 25.40] | 23.00 [21.10, 26.70] | 0.727 |
| Nulliparous, n (%) ‡ | 7380 (49.9) | 68 (82.9) | 561 (81.3) | <0.001 |
| Previous vaginal delivery, n (%) ‡ | 4389 (29.7) | 8 (9.8) | 43 (6.2) | |
| Previous cesarean section, n (%) ‡ | 3011 (20.4) | 6 (7.3) | 86 (12.5) | |
| Pre-existing diabetes mellitus (I or II), n (%) ‡ | 59 (0.4) | 0 (0.0) | 9 (1.3) | 0.002 |
| Thyroid disease, n (%) ‡ | 1068 (7.2) | 8 (9.8) | 96 (13.9) | <0.001 |
| Smoker, n (%) ‡ | 2018 (13.7) | 3 (3.7) | 49 (7.1) | <0.001 |
| Gestational diabetes mellitus, n (%) ‡ | 1285 (8.7) | 8 (9.8) | 108 (15.7) | <0.001 |
| Gestational age in weeks, median [IQR] † | 22.14 [21.71, 22.57] | 22.00 [21.71, 22.29] | 22.14 [21.71, 22.43] | 0.023 |
| No early diastolic notching, n (%) ‡ | 13,910 (94.1) | 74 (90.2) | 657 (95.2) | 0.517 |
| Unilateral early diastolic notching, n (%) ‡ | 646 (4.4) | 5 (6.1) | 25 (3.6) | |
| Bilateral early diastolic notching, n (%) ‡ | 216 (1.5) | 3 (3.7) | 8 (1.2) | |
| Birthweight in grams, mean (SD) * | 3232.35 (500.10) | 3075.41 (581.97) | 3094.09 (588.70) | <0.001 |
| Forced Entry Initial Model | Final Model | |||
|---|---|---|---|---|
| Variables | Estimate (95% CI) | p-Value | Estimate (95% CI) | p-Value |
| Intercept | 6.183 (3.303, 9.062) | <0.001 | 6.194 (3.315, 9.073) | <0.001 |
| Method of conception | ||||
| Spontaneous conception | (Reference) | (Reference) | ||
| OI or IUI | 0.035 (−0.020, 0.090) | 0.213 | 0.035 (−0.020, 0.090) | 0.214 |
| IVF or ICSI | −0.076 (−0.096, −0.056) | <0.001 | −0.076 (−0.096, −0.056) | <0.001 |
| Gestational age | −0.492 (−0.750, −0.233) | <0.001 | −0.493 (−0.751, −0.234) | <0.001 |
| Gestational age2 | 0.010 (0.004, 0.016) | 0.001 | 0.010 (0.004, 0.016) | 0.001 |
| Weight | −0.002 (−0.004, −0.000) | 0.018 | −0.002 (−0.004, −0.000) | 0.017 |
| Weight2 | 0.000 (0.000, 0.000) | 0.007 | 0.000 (0.000, 0.000) | 0.007 |
| Height | −0.002 (−0.002, −0.001) | <0.001 | −0.002 (−0.002, −0.001) | <0.001 |
| Maternal age | 0.001 (0.000, 0.002) | 0.007 | 0.001 (0.000, 0.002) | 0.006 |
| Parity | ||||
| Nulliparous | (Reference) | (Reference) | ||
| Previous vaginal delivery | 0.017 (0.007, 0.027) | 0.001 | 0.017 (0.007, 0.027) | 0.001 |
| Previous cesarean section | −0.003 (−0.014, 0.008) | 0.610 | −0.003 (−0.014, 0.008) | 0.600 |
| Smoking | 0.003 (−0.009, 0.015) | 0.577 | - | - |
| Pre-existing diabetes mellitus (I or II) | −0.021 (−0.081, 0.039) | 0.492 | - | - |
| Thyroid disease | 0.006 (−0.009, 0.020) | 0.464 | - | - |
| Standard deviation | 0.246 | 0.246 | ||
| R2 | 0.017 | 0.017 | ||
| AIC | 516.7 | 511 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siargkas, A.; Tsakiridis, I.; Kappou, D.; Mamopoulos, A.; Papastefanou, I.; Dagklis, T. The Association Between Uterine Artery Pulsatility Index at Mid-Gestation and the Method of Conception: A Cohort Study. Medicina 2025, 61, 1093. https://doi.org/10.3390/medicina61061093
Siargkas A, Tsakiridis I, Kappou D, Mamopoulos A, Papastefanou I, Dagklis T. The Association Between Uterine Artery Pulsatility Index at Mid-Gestation and the Method of Conception: A Cohort Study. Medicina. 2025; 61(6):1093. https://doi.org/10.3390/medicina61061093
Chicago/Turabian StyleSiargkas, Antonios, Ioannis Tsakiridis, Dimitra Kappou, Apostolos Mamopoulos, Ioannis Papastefanou, and Themistoklis Dagklis. 2025. "The Association Between Uterine Artery Pulsatility Index at Mid-Gestation and the Method of Conception: A Cohort Study" Medicina 61, no. 6: 1093. https://doi.org/10.3390/medicina61061093
APA StyleSiargkas, A., Tsakiridis, I., Kappou, D., Mamopoulos, A., Papastefanou, I., & Dagklis, T. (2025). The Association Between Uterine Artery Pulsatility Index at Mid-Gestation and the Method of Conception: A Cohort Study. Medicina, 61(6), 1093. https://doi.org/10.3390/medicina61061093







