Use of JAK Inhibitors in Lichen Planus: An Update
Abstract
1. Introduction
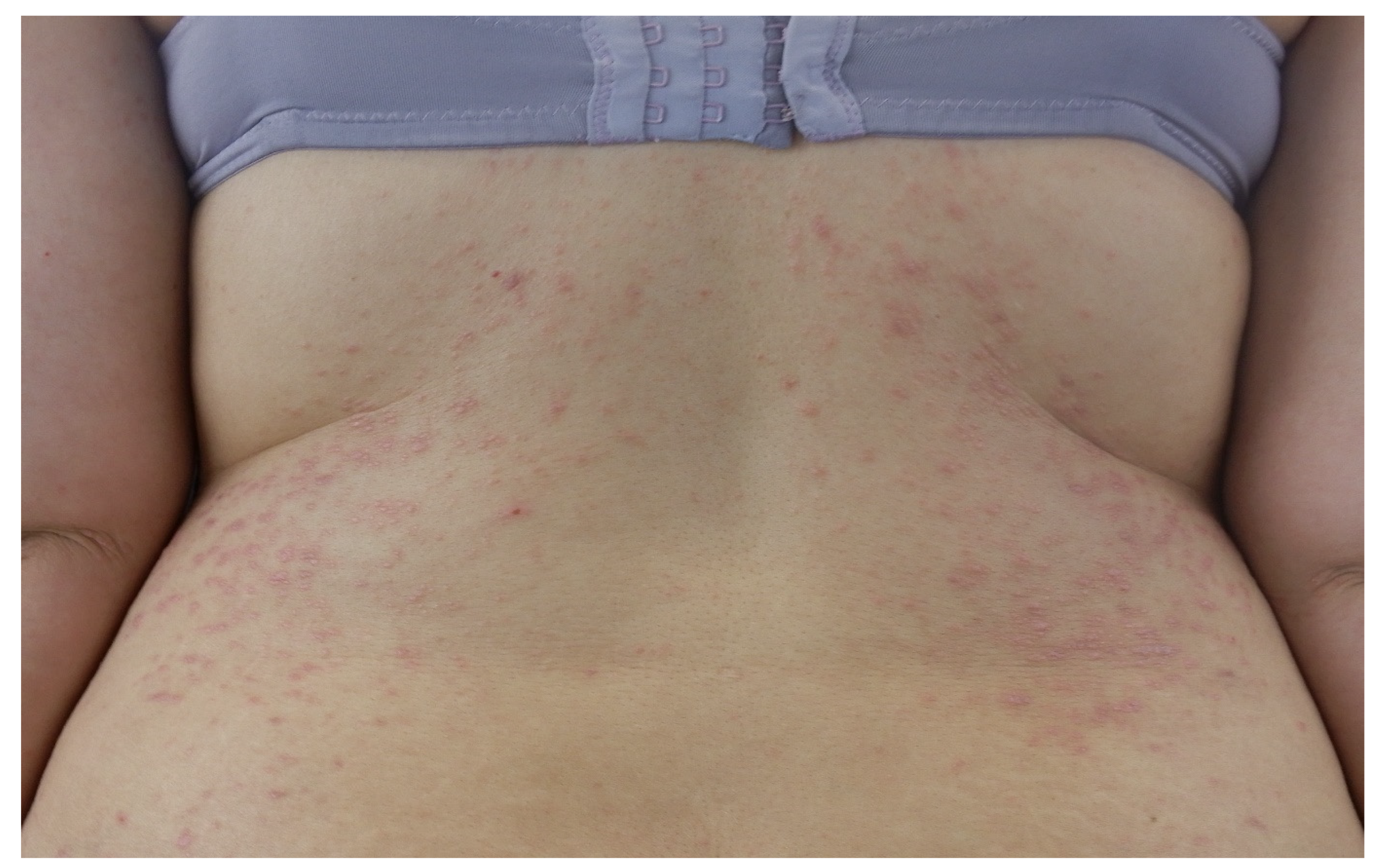
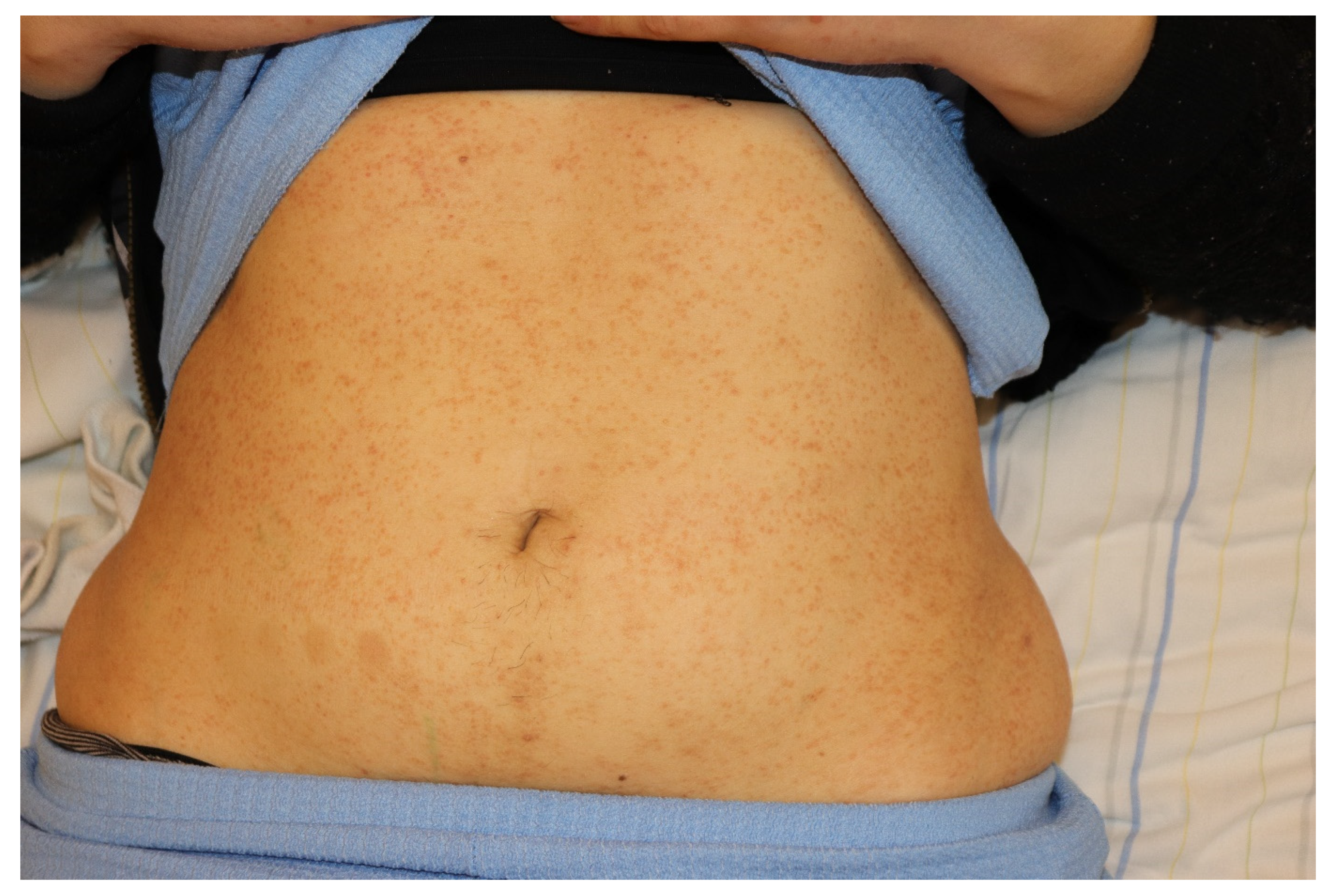
2. Clinical Features of Cutaneous Lichen Planus
3. Clinical Features of Oral Lichen Planus
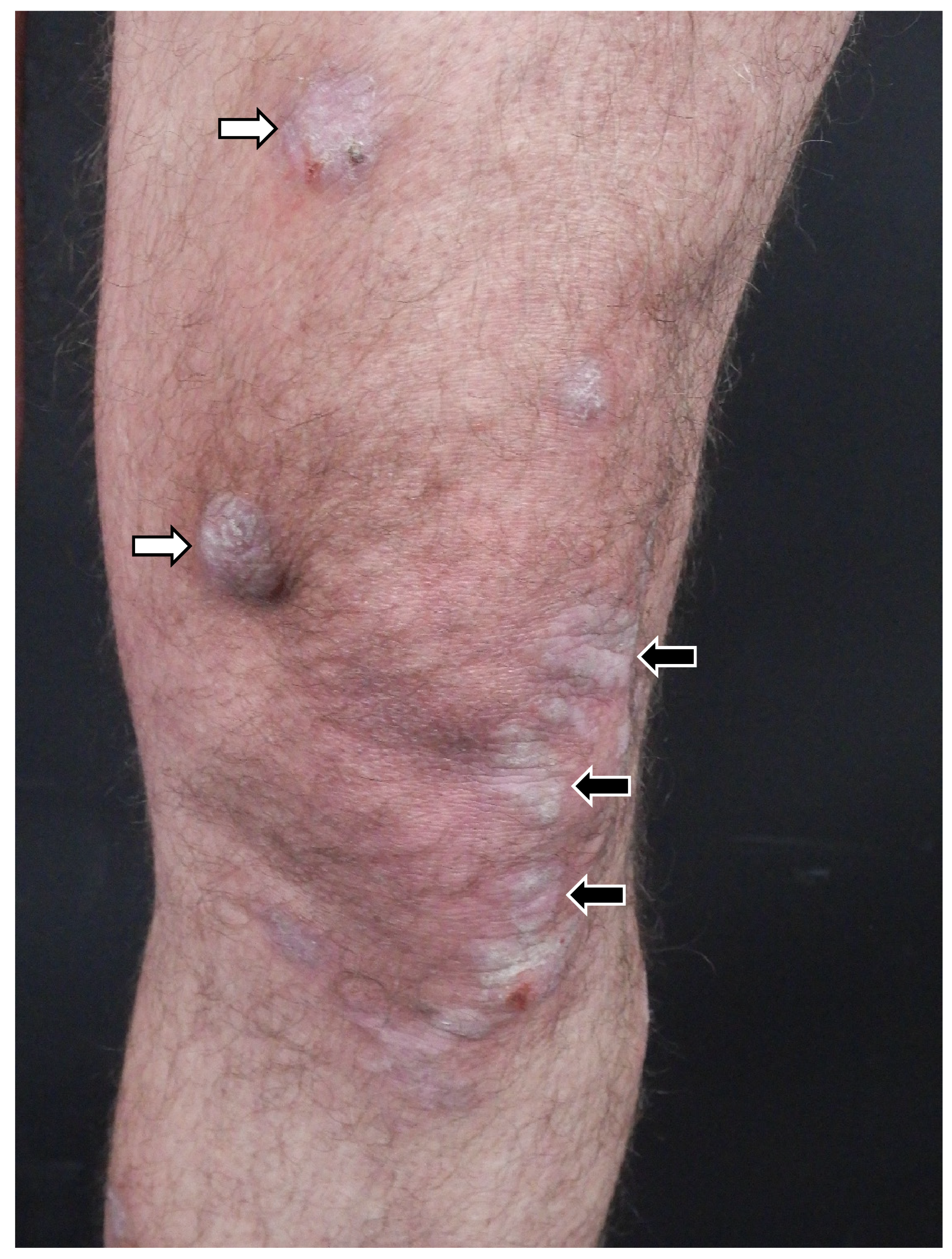
4. Clinical Features of Lichen Planus of Appendages
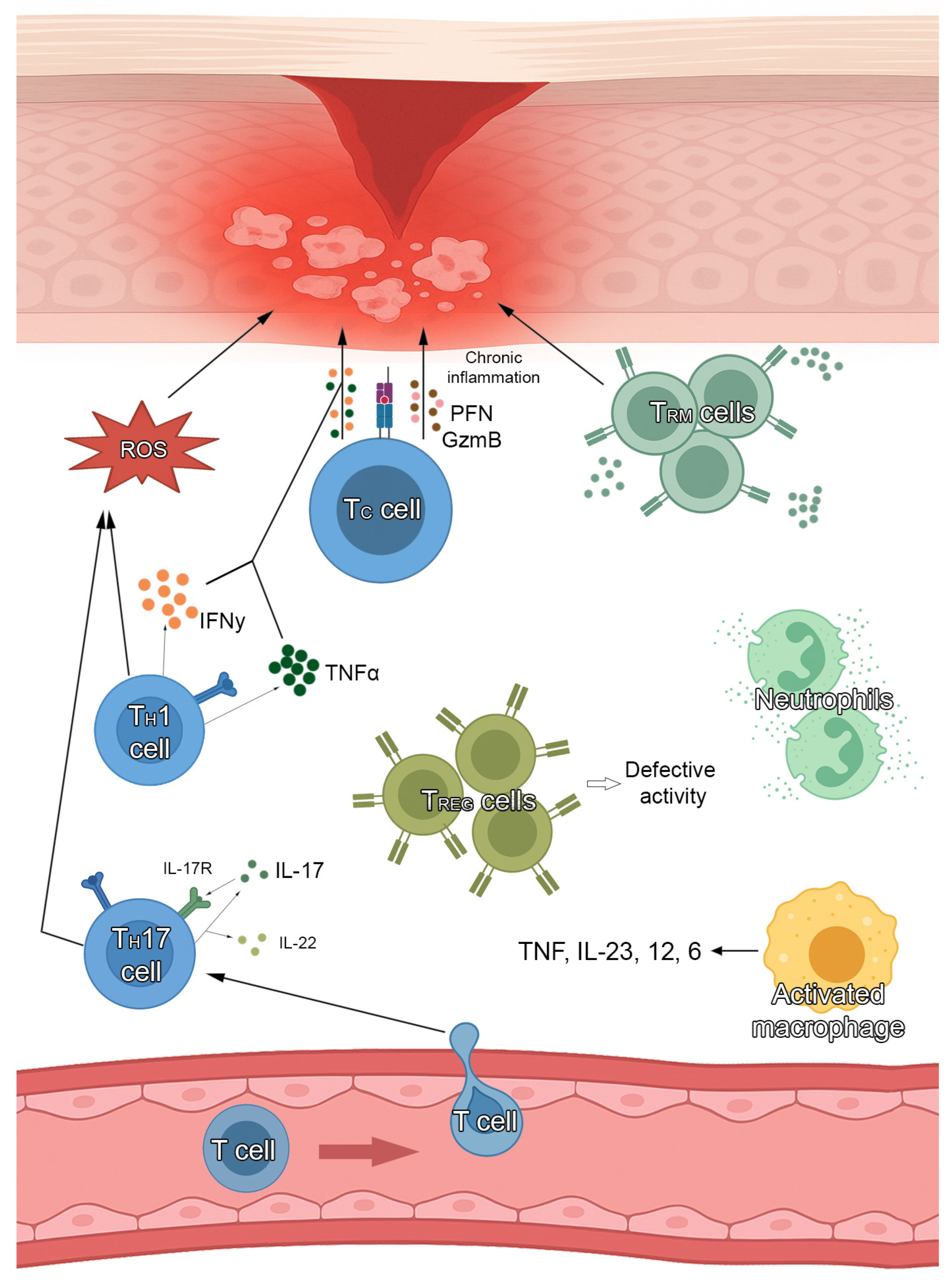
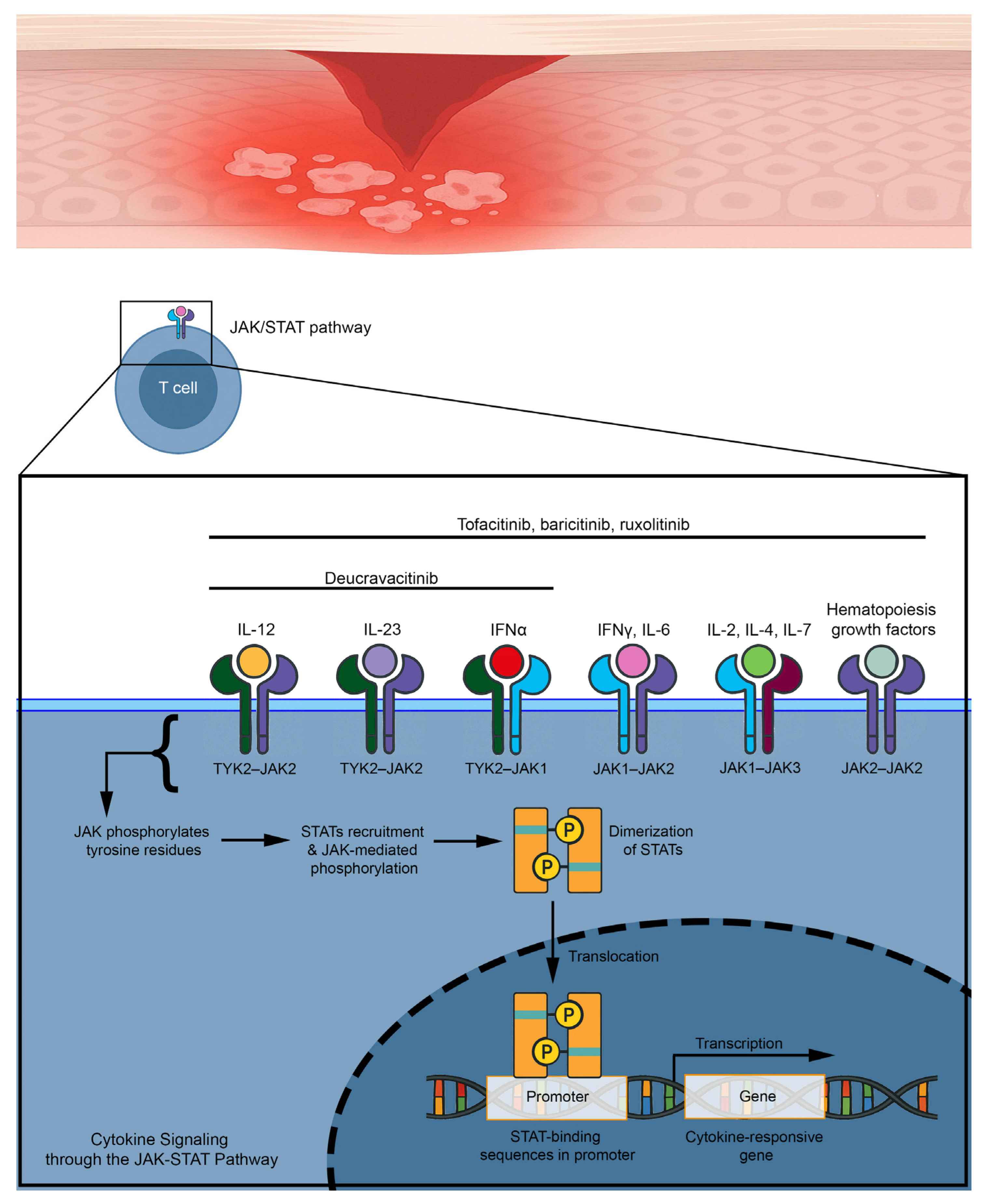
5. JAK Inhibitors and Their Rationale for the Treatment of Lichen Planus
6. Use of JAK Inhibitors for Cutaneous Lichen Planus
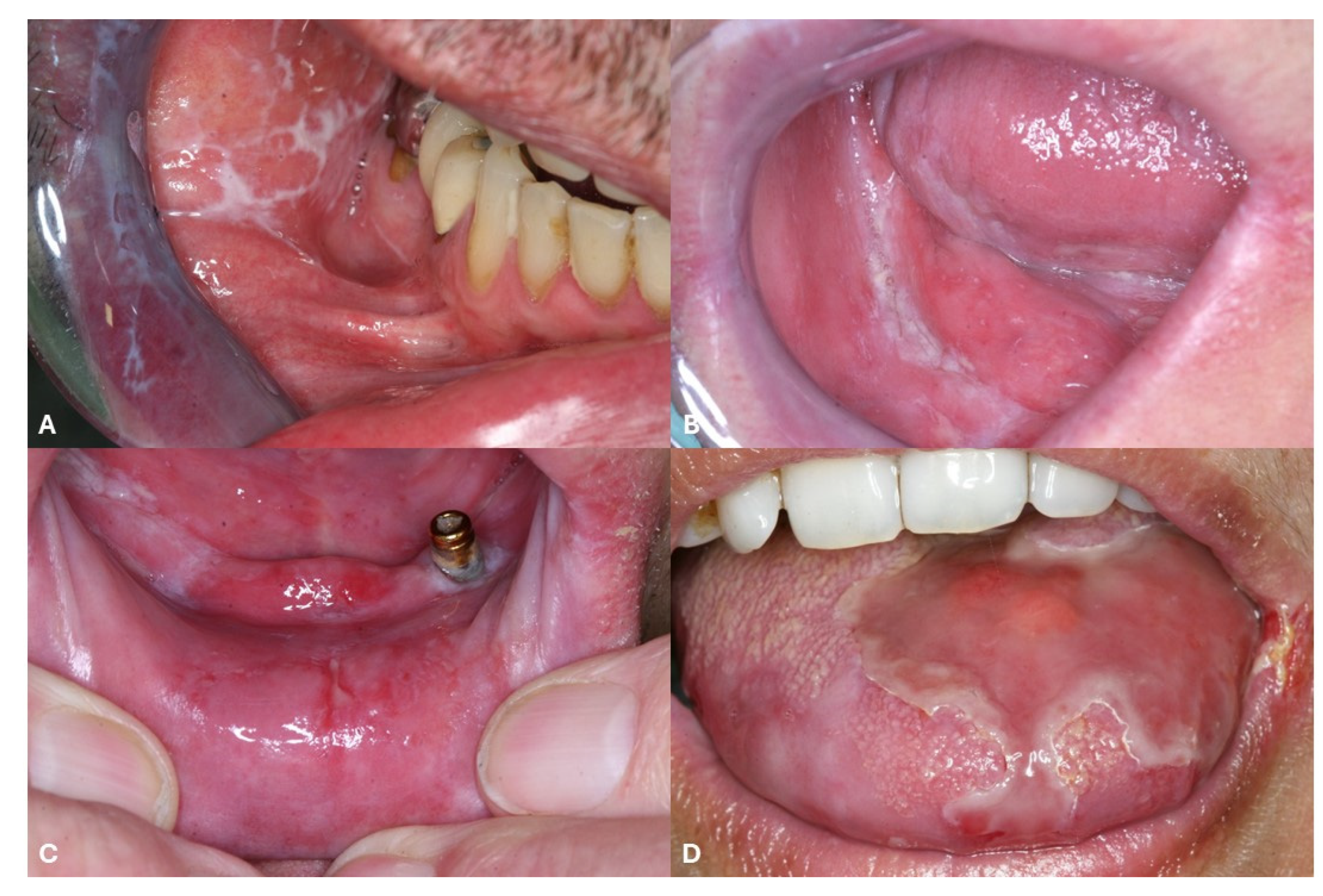
7. Use of JAK Inhibitors for Oral Lichen Planus
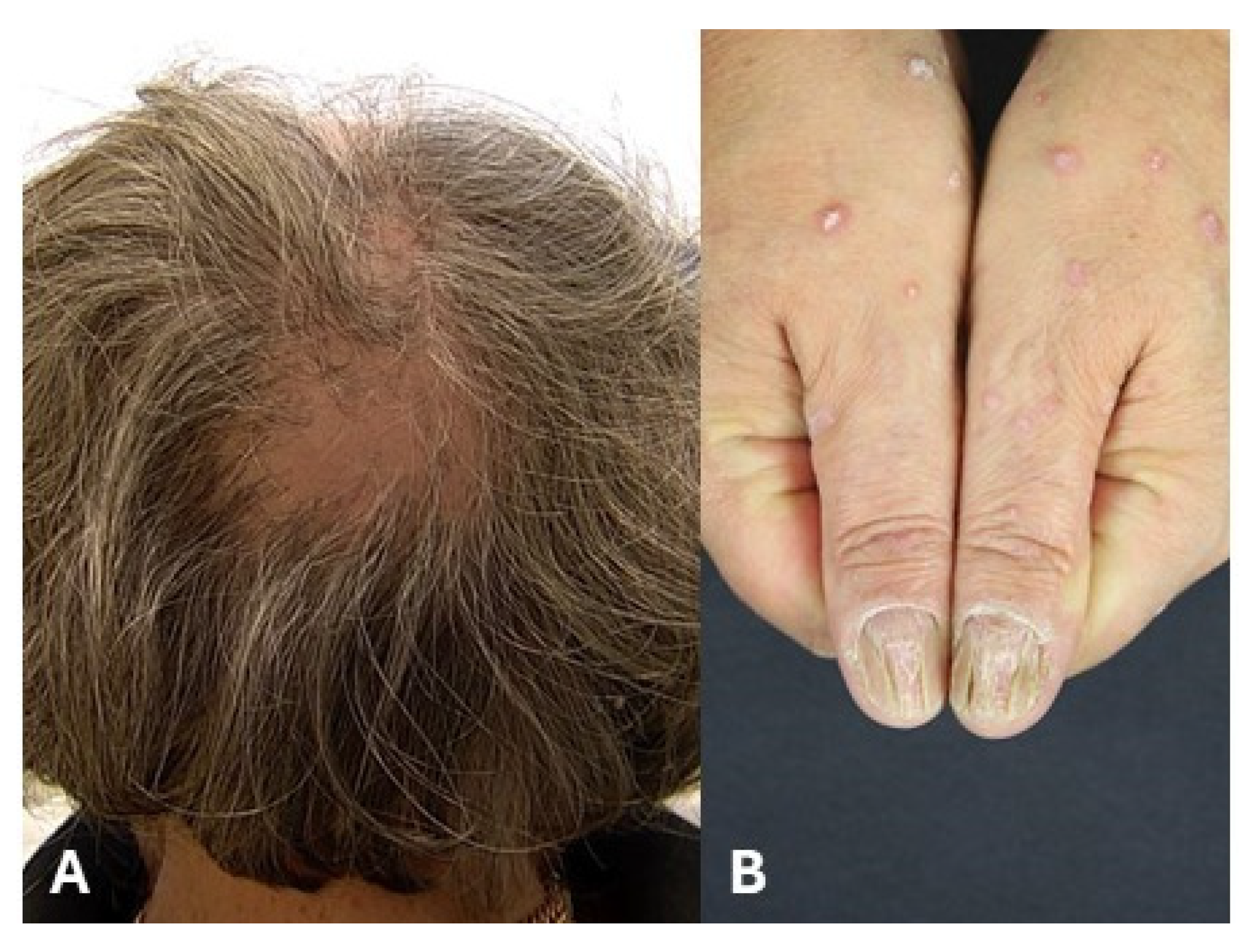
8. Use of JAK Inhibitors for Lichen Planopilaris
9. Use of JAKIs for Nail Lichen Planus
10. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solimani, F.; Forchhammer, S.; Schloegl, A.; Ghoreschi, K.; Meier, K. Lichen planus—A clinical guide. J. Dtsch. Dermatol. Ges. 2021, 19, 864–882. [Google Scholar] [CrossRef] [PubMed]
- Le Cleach, L.; Chosidow, O. Clinical practice. Lichen planus. N. Engl. J. Med. 2012, 366, 723–732. [Google Scholar] [CrossRef]
- Li, C.; Tang, X.; Zheng, X.; Ge, S.; Wen, H.; Lin, X.; Chen, Z.; Lu, L. Global Prevalence and Incidence Estimates of Oral Lichen Planus: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Vičić, M.; Hlača, N.; Kaštelan, M.; Brajac, I.; Sotošek, V.; Prpić Massari, L. Comprehensive Insight into Lichen Planus Immunopathogenesis. Int. J. Mol. Sci. 2023, 24, 3038. [Google Scholar] [CrossRef]
- Solimani, F.; Ghoreschi, K. Januskinaseinhibitoren für dermatologische Erkrankungen. Dermatologie 2024, 75, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Miot, H.A.; Criado, P.R.; de Castro, C.C.S.; Ianhez, M.; Talhari, C.; Ramos, P.M. JAK-STAT pathway inhibitors in dermatology. An. Bras. Dermatol. 2023, 98, 656–677. [Google Scholar] [CrossRef]
- Steffen, C.; Dupree, M.L. Louis-Frédéric Wickham and the Wickham’s striae of lichen planus. Skinmed 2004, 3, 287–289. [Google Scholar] [CrossRef]
- Wagner, G.; Rose, C.; Sachse, M.M. Clinical variants of lichen planus. J. Dtsch. Dermatol. Ges. 2013, 11, 309–319. [Google Scholar] [CrossRef]
- Whittington, C.P.; Saleh, J.S.; Bresler, S.C.; Patel, R.M. Hypertrophic Lichen Planus: An Up-to-Date Review and Differential Diagnosis. Arch. Pathol. Lab. Med. 2024, 148, 659–665. [Google Scholar] [CrossRef]
