Do Colorectal Serrated and Non-Serrated Adenocarcinomas Differ in Somatic Mutations and Clinicopathologic Features?
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
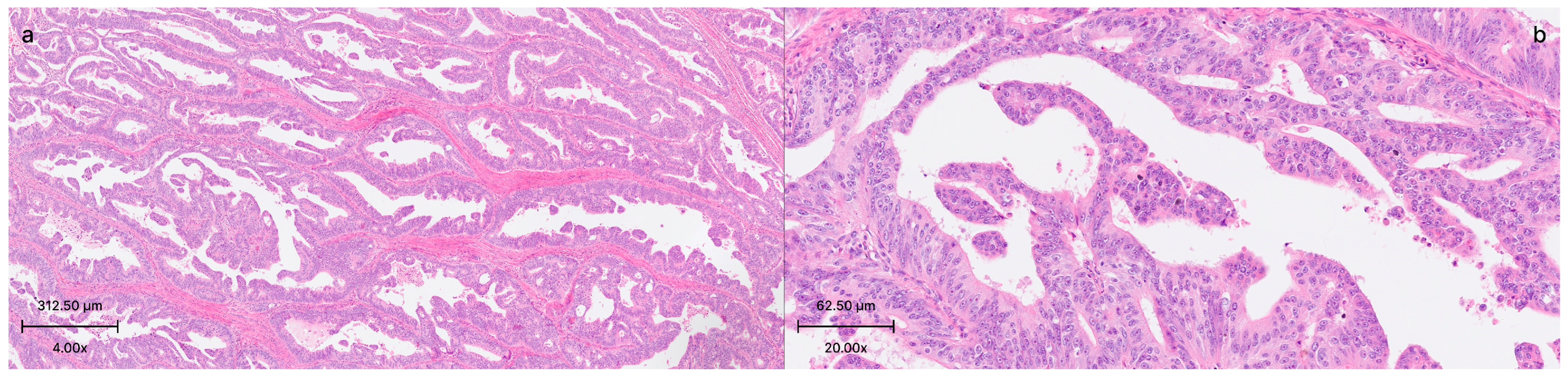
2.2. Histological and Clinical Findings
2.3. DNA/RNA Extraction
2.4. Next Generating Sequencing
2.5. Variant Detection
2.6. Statistical Analysis
2.7. In Silico Analysis
3. Results
3.1. Clinicopathological Characteristics
3.2. Mutations and Microsatellite Instability
3.3. In Silico Analysis
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| CIMP-H | Cpg Island Methylator Phenotype-High |
| CIMP-L | CpG Island Methylator Phenotype-Low |
| FFPE | Formalin-Fixed and Paraffin-Embedded |
| IHC | Immunohistochemical |
| MAPK | Mitogen-activated protein kinase |
| MSI | Microsatellite instability |
| MSI-L | Microsatellite instability-low |
| MSI-H | Microsatellite instability-high |
| MSS | Microsatellite stable |
| NGS | Next-generation sequencing |
| non-SACs | Non-serrated adenocarcinomas |
| SAC | Serrated adenocarcinoma |
| SSL | Sessile serrated lesion |
| TSA | Traditional serrated adenoma |
| WHO | World Health Organization |
References
- Pai, R.K.; Bettington, M.; Srivastava, A.; Rosty, C. An update on the morphology and molecular pathology of serrated colorectal polyps and associated carcinomas. Mod. Pathol. 2019, 32, 1390–1415. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R.; Smith, M. Sialic acid and epithelial differentiation in colorectal polyps and cancer—A morphological, mucin and lectin histochemical study. Pathology 1992, 24, 233–242. [Google Scholar] [CrossRef]
- Aust, D.E. WHO classification 2010 for the lower gastrointestinal tract: What is new? Pathologe 2011, 32, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, K.; Mäkinen, J.M.; Junttila, O.; Liakka, A.; Kyllönen, A.P.; Tuominen, H.; Karttunen, T.J.; Mäkinen, M.J. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J. Pathol. 2005, 207, 285–294. [Google Scholar] [CrossRef]
- Remo, A.; Fassan, M.; Vanoli, A.; Bonetti, L.R.; Barresi, V.; Tatangelo, F.; Gafà, R.; Giordano, G.; Pancione, M.; Grillo, F.; et al. Morphology and Molecular Features of Rare Colorectal Carcinoma Histotypes. Cancers 2019, 11, 1036. [Google Scholar] [CrossRef]
- Stefanius, K.; Ylitalo, L.; Tuomisto, A.; Kuivila, R.; Kantola, T.; Sirniö, P.; Karttunen, T.J.; Mäkinen, M.J. Frequent mutations of KRAS in addition to BRAF in colorectal serrated adenocarcinoma. Histopathology 2011, 58, 679–992. [Google Scholar] [CrossRef]
