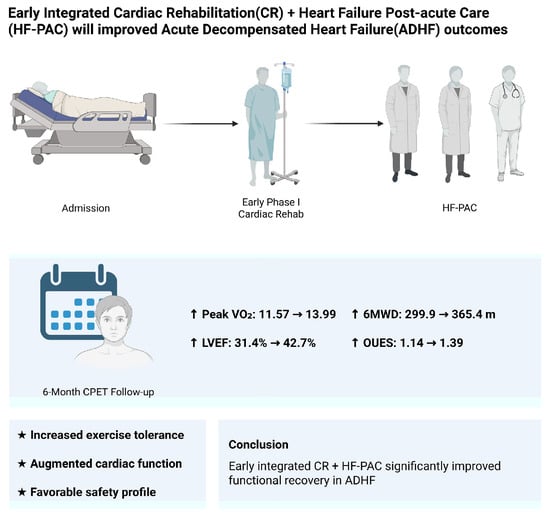
Early Phase I Cardiac Rehabilitation Integrated with Multidisciplinary Post-Acute Care in Decompensated Heart Failure: Insights from Serial Cardiopulmonary Exercise Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Intervention
2.2. Exercise Testing
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWD | 6 min walk distance |
| 6MWT | 6 min walk test |
| ACSM | American College of Sports Medicine |
| ADHF | acute decompensated heart failure |
| AT | anaerobic threshold |
| BNP | brain natriuretic peptide |
| CAD | coronary artery disease |
| COPD | chronic obstructive pulmonary disease |
| CPET | cardiopulmonary exercise testing |
| CR | cardiac rehabilitation |
| DCM | dilated cardiomyopathy |
| DM | diabetes mellitus |
| ESRD | end-stage renal disease |
| HF | heart failure |
| HF-PAC | heart failure post-acute care |
| HRR | heart rate recovery |
| LVEF | left ventricular ejection fraction |
| MDT | multidisciplinary team |
| MR | mitral regurgitation |
| NYHA | New York Heart Association |
| OUES | oxygen uptake efficiency slope |
| PAOD | peripheral arterial occlusion disease |
| Peak VO2 | peak oxygen uptake |
| RER | respiratory exchange ratio |
| SD | standard deviation |
| VCO2 | carbon dioxide production |
| VE | minute ventilation |
| VO2 | oxygen consumption |
| WR | work rate |
References
- Ahmad, T.; Baker, W.L.; Teerlink, J.R.; Lee, C.; Stevenson, L.W.; Bozkurt, B.; Stehlik, J.; Khush, K.K.; Koelling, T.; Page, R.L.; et al. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412–1451. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D.; Huang, S.T.; Wang, C.Y.; Lin, F.J.; Chen, H.M.; Hsiao, F.Y. Nationwide trends in incidence, healthcare utilization, and mortality in hospitalized heart failure patients in Taiwan. ESC Heart Fail. 2020, 7, 3653–3666. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Hung, P.L.; Liao, C.T.; Hsu, C.Y.; Liao, Y.C.; Lu, K.H.; Wang, C.C. Assessing the facilities and healthcare services for heart failure: Taiwan versus European countries. J. Formos. Med. Assoc. 2022, 121, 258–268. [Google Scholar] [CrossRef]
- Bozkurt, B.; Fonarow, G.C.; Goldberg, L.R.; Guglin, M.; Josephson, R.A.; Forman, D.E.; Lin, G.; Lindenfeld, J.; O’Connor, C.; Panjrath, G.; et al. Cardiac Rehabilitation for Patients With Heart Failure: JACC Expert Panel. J. Am. Coll. Cardiol. 2021, 77, 1454–1469. [Google Scholar] [CrossRef]
- Sokos, G.; Kido, K.; Panjrath, G.; Benton, E.; Page, R., 2nd; Patel, J.; Smith, P.J.; Korous, S.; Guglin, M. Multidisciplinary Care in Heart Failure Services. J. Card. Fail. 2023, 29, 943–958. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Zhao, N.; Zhang, M.; Zhou, L.; Ren, X.; Zhang, T.; Zhao, P.; Hu, D.; Pang, X.; et al. Early Exercise-Based Rehabilitation for Patients with Acute Decompensated Heart Failure: A Systemic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2022, 23, 356. [Google Scholar] [CrossRef]
- Kamiya, K.; Tanaka, S.; Saito, H.; Yamashita, M.; Yonezawa, R.; Hamazaki, N.; Matsuzawa, R.; Nozaki, K.; Endo, Y.; Wakaume, K.; et al. Effects of Acute Phase Intensive Exercise Training in Patients With Acute Decompensated Heart Failure. JACC Heart Fail. 2025, 13, 912–922. [Google Scholar] [CrossRef]
- Kaneko, H.; Itoh, H.; Kamiya, K.; Morita, K.; Sugimoto, T.; Konishi, M.; Kiriyama, H.; Kamon, T.; Fujiu, K.; Michihata, N.; et al. Acute-phase initiation of cardiac rehabilitation and clinical outcomes in hospitalized patients for acute heart failure. Int. J. Cardiol. 2021, 340, 36–41. [Google Scholar] [CrossRef]