- Manz, B.; Paasch, U.; Sticherling, M. Squamous cell carcinoma as a complication of long-standing hypertrophic lichen planus. Int. J. Dermatol. 2005, 44, 773–774. [Google Scholar] [CrossRef]
- Tonsager, M.; Crutchfield, C.E. Atrophic lichen planus. Dermatol. Nurs. 2004, 16, 73–74. [Google Scholar] [PubMed]
- Robles-Méndez, J.C.; Rizo-Frías, P.; Herz-Ruelas, M.E.; Pandya, A.G.; Ocampo Candiani, J. Lichen planus pigmentosus and its variants: Review and update. Int. J. Dermatol. 2018, 57, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, T.; Otake, N.; Nagai, M. Eruptive lichen planus. J. Dermatol. 1992, 19, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Didona, D.; Caposiena Caro, R.D.; Sequeira Santos, A.M.; Solimani, F.; Hertl, M. Therapeutic strategies for oral lichen planus: State of the art and new insights. Front. Med. 2022, 9, 997190. [Google Scholar] [CrossRef]
- Didona, D.; Hinterseher, J.; Eming, R. Bullöse Autoimmundermatosen der Schleimhaut. Dermatologie 2022, 73, 692–700. [Google Scholar] [CrossRef]
- Didona, D.; Hertl, M. Detection of anti-desmoglein antibodies in oral lichen planus: What do we know so far. Front. Immunol. 2022, 13, 1001970. [Google Scholar] [CrossRef]
- Didona, D.; Schmidt, M.F.; Meier, K.; Mesas-Fernandez, A.; Maglie, R.; Antiga, E.; Klemp, M.; Yazdi, A.S.; Ghoreschi, K.; Hertl, M.; et al. Pathogenic relevance of antibodies against desmoglein 3 in patients with oral lichen planus. J. Dtsch. Dermatol. Ges. 2024, 22, 1392–1399. [Google Scholar] [CrossRef]
- Fitzpatrick, S.G.; Hirsch, S.A.; Gordon, S.C. The malignant transformation of oral lichen planus and oral lichenoid lesions: A systematic review. J. Am. Dent. Assoc. 2014, 145, 45–56. [Google Scholar] [CrossRef]
- Georgakopoulou, E.A.; Troupis, T.G.; Troupis, G.; Gorgoulis, V.G. Update of the cancer-associated molecular mechanisms in oral lichen planus, a disease with possible premalignant nature. J. BUON 2011, 16, 613–616. [Google Scholar]
- Kerkemeyer, K.L.S.; Eisman, S.; Bhoyrul, B.; Pinczewski, J.; Sinclair, R.D. Frontal fibrosing alopecia. Clin. Dermatol. 2021, 39, 183–193. [Google Scholar] [CrossRef]
- Wagner, G.; Meyer, V.; Sachse, M.M. Frontal fibrosierende Alopezie Kossard. Hautarzt 2016, 67, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Verma, G. Graham-Little-Piccardi-Lasseur Syndrome. Indian Dermatol. Online J. 2019, 10, 180–181. [Google Scholar] [CrossRef]