- Shida, Y.; Fujimori, T.; Tanaka, H.; Fujimori, Y.; Kimura, R.; Ueda, H.; Ichikawa, K.; Tomita, S.; Nagata, H.; Kubota, K.; et al. Clinicopathological Features of Serrated Adenocarcinoma Defined by Mäkinen in Dukes’ B Colorectal Carcinoma. Pathobiology 2012, 79, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Laiho, P.; Kokko, A.; Vanharanta, S.; Salovaara, R.; Sammalkorpi, H.; Järvinen, H.; Mecklin, J.P.; Karttunen, T.J.; Tuppurainen, K.; Davalos, V.; et al. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene 2007, 26, 312–320. [Google Scholar] [CrossRef]
- Mäkinen, M.J.; George, S.M.C.; Jernvall, P.; Mäkelä, J.; Vihko, P.; Karttunen, T.J. Colorectal carcinoma associated with serrated adenoma—Prevalence, histological features, and prognosis. J. Pathol. 2001, 193, 286–294. [Google Scholar] [CrossRef]
- Conesa-Zamora, P.; García-Solano, J.; García-García, F.; Turpin Mdel, C.; Trujillo-Santos, J.; Torres-Moreno, D.; Oviedo-Ramírez, I.; Carbonell-Muñoz, R.; Muñoz-Delgado, E.; Rodriguez-Braun, E.; et al. Expression profiling shows differential molecular pathways and provides potential new diagnostic biomarkers for colorectal serrated adenocarcinoma. Int. J. Cancer 2013, 132, 297–307. [Google Scholar] [CrossRef]
- Marquet, B.; Marchal Bressenot, A.; Fichel, C.; Bouland, N.; Barbe, C.; Bouché, O.; Kianmanesh, R.; Diebold, M.D.; Boulagnon-Rombi, C. Expression of the Serrated Markers Annexin A10 or Gremlin1 in Colonic Adenocarcinomas: Morphology and Prognostic Values. Pathol. Oncol. Res. 2020, 26, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- Hirano, D.; Urabe, Y.; Tanaka, S.; Nakamura, K.; Ninomiya, Y.; Yuge, R.; Hayashi, R.; Oka, S.; Kitadai, Y.; Shimamoto, F.; et al. Early-stage serrated adenocarcinomas are divided into several molecularly distinct subtypes. PLoS ONE 2019, 14, e0211477. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, M.J. Colorectal serrated adenocarcinoma. Histopathology 2007, 50, 131–150. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Yang, S.; Mack, C.; Xu, H.; Huang, C.S.; Mulcahy, E.; Amorosino, M.; Farraye, F.A. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am. J. Surg. Pathol. 2006, 30, 1491–1501. [Google Scholar] [CrossRef]
- De Palma, F.; D’Argenio, V.; Pol, J.; Kroemer, G.; Maiuri, M.; Salvatore, F. The Molecular Hallmarks of the Serrated Pathway in Colorectal Cancer. Cancers 2019, 11, 1017. [Google Scholar] [CrossRef]
- Bae, J.M.; Kim, J.H.; Kang, G.H. Molecular subtypes of colorectal cancer and their clinicopathologic features, with an emphasis on the serrated neoplasia pathway. Arch. Pathol. Lab. Med. 2016, 140, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Nosho, K.; Igarashi, H.; Ito, M.; Mitsuhashi, K.; Naito, T.; Yamamoto, E.; Tanuma, T.; Nomura, M.; Maguchi, H.; et al. MicroRNA-31 expression in colorectal serrated pathway progression. World J. Gastroenterol. 2014, 20, 12346–12349. [Google Scholar] [CrossRef]