- Wang, P.Y.; Lin, W.C.; Hsieh, P.C.; Lin, S.H.; Liu, P.Y.; Chao, T.H.; Hsu, C.H. The Effects of Post-Acute Care in Patients with Heart Failure in Taiwan: A Single Center Experience. Acta Cardiol. Sin. 2023, 39, 287–296. [Google Scholar] [CrossRef]
- Baccanelli, G.; Tomaselli, M.; Ferri, U.; Giglio, A.; Munforti, C.; Parati, G.; Facchini, M.; Crotti, L.; Malfatto, G. Effects of cardiac rehabilitation on cardiopulmonary test parameters in heart failure: A real world experience. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 17, 200178. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.R.; Guglin, M. Prognostic value of 6-minute walk test and cardiopulmonary exercise test in acute heart failure (from the ESCAPE trial). Am. Heart J. Plus 2021, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Interpretation of the Correlation Coefficient: A Basic Review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef]
- Frankenstein, L.; Fröhlich, H.; Cleland, J.G. Multidisciplinary Approach for Patients Hospitalized With Heart Failure. Rev. Esp. Cardiol. (Engl. Ed.) 2015, 68, 885–891. [Google Scholar] [CrossRef]
- Comín-Colet, J.; Enjuanes, C.; Lupón, J.; Cainzos-Achirica, M.; Badosa, N.; Verdú, J.M. Transitions of Care Between Acute and Chronic Heart Failure: Critical Steps in the Design of a Multidisciplinary Care Model for the Prevention of Rehospitalization. Rev. Esp. Cardiol. (Engl. Ed.) 2016, 69, 951–961. [Google Scholar] [CrossRef]
- Meng, Y.; Zhuge, W.; Huang, H.; Zhang, T.; Ge, X. The effects of early exercise on cardiac rehabilitation-related outcome in acute heart failure patients: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2022, 130, 104237. [Google Scholar] [CrossRef]
- Takada, S.; Kondo, T.; Yasunaga, M.; Watanabe, S.; Kinoshita, H.; Fukuhara, S.; Yamamoto, Y. Early rehabilitation in older patients hospitalized with acute decompensated heart failure: A retrospective cohort study. Am. Heart J. 2020, 230, 44–53. [Google Scholar] [CrossRef]
- Motoki, H.; Nishimura, M.; Kanai, M.; Kimura, K.; Minamisawa, M.; Yamamoto, S.; Saigusa, T.; Ebisawa, S.; Okada, A.; Kuwahara, K. Impact of inpatient cardiac rehabilitation on Barthel Index score and prognosis in patients with acute decompensated heart failure. Int. J. Cardiol. 2019, 293, 125–130. [Google Scholar] [CrossRef]
- Hoshika, Y.; Kubota, Y.; Nishino, T.; Shiomura, R.; Shibuya, J.; Nakata, J.; Miyachi, H.; Tara, S.; Iwasaki, Y.K.; Yamamoto, T.; et al. Prognostic impact of plasma volume status during hospital admission in patients with acute decompensated heart failure. ESC Heart Fail. 2024, 11, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, P.; Ciccone, M.M.; Iacoviello, M.; Guida, P.; De Palo, M.; Potenza, A.; Basile, M.; Sasanelli, P.; Trotta, F.; Sanasi, M.; et al. Respiratory failure and bioelectrical phase angle are independent predictors for long-term survival in acute heart failure. Scand. Cardiovasc. J. 2022, 56, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Sovetova, S.A.; Nikiforova, T.A.; Charaya, K.V.; Shchekochikhin, D.Y.; Kulikov, V.M.; Dubovitsky, A.M.; Suchkova, S.A.; Bogdanova, A.A.; Ananicheva, N.A.; Andreev, D.A. Hemodynamic Changes in Intrarenal Blood Flow are Associated With Poor Prognosis in Patients With Acute Decompensated Heart Failure. Kardiologiia 2024, 64, 38–44. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Stelken, A.M.; Younis, L.T.; Jennison, S.H.; Miller, D.D.; Miller, L.W.; Shaw, L.J.; Kargl, D.; Chaitman, B.R. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J. Am. Coll. Cardiol. 1996, 27, 345–352. [Google Scholar] [CrossRef]