- Gupta, M.K.; Lipner, S.R. Review of Nail Lichen Planus: Epidemiology, Pathogenesis, Diagnosis, and Treatment. Dermatol. Clin. 2021, 39, 221–230. [Google Scholar] [CrossRef]
- Hwang, J.K.; Grover, C.; Iorizzo, M.; Lebwohl, M.G.; Piraccini, B.M.; Rigopoulos, D.G.; Lipner, S.R. Nail psoriasis and nail lichen planus: Updates on diagnosis and management. J. Am. Acad. Dermatol. 2024, 90, 585–596. [Google Scholar] [CrossRef] [PubMed]
- McLornan, D.P.; Pope, J.E.; Gotlib, J.; Harrison, C.N. Current and future status of JAK inhibitors. Lancet 2021, 398, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Selectivity and therapeutic inhibition of kinases: To be or not to be? Nat. Immunol. 2009, 10, 356–360. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Pietschke, K.; Holstein, J.; Meier, K.; Schäfer, I.; Müller-Hermelink, E.; Gonzalez-Menendez, I.; Quintanilla-Martinez, L.; Ghoreschi, F.C.; Solimani, F.; Ghoreschi, K. The inflammation in cutaneous lichen planus is dominated by IFN-ϒ and IL-21-A basis for therapeutic JAK1 inhibition. Exp. Dermatol. 2021, 30, 262–270. [Google Scholar] [CrossRef]
- Shao, S.; Tsoi, L.C.; Sarkar, M.K.; Xing, X.; Xue, K.; Uppala, R.; Berthier, C.C.; Zeng, C.; Patrick, M.; Billi, A.C.; et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci. Transl. Med. 2019, 11, eaav7561. [Google Scholar] [CrossRef]
- Schmidt, T.; Solimani, F.; Pollmann, R.; Stein, R.; Schmidt, A.; Stulberg, I.; Kühn, K.; Eming, R.; Eubel, V.; Kind, P.; et al. TH1/TH17 cell recognition of desmoglein 3 and bullous pemphigoid antigen 180 in patients with lichen planus. J. Allergy Clin. Immunol. 2018, 142, 669–672.e7. [Google Scholar] [CrossRef]
- Tian, Y.; Zajac, A.J. IL-21 and T Cell Differentiation: Consider the Context. Trends Immunol. 2016, 37, 557–568. [Google Scholar] [CrossRef]
- Motamed-Sanaye, A.; Khazaee, Y.F.; Shokrgozar, M.; Alishahi, M.; Ahramiyanpour, N.; Amani, M. JAK inhibitors in lichen planus: A review of pathogenesis and treatments. J. Dermatol. Treat. 2022, 33, 3098–3103. [Google Scholar] [CrossRef]
- Seiringer, P.; Lauffer, F.; Pilz, A.C.; Boehmer, D.; Biedermann, T.; Eyerich, K. Tofacitinib in Hypertrophic Lichen Planus. Acta Derm. Venereol. 2020, 100, adv00220. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.; Bordone, L.A. Oral tofacitinib effectively treating eruptive and hypertrophic cutaneous lichen planus. JAAD Case Rep. 2023, 37, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zundell, M.P.; Kaminetsky, J.; Lebwohl, M.; Gottlieb, A.B. Successful Treatment of Lichen Planus with Oral Upadacitinib. J. Drugs Dermatol. 2023, 22, 1058–1060. [Google Scholar] [CrossRef]