- Nosho, K.; Igarashi, H.; Nojima, M.; Ito, M.; Maruyama, R.; Yoshii, S.; Naito, T.; Sukawa, Y.; Mikami, M.; Sumioka, W.; et al. Association of microRNA-31 with BRAF mutation, colorectal cancer survival and serrated pathway. Carcinogenesis 2014, 35, 776–783. [Google Scholar] [CrossRef]
- García-Solano, J.; Pérez-Guillermo, M.; Conesa-Zamora, P.; Acosta-Ortega, J.; Trujillo-Santos, J.; Cerezuela-Fuentes, P.; Mäkinen, M.J. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: Further insights into the full recognition of a new subset of colorectal carcinoma. Hum. Pathol. 2010, 41, 1359–1368. [Google Scholar] [CrossRef]
- Yılmaz, O.; Crabbe, A.; Neyaz, A.; Pankaj, A.; Lee, S.H.; Hosseini, S.; Rickelt, S.; Cerda, S.; Zhao, Q.; Leijsen, L.; et al. Clinical, pathological genetics and intratumoral immune milieu of serrated adenocarcinoma of the colon. Histopathology 2022, 81, 380–388. [Google Scholar] [CrossRef]
- g:Profiler. Available online: https://biit.cs.ut.ee/gprofiler/gost (accessed on 27 May 2025).
- Cercek, A.; Chatila, W.K.; Yaeger, R.; Walch, H.; Fernandes, G.D.S.; Krishnan, A.; Palmaira, L.; Maio, A.; Kemel, Y.; Srinivasan, P. A Comprehensive Comparison of Early-Onset and Average-Onset Colorectal Cancers. JNCI J. Natl. Cancer Inst. 2021, 113, 1683–1692. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, 269. [Google Scholar] [CrossRef]
- de Bruijn, I.; Kundra, R.; Mastrogiacomo, B.; Tran, T.N.; Sikina, L.; Mazor, T.; Li, X.; Ochoa, A.; Zhao, G.; Lai, B.; et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023, 83, 3861–3867. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qin, H.; Huang, Z.; Li, S.; Zhu, X.; He, J.; Yang, J.; Yu, X.; Yi, X. Clinical significance of programmed death ligand-1 (PD-L1) in colorectal serrated adenocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 9351–9359. [Google Scholar] [PubMed]
- García-Solano, J.; Conesa-Zamora, P.; Carbonell, P.; Trujillo-Santos, J.; Torres-Moreno, D.D.; Pagán-Gómez, I.; Rodríguez-Braun, E.; Pérez-Guillermo, M. Colorectal serrated adenocarcinoma shows a different profile of oncogene mutations, MSI status and DNA repair protein expression compared to conventional and sporadic MSI-H carcinomas. Int. J. Cancer 2012, 131, 1790–1799. [Google Scholar] [CrossRef]
- Sugai, T.; Eizuka, M.; Fujita, Y.; Kawasaki, K.; Yamamoto, E.; Ishida, K.; Yamano, H.; Suzuki, H.; Matsumoto, T. Molecular Profiling Based on KRAS/BRAF Mutation, Methylation, and Microsatellite Statuses in Serrated Lesions. Dig. Dis. Sci. 2018, 63, 2626–2638. [Google Scholar] [CrossRef]