- Chase, P.J.; Kenjale, A.; Cahalin, L.P.; Arena, R.; Davis, P.G.; Myers, J.; Guazzi, M.; Forman, D.E.; Ashley, E.; Peberdy, M.A.; et al. Effects of respiratory exchange ratio on the prognostic value of peak oxygen consumption and ventilatory efficiency in patients with systolic heart failure. JACC Heart Fail. 2013, 1, 427–432. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Guazzi, M. Cardiopulmonary exercise testing is a core assessment for patients with heart failure. Congest. Heart Fail. 2011, 17, 115–119. [Google Scholar] [CrossRef]
- Santos, M.; Ribeiro, F.; Lopes, I.; Magalhães, S.; Schmidt, C.; Dores, H.; Oliveira, M.I.; Couto, D.S. Exercise intensity prescription in heart failure: A comparison of different physiological parameters. Rev. Port. Cardiol. 2025, 44, 361–371. [Google Scholar] [CrossRef]
- Agostoni, P.; Dumitrescu, D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int. J. Cardiol. 2019, 288, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Psotka, M.A.; Abraham, W.T.; Fiuzat, M.; Filippatos, G.; Lindenfeld, J.; Ahmad, T.; Felker, G.M.; Jacob, R.; Kitzman, D.W.; Leifer, E.S.; et al. Functional and Symptomatic Clinical Trial Endpoints. JACC Heart Failure 2022, 10, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.J.; Curtis, A.B.; Vangsnes, E.; Dickinson, M.G. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm. Phys. Ther. J. 2013, 24, 21–29. [Google Scholar] [CrossRef]
- Gitt, A.K.; Wasserman, K.; Kilkowski, C.; Kleemann, T.; Kilkowski, A.; Bangert, M.; Schneider, S.; Schwarz, A.; Senges, J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002, 106, 3079–3084. [Google Scholar] [CrossRef]
- Garcia Brás, P.; Gonçalves, A.V.; Reis, J.F.; Moreira, R.I.; Pereira-da-Silva, T.; Rio, P.; Timóteo, A.T.; Silva, S.; Soares, R.M.; Ferreira, R.C. Age Differences in Cardiopulmonary Exercise Testing Parameters in Heart Failure with Reduced Ejection Fraction. Medicina 2023, 59, 1685. [Google Scholar] [CrossRef]
- Cahalin, L.P.; Mathier, M.A.; Semigran, M.J.; Dec, G.W.; DiSalvo, T.G. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 1996, 110, 325–332. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Huang, I.C.; Chen, Y.J.; Chen, C.H.; Huang, W.C.; Lin, K.L. The Pre-Discharge Oxygen Uptake Efficiency Slope Predicts One-Year Cardiovascular Events in Acute Decompensated Heart Failure Patients. Life 2022, 12, 1449. [Google Scholar] [CrossRef]
- Pugliatti, P.; Trimarchi, G.; Barocelli, F.; Pizzino, F.; Di Spigno, F.; Tedeschi, A.; Piccione, M.C.; Irrera, P.; Aschieri, D.; Niccoli, G.; et al. Advancing Cardiac Amyloidosis Care Through Insights from Cardiopulmonary Exercise Testing. J. Clin. Med. 2024, 13, 7285. [Google Scholar] [CrossRef]
- Wu, G.; Sanderson, B.; Bittner, V. The 6-minute walk test: How important is the learning effect? Am. Heart J. 2003, 146, 129–133. [Google Scholar] [CrossRef]
| All Patients (n = 90) | ||
|---|---|---|
| n (%) | Mean ± SD | |
| Age (year) | 58.38 ± 14.70 | |
| Gender | ||
| Male | 67 (74.4) | |
| Female | 23 (25.5) | |
| Height (cm) | 165.08 ± 8.79 | |
| Weight (kg) | 70.29 ± 23.21 | |
| BMI (kg/m2) | 25.46 ± 6.72 | |
| NYHA classification | ||
| I | 1 (1.1) | |
| II | 30 (33.3) | |
| III | 54 (60.0) | |
| IV | 5 (5.5) | |
| ADHF etiology | ||