- Böll, S.L.; Zahn, C.A.; Schlapbach, C. Rapid and sustained improvement of cutaneous lichen planus with oral JAK1 inhibitors. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e82–e85. [Google Scholar] [CrossRef]
- Ball, G.D.; Golant, A. A Case of Extensive Lichen Planus Treated with Deucravacitinib. Cureus 2024, 16, e71951. [Google Scholar] [CrossRef] [PubMed]
- Sharath, S.; Sardana, K.; Khurana, A.; Yadav, A.; Singh, A. Therapy resistant hypertrophic lichen planus and its response to oral tofacitinib with a priori tissue cytokine expression: A real-world hospital-based study. Arch. Dermatol. Res. 2025, 317, 590. [Google Scholar] [CrossRef]
- Popa, C.; Sciuca, A.M.; Onofrei, B.-A.; Toader, S.; Condurache Hritcu, O.M.; Boțoc Colac, C.; Porumb Andrese, E.; Brănișteanu, D.E.; Toader, M.P. Integrative Approaches for the Diagnosis and Management of Erosive Oral Lichen Planus. Diagnostics 2024, 14, 692. [Google Scholar] [CrossRef]
- Wu, T.; Bai, Y.; Jing, Y.; Chen, F. What can we learn from treatments of oral lichen planus? Front. Cell. Infect. Microbiol. 2024, 14, 1279220. [Google Scholar] [CrossRef]
- Kooybaran, N.R.; Petzold, G.; Ströbel, P.; Schön, M.P.; Mössner, R. Alleviation of erosive oral and esophageal lichen planus by the JAK1 inhibitor upadacitinib. J. Dtsch. Dermatol. Ges. 2021, 19, 1778–1780. [Google Scholar] [CrossRef] [PubMed]
- Balestri, R.; Bortolotti, R.; Rech, G.; Girardelli, C.R.; Zorzi, M.G.; Magnano, M. Treatment of Oral Erosive Lichen Planus with Upadacitinib. JAMA Dermatol. 2022, 158, 457–458. [Google Scholar] [CrossRef]
- Moussa, A.; Colla, T.; Morrison, B.; Sinclair, R. Effective treatment of oral lichen planus with the JAK inhibitor baricitinib. Australas. J. Dermatol. 2022, 63, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Landells, F.M.; Goudie, S.; McGrath, J.; Tibbo, J.; Landells, I. Successful treatment of erosive lichen planus with Upadacitinib complicated by oral squamous cell carcinoma. SAGE Open Med. Case Rep. 2023, 11, 2050313X231213144. [Google Scholar] [CrossRef]
- Slater, K.; Halash, K.; Kartono, F. Oral Lichen Planus Successfully Treated With Upadacitinib. J. Drugs Dermatol. 2024, 23, e104–e106. [Google Scholar] [CrossRef]