- Kim, K.M.; Ahn, A.R.; Park, H.S.; Jang, K.Y.; Moon, W.S.; Kang, M.J.; Ha, G.W.; Lee, M.R.; Chung, M.J. Clinical significance of p53 protein expression and TP53 variation status in colorectal cancer. BMC Cancer 2022, 22, 940. [Google Scholar] [CrossRef]
- East, J.E.; Saunders, B.P.; Jass, J.R. Sporadic and Syndromic Hyperplastic Polyps and Serrated Adenomas of the Colon: Classification, Molecular Genetics, Natural History, and Clinical Management. Gastroenterol. Clin. N. Am. 2008, 37, 25–46. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Martini, M.; Molinari, F.; Sartore-Bianchi, A.; Arena, S.; Saletti, P.; De Dosso, S.; Mazzucchelli, L.; Frattini, M.; Siena, S.; et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 5705–5712. [Google Scholar] [CrossRef]
- Leggett, B.; Whitehall, V. Role of the Serrated Pathway in Colorectal Cancer Pathogenesis. Gastroenterology 2010, 138, 2088–2100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Huang, Y.C.; Hung, L.Y.; Chow, N.H.; Su, P.F.; Ho, C.L.; Tsai, H.W.; Chen, Y.L.; Lin, S.C.; Lin, B.W.; et al. Serrated adenocarcinoma morphology in colorectal mucinous adenocarcinoma is associated with improved patient survival. Oncotarget 2017, 8, 35165–35175. [Google Scholar] [CrossRef] [PubMed]

| Serrated Adenocarcinomas | Non-Serrated Adenocarcinomas | p | |
|---|---|---|---|
| Gender | 0.65 | ||
| Male | 73.9% (17/23) | 66.2% (90/136) | |
| Female | 26.1% (6/23) | 33.8% (46/136) | |
| Age | 0.72 | ||
| Mean ± SD * | 62.5 ± 15.4 | 61.6 ± 11.0 | |
| Location | 0.15 | ||
| Sigmoid colon | 27.8% (5/18) | 30% (36/120) | |
| Rectum | 27.8% (5/18) | 30% (36/120) | |
| Ascending colon | 11.1% (2/18) | 13.3% (16/120) | |
| Descending colon | 11.1% (2/18) | 8.3% (10/120) | |
| Transverse colon | 22.2% (4/18) | 3.3% (4/120) | |
| Splenic flexura | 0 | 3.3% (4/120) | |
| Hepatic flexura | 0 | 2.5% (3/120) | |
| Cecum | 0 | 9.2% (11/120) | |
| Histological Grade | 0.019 | ||
| Grade 1 | 4.3% (1/23) | 8.1% (11/136) | |
| Grade 2 | 95.7% (22/23) | 71.3% (97/136) | |
| Grade 3 | 0 | 20.6% (28/136) | |
| pT stage | 0.49 | ||
| pT1 | 0 | 1.6% (2/124) | |
| pT2 | 0 | 1.6% (2/124) | |
| pT3 | 78.9% (15/19) | 68.9% (73/124) | |
| pT4a | 10.5% (2/19) | 27.4% (34/124) | |
| pT4b | 10.5% (2/19) | 10.5% (13/124) | |
| pN stage | 0.08 | ||
| pN0 | 36.8% (7/19) | 26.6% (33/124) | |
| pN1a | 5.3% (1/19) | 14.5% (18/124) | |
| pN1b | 10.5% (2/19) | 19.4% (24/124) | |
| pN1c | 15.8% (3/19) | 2.4% (3/124) | |
| pN2a | 15.8% (3/19) | 14.5% (18/124) | |
| pN2b | 15.8% (3/19) | 22.6% (28/124) | |
| Tumor deposits | 0.25 | ||
| Present | 35.7% (5/14) | 52.3% (46/88) | |
| Absent | 64.3% (9/14) | 47.7% (42/88) | |
| Lymphovascular invasion | 0.70 | ||
| Present | 60.9% (14/23) | 56.6% (77/136) | |