| DCM | 13 (14.4) | |
| MR | 13 (14.4) | |
| CAD † | 42 (46.7) | |
| Myocarditis | 4 (4.4) | |
| Thrombus | 2 (2.2) | |
| Other ‡ | 2 (2.2) | |
| Unknown | 14 (15.6) | |
| Comorbidities | ||
| Hypertension | 56 (62.2) | |
| COPD | 6 (6.6) | |
| DM | 35 (38.8) | |
| PAOD | 2 (2.2) | |
| ESRD | 3 (3.3) | |
| Dyslipidemia | 45 (50) | |
| Number | Baseline | After Six Months | Mean Difference (95% CI) | p-Value | |
|---|---|---|---|---|---|
| n | Mean ± SD | Mean ± SD | |||
| Peak VO2 (mL/min/kg) | 90 | 11.57 ± 3.33 | 13.99 ± 4.2 | +2.42 (+1.75 to +3.07) | p < 0.001 |
| Peak VE (L/min) | 90 | 34.06 ± 11.45 | 37.88 ± 12.57 | +3.82 (+1.63 to +6.01) | p = 0.001 |
| Peak HR | 90 | 107.8 ± 21.26 | 115.81 ± 22.3 | +8.01 (+3.73 to +12.28) | p < 0.001 |
| Peak RER | 90 | 1.08 ± 0.09 | 1.09 ± 0.08 | +0.01 (−0.01 to +0.03) | p = 0.298 |
| 6MWD (m) | 87 | 299.96 ± 107.56 | 365.44 ± 103.26 | +65.48 (+47.94 to +83.01) | p = 0.010 |
| HRR | 89 | 11.6 ± 8.49 | 14.74 ± 9.95 | +3.14 (+0.64 to +5.62) | p = 0.014 |
| ATVO2 (mL/min/kg) | 89 | 8.17 ± 2.38 | 9.98 ± 2.97 | +1.81 (+1.30 to +2.30) | p < 0.001 |
| VE–VCO2 slope | 89 | 37.78 ± 11 | 34 ± 14.67 | −3.78 (−0.88 to −6.66) | p = 0.011 |
| OUES | 89 | 1.14 ± 0.44 | 1.39 ± 0.61 | +0.25 (+0.14 to +0.36) | p = 0.001 |
| LVEF (%) | 90 | 31.38 ± 8.31 | 42.67 ±11.09 | +11.29 (+9.02 to +13.54) | p < 0.001 |
| VO2–WR slope | 89 | 7.44 ± 2.9 | 7.79 ± 3.08 | +0.35 (−0.38 to +1.07) | p = 0.349 |
| Maximal workload (W) | 90 | 48.44 ± 20.24 | 63.63 ± 29.01 | +15.19 (+10.62 to +19.75) | p < 0.001 |
| Variable | n | r | p-Value |
|---|---|---|---|
| ΔATVO2 (mL/min/kg) | 86 | 0.169 | 0.120 |
| ΔPeak RER | 87 | 0.314 | 0.003 * |
| ΔHRR | 86 | 0.020 | 0.855 |
| ΔVE–VCO2 slope | 86 | −0.197 | 0.069 |
| ΔOUES | 86 | 0.166 | 0.127 |
| ΔLVEF (%) | 86 | 0.169 | 0.120 |
| ΔPeak VO2 (mL/kg/min) | 87 | 0.346 | 0.001 * |
| Variable | n | r | p-Value |
|---|---|---|---|
| ΔATVO2 (mL/min/kg) | 89 | 0.677 | <0.01 * |
| ΔPeak RER | 90 | 0.180 | 0.090 |
| ΔHRR | 89 | 0.259 | 0.014 * |
| ΔVE–VCO2 slope | 89 | −0.249 | 0.019 * |
| ΔOUES | 89 | 0.461 | <0.01 * |
| ΔLVEF (%) | 89 | 0.677 | <0.01 * |
| Δ6MWT (m) | 87 | 0.346 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, R.-S.; Lin, K.-L.; Wang, W.-H.; Huang, M.-H.; Liou, I.-H. Early Phase I Cardiac Rehabilitation Integrated with Multidisciplinary Post-Acute Care in Decompensated Heart Failure: Insights from Serial Cardiopulmonary Exercise Testing. Medicina 2025, 61, 1080. https://doi.org/10.3390/medicina61061080
Ding R-S, Lin K-L, Wang W-H, Huang M-H, Liou I-H. Early Phase I Cardiac Rehabilitation Integrated with Multidisciplinary Post-Acute Care in Decompensated Heart Failure: Insights from Serial Cardiopulmonary Exercise Testing. Medicina. 2025; 61(6):1080. https://doi.org/10.3390/medicina61061080
Chicago/Turabian StyleDing, Ruei-Sian, Ko-Long Lin, Wen-Hwa Wang, Ming-Hsuan Huang, and I-Hsiu Liou. 2025. "Early Phase I Cardiac Rehabilitation Integrated with Multidisciplinary Post-Acute Care in Decompensated Heart Failure: Insights from Serial Cardiopulmonary Exercise Testing" Medicina 61, no. 6: 1080. https://doi.org/10.3390/medicina61061080
APA StyleDing, R.-S., Lin, K.-L., Wang, W.-H., Huang, M.-H., & Liou, I.-H. (2025). Early Phase I Cardiac Rehabilitation Integrated with Multidisciplinary Post-Acute Care in Decompensated Heart Failure: Insights from Serial Cardiopulmonary Exercise Testing. Medicina, 61(6), 1080. https://doi.org/10.3390/medicina61061080