- Stolte, K.N.; Mesas-Fernández, A.; Meier, K.; Klein, E.K.; Dommisch, H.; Ghoreschi, K.; Solimani, F. TYK2 inhibition with deucravacitinib ameliorates erosive oral lichen planus. Exp. Dermatol. 2024, 33, e15080. [Google Scholar] [CrossRef]
- Gowda, S.K.; Thakur, V.; Behera, B.; Garg, S.; Sahu, D.K.; Sethy, M.; Ayyanar, P. The efficacy and safety of oral tofacitinib in oral erosive lichen planus: A case series. Int. J. Dermatol. 2024, 63, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, P.; Jafari, M.A.; Chalangari, R.; Roohaninasab, M.; Goodarzi, A. Successful Treatment of Erosive Lichen Planus With Tofacitinib: A Case Series and Review of the Literature. Clin. Med. Insights Case Rep. 2024, 17, 11795476241237350. [Google Scholar] [CrossRef]
- Yang, C.C.; Khanna, T.; Sallee, B.; Christiano, A.M.; Bordone, L.A. Tofacitinib for the treatment of lichen planopilaris: A case series. Dermatol. Ther. 2018, 31, e12656. [Google Scholar] [CrossRef]
- Plante, J.; Eason, C.; Snyder, A.; Elston, D. Tofacitinib in the treatment of lichen planopilaris: A retrospective review. J. Am. Acad. Dermatol. 2020, 83, 1487–1489. [Google Scholar] [CrossRef]
- Moussa, A.; Bhoyrul, B.; Asfour, L.; Kazmi, A.; Eisman, S.; Sinclair, R.D. Treatment of lichen planopilaris with baricitinib: A retrospective study. J. Am. Acad. Dermatol. 2022, 87, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Griffith, V.; Coican, A.; Dane, A.; Chow, W.; Aneja, S.; Nathoo, R.; Leavitt, A.; Hawkins, S.D. Janus kinase inhibition for the treatment of refractory frontal fibrosing alopecia: A case series and review of the literature. JAAD Case Rep. 2023, 40, 47–52. [Google Scholar] [CrossRef]
- Chen, L.-C.; Ogbutor, C.; Kelley, K.J.; Pickford, J.R.; Senna, M.M. Prevalence of lichen planopilaris and frontal fibrosing alopecia in the United States: An All of Us database analysis. Int. J. Dermatol. 2024, 63, 1099–1101. [Google Scholar] [CrossRef]
- Gonzalez Matheus, G.A.; Khosrotehrani, K. Treatment of lichen planopilaris with Janus kinase inhibitors. Australas. J. Dermatol. 2024, 65, 381–383. [Google Scholar] [CrossRef]
- Goodarzi, M.; Dadkhahfar, S.; Yahyaei, F.; Razzaghi, Z.; Moravvej, H. Efficacy and Safety of Tofacitinib in Lichen Planopilaris: A Retrospective Series of 74 Patients. Arch. Iran. Med. 2025, 28, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.N.; Perez, S.M.; Burroway, B.; Tosti, A. Topical ruxolitinib in the management of frontal fibrosing alopecia and/or lichen planopilaris: A single-center retrospective cohort study. J. Am. Acad. Dermatol. 2025, 92, 170–172. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, Y. Efficacy and safety of JAK inhibitors in treating lichen planopilaris or frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2025; online ahead of print. [Google Scholar] [CrossRef]