| Absent | 39.1% (9/23) | 44.3% (59/136) | |
| Perineural invasion | 0.96 | ||
| Present | 30.4% (7/23) | 30.9% (42/136) | |
| Absent | 69.6% (16/23) | 69.1% (94/136) | |
| Metastasis (pM) | 0.32 | ||
| Present | 100% (12/12) | 92.2% (95/103) | |
| Absent | 0 | 7.8% (8/103) |
| Serrated Adenocarcinomas | Non-Serrated Adenocarcinomas | p | |
|---|---|---|---|
| TP53 | 61.1% (11/18) | 67.7% (67/99) | 0.58 |
| KRAS | 39.1% (9/23) | 44.9% (61/136) | 0.60 |
| PIK3CA | 26.1% (6/23) | 20.6% (28/136) | 0.58 |
| BRAF | 4.3% (1/23) | 8.1% (11/136) | >0.99 |
| NRAS | 4.3% (1/23) | 3.7% (5/136) | >0.99 |
| KIT | 4.3% (1/23) | 0 (0/136) | 0.14 |
| FBXW7 | 0 (0/1) | 15.4% (2/13) | >0.99 |
| SMAD4 | 0 (0/1) | 7.1% (1/14) | >0.99 |
| FGFR1 | 0 (0/1) | 7.1% (1/14) | >0.99 |
| CTNNB1 | 0 (0/1) | 7.1% (1/14) | >0.99 |
| ERBB3 | 0 (0/3) | 6.7% (1/15) | >0.99 |
| MAP2K1 | 0 (0/1) | 6.7% (1/15) | >0.99 |
| ERBB2 | 0 (0/23) | 4.4% (6/136) | 0.59 |
| BCOR | 0 (0/5) | 3.8% (1/26) | >0.99 |
| POLE | 0 (0/10) | 3.6% (2/56) | >0.99 |
| PTEN | 0 (0/20) | 3.3% (4/122) | >0.99 |
| BRCA2 | 0 (0/20) | 2.5% (3/121) | >0.99 |
| TERT | 0 (0/16) | 1% (1/102) | >0.99 |
| IDH1 | 0 (0/20) | 0.8% (1/121) | >0.99 |
| ROS1 | 0 (0/20) | 0.8% (1/121) | >0.99 |
| HRAS | 0 (0/20) | 0.8% (1/121) | >0.99 |
| EGFR | 0 (0/23) | 0.7% (1/136) | >0.99 |
| Microsatellite instability | MSS—81.8% (9/11) | MSS—78.8% (63/80) | 0.399 |
| MSI-L—18.2% (2/11) | MSI-L—10% (8/80) | ||
| MSI-H—0 (0/11) | MSI-H—11.3% (9/80) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagnak Yilmaz, Z.; Demir Kececi, S.; Aydin Mungan, S.; Saygin, I.; Sagol, O.; Sarioglu, S. Do Colorectal Serrated and Non-Serrated Adenocarcinomas Differ in Somatic Mutations and Clinicopathologic Features? Medicina 2025, 61, 1032. https://doi.org/10.3390/medicina61061032
Sagnak Yilmaz Z, Demir Kececi S, Aydin Mungan S, Saygin I, Sagol O, Sarioglu S. Do Colorectal Serrated and Non-Serrated Adenocarcinomas Differ in Somatic Mutations and Clinicopathologic Features? Medicina. 2025; 61(6):1032. https://doi.org/10.3390/medicina61061032
Chicago/Turabian StyleSagnak Yilmaz, Zeynep, Sibel Demir Kececi, Sevdegul Aydin Mungan, Ismail Saygin, Ozgul Sagol, and Sulen Sarioglu. 2025. "Do Colorectal Serrated and Non-Serrated Adenocarcinomas Differ in Somatic Mutations and Clinicopathologic Features?" Medicina 61, no. 6: 1032. https://doi.org/10.3390/medicina61061032
APA StyleSagnak Yilmaz, Z., Demir Kececi, S., Aydin Mungan, S., Saygin, I., Sagol, O., & Sarioglu, S. (2025). Do Colorectal Serrated and Non-Serrated Adenocarcinomas Differ in Somatic Mutations and Clinicopathologic Features? Medicina, 61(6), 1032. https://doi.org/10.3390/medicina61061032