- David, E.; Shokrian, N.; Del Duca, E.; Meariman, M.; Dubin, C.; Hawkins, K.; Andrews, E.; Sikand, S.; Singer, G.; Oemar, B.; et al. A phase 2a trial of brepocitinib for cicatricial alopecia. J. Am. Acad. Dermatol. 2025, 92, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Haneke, E. Tofacitinib as Treatment for Nail Lichen Planus Associated with Alopecia Universalis. JAMA Dermatol. 2021, 157, 352–353. [Google Scholar] [CrossRef]
- Pünchera, J.; Laffitte, E. Treatment of Severe Nail Lichen Planus with Baricitinib. JAMA Dermatol. 2022, 158, 107–108. [Google Scholar] [CrossRef]
- Huang, J.; Shi, W. Successful treatment of nail lichen planus with tofacitinib: A case report and review of the literature. Front. Med. 2023, 10, 1301123. [Google Scholar] [CrossRef]
- He, J.; Weng, T.; Zhu, W.; Yang, Y.; Li, C. Alleviation of isolated nail lichen planus by the JAK1/2 inhibitor Baricitinib: A case report. J. Dermatol. Treat. 2023, 34, 2274816. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, Y. Janus kinase 1 inhibitor abrocitinib for isolated nail lichen planus: A case report and literature review. J. Dermatol. Treat. 2024, 35, 2434094. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Study Type | JAK Inhibitor | Result |
|---|---|---|---|---|
| Seiringer et al. [33] | 2020 | Case report | Oral tofacitinib | Reduction of pruritus on a NRS scale from 10/10 (baseline) to 0/10 (week 20) |
| Youssef et al. [34] | 2023 | Case report | Oral tofacitinib | Resolution of pruritus and improvement of patches at week 4 |
| Zundell et al. [35] | 2023 | Case report | Oral upadacitinib | Successfully treated |
| Böll et al. [36] | 2024 | Case series | Oral abrocitinib or upadacitinib | Two patients obtained complete relief from pruritus within the first days; 7 out of 7 patients had >4 points reduction in NRS pruritus by day 21 |
| Ball et al. [37] | 2024 | Case report | Oral deucravacitinib | Reduction of BSA involvement from 80% to 20% at week 8 |
| Sharath et al. [38] | 2025 | Case series | Oral tofacitinib | Pruritus resolved in a mean of 8.6 days; mean time to achieve resolution of lesions was 4.7 weeks |
| Author | Year | Study Type | JAK Inhibitor | Result |
|---|---|---|---|---|
| Kooybaran et al. [41] | 2021 | Case report | Oral upadacitinib | Pain at baseline 7/10 on NRS; pain at week three 5/10 on NRS |
| Balestri et al. [42] | 2022 | Case report | Oral upadacitinib | Complete healing of the oral lesions after 7 days |
| Moussa et al. [43] | 2022 | Case report | Oral baricitinib | Near complete resolution of the oral irritation and discomfort at week 16 |
| Landells et al. [44] | 2023 | Case report | Oral upadacitinib | Almost complete clearance of oral lesions |
| Slater et al. [45] | 2024 | Case report | Oral upadacitinib | A 70% improvement within 4 weeks |
| Stolte et al. [46] | 2024 | Case series | Oral deucravacitinib | Improvement of oral lesions at week 12 |
| Gowda et al. [47] | 2024 | Case series | Oral tofacitinib | Reduction in ODSS from the baseline was 70% at week 12 |
| Mansouri et al. [48] | 2024 | Case series | Oral tofacitinib | The mean pain alleviation score was 9.16 on the VAS; symptom improvement began 1.33 months after starting treatment (mean value) |
| Author | Year | Study Type | JAK Inhibitor | Result |
|---|---|---|---|---|
| Yang et al. [49] | 2018 | Retrospective | Oral tofacitinib | Improvement in 8 out of 10 patients; LPPAI before and after treatment was measured in 7 patients (6.22 before treatment, 3.08 after treatment) |
| Plante et al. [50] | 2020 | Retrospective | Oral tofacitinib and topical tofacitinib | Improvement in 2 out of 3 patients on topical therapy and in 5 out of 6 patients on oral therapy |
| Moussa et al. [51] | 2022 | Retrospective | Oral baricitinib | Five of the 7 patients with LPP demonstrated an initial reduction in the median LPPAI score of 2.8 (46.5%); 3 of the 5 patients with FFA demonstrated an initial reduction in the median LPPAI score of 5.6 (83.8%) |
| Dunn et al. [52] | 2023 | Case series | Topical ruxolitinib 1.5% or oral baricitinib | Case 1 (topical ruxolitinib 1.5%): Initial LPPAI of 7; at week 12, LPPAI score of 2 |
| Case 2 (topical ruxolitinib 1.5%): Initial LPPA of 8; at week 15, LPPAI of 3 | ||||
| Case 3 (oral baricitinib): Initial LPPAI of 7; at week 4, LPPAI of 2 | ||||
| Chen et al. [53] | 2024 | Retrospective | Topical tofacitinib | Out of 38 patients, 92.1% showed an improvement |
| Gonzalez Matheus et al. [54] | 2024 | Case series | Oral tofacitinib and oral baricitinib | Clinical hair regrowth in 3 patients, reduced patch size in 1 patient and reduced itching and burning symptoms in 1 patient |
| Goodarzi et al. [55] | 2025 | Retrospective | Oral tofacitinib | Nine (12.2%) patients did not respond; 16 (21.6%) patients responded within 1–3 months; 18 (24.3%) patients responded within 3–6 months; 25 (33.8%) patients responded within 6–12 months; 6 (8.1%) patients responded within 12–24 months. |
| Williams et al. [56] | 2025 | Retrospective | Topical ruxolitinib | The average percentage reduction in LPPAI score was 34%, with 7 patients demonstrating a <25% reduction, 9 demonstrating a 25% to 75% reduction, and 4 demonstrating a >75% reduction. |
| Author | Year | Study Type | Number of Patients | JAK Inhibitor | Result |
|---|---|---|---|---|---|
| Iorizzo et al. [59] | 2021 | Case report | 1 | Tofacitinib 5 mg twice per day | Significant improvement in 6 months |
| Pünchera et al. [60] | 2022 | Case report | 1 | Baricitinib 4 mg | Complete remission in 6 months |
| Huang et al. [61] | 2023 | Case report | 1 | Tofacitinib 5 mg twice per day | Significant improvement in 6 months |
| He et al. [62] | 2023 | Case report | 1 | Baricitinib 4 mg | Complete remission in 6 months |
| He et al. [63] | 2024 | Case report | 1 | Abrocitinib 100 mg | Significant improvement in 6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didona, D.; Caposiena Caro, R.D.; Calabrese, L.; D’Onghia, M.; Galluccio, G.; Di Nicola, M.R.; Rallo, A.; Paolino, G. Use of JAK Inhibitors in Lichen Planus: An Update. Medicina 2025, 61, 1056. https://doi.org/10.3390/medicina61061056
Didona D, Caposiena Caro RD, Calabrese L, D’Onghia M, Galluccio G, Di Nicola MR, Rallo A, Paolino G. Use of JAK Inhibitors in Lichen Planus: An Update. Medicina. 2025; 61(6):1056. https://doi.org/10.3390/medicina61061056
Chicago/Turabian StyleDidona, Dario, Raffaele Dante Caposiena Caro, Laura Calabrese, Martina D’Onghia, Giulia Galluccio, Matteo Riccardo Di Nicola, Alessandra Rallo, and Giovanni Paolino. 2025. "Use of JAK Inhibitors in Lichen Planus: An Update" Medicina 61, no. 6: 1056. https://doi.org/10.3390/medicina61061056
APA StyleDidona, D., Caposiena Caro, R. D., Calabrese, L., D’Onghia, M., Galluccio, G., Di Nicola, M. R., Rallo, A., & Paolino, G. (2025). Use of JAK Inhibitors in Lichen Planus: An Update. Medicina, 61(6), 1056. https://doi.org/10.3390/medicina61061056







