Metformin: A Dual-Role Player in Cancer Treatment and Prevention: A Comprehensive Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
| Cancer |
| Metformin |
| Neoplasms |
| Prevention |
| Carcinoma |
| Risk |
| Incidence |
| Bisguanides |
| Type two Diabetes |
| Hypoglycaemic agents |
| Chemotherapy |
| Metformin monotherapy |
| Mortality |
- Compliance with Reporting Guidelines:
2.2. Inclusion Criteria
- RCTs and observational studies.
- Patients exposed to metformin compared to placebo, other therapies, or no therapy.
- Outcomes: cancer incidence, mortality, or treatment response.
- Reported effect estimates (RR, OR, and HR) with 95% CI.
2.3. Exclusion Criteria
- Animal studies.
- Reviews, commentaries, and incomplete abstracts.
- Studies lacking sufficient data for effect estimates.
2.4. Study Selection and Data Extraction
- Risk of Bias Assessment
- RCTs: evaluated using the RoB 2 tool.
- Observational studies: evaluated using the ROBINS-I tool.
- Certainty of evidence: the GRADE methodology was used to assess the certainty of evidence for each major cancer outcome.
2.5. Data Synthesis and Statistical Analysis
3. Results
3.1. Results of Literature Search
3.1.1. Study Identification Through Databases and Registrations
3.1.2. Identification of Studies via Other Methods
3.1.3. Overview of Included Studies
3.2. Qualitative Analysis
3.2.1. Role of Metformin in Cancer Prevention, Risk of Cancer Development, and Cancer Treatment in All Types of Cancer
3.2.2. Metformin Role in Cancer Risk, Treatment, and Prevention in Breast Cancer
3.2.3. Metformin Role in Cancer Risk, Treatment, and Prevention in Prostate Cancer
3.2.4. Metformin Role in Cancer Risk, Treatment, and Prevention in Pancreatic Cancer
3.2.5. Metformin Role in Cancer Risk, Treatment, and Prevention in Colorectal Cancer
3.2.6. Metformin Role in Cancer Risk, Treatment and Prevention in Other Cancers
3.3. Quantitative Analysis
3.4. Results of Risk of Bias Assessment
- Sensitivity Analyses
- Subgroup Analyses
- Meta-Regression
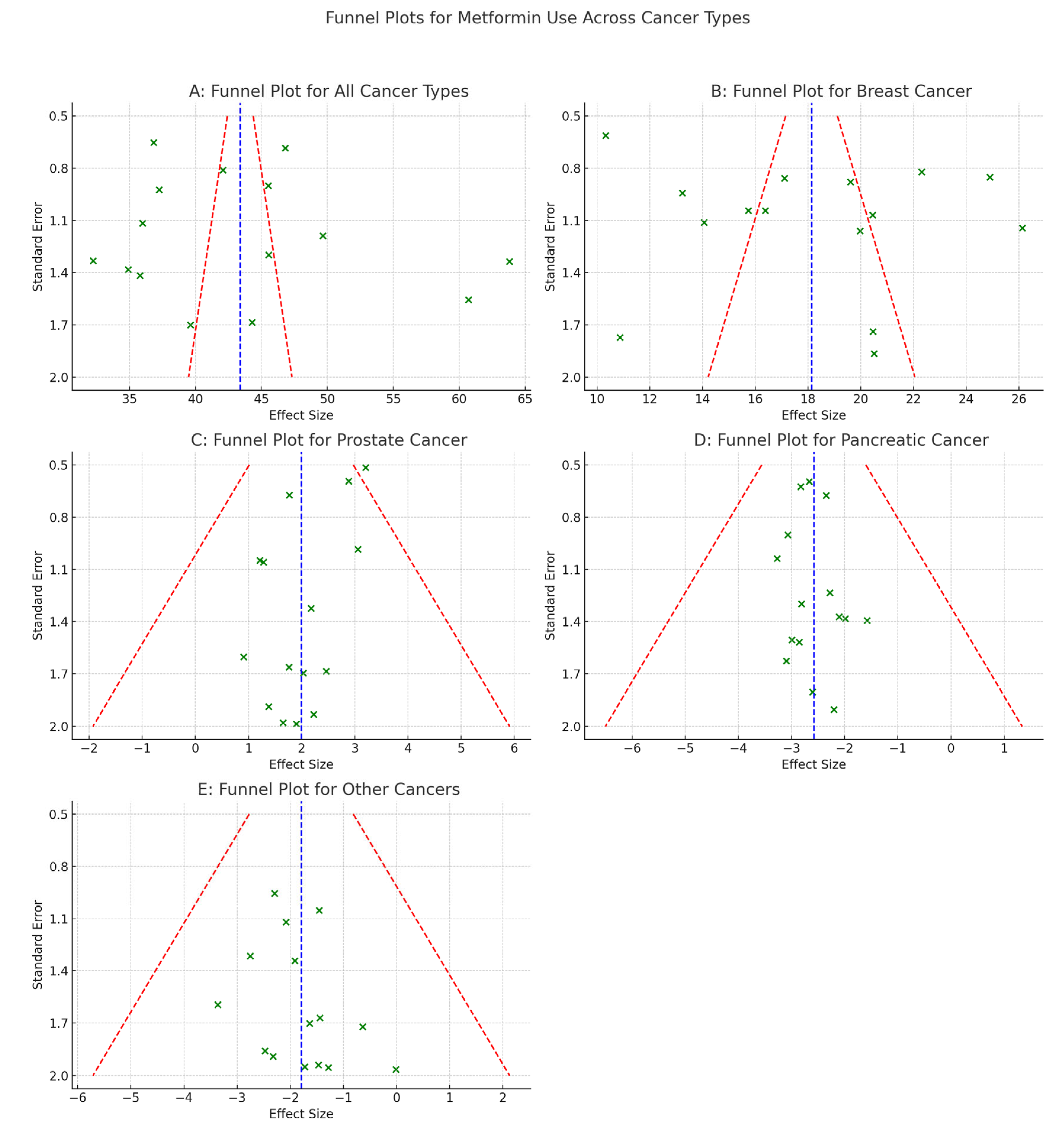
- Publication Bias
- Funnel plot asymmetry detected.
- Egger’s test significant for overall cancer (p < 0.05).
- Trim-and-fill adjustment slightly increased pooled RRs but preserved significance.
4. Discussion
Limitations of Systematic Review and Meta-Analysis
- Predominance of observational studies with residual confounding.
- Possible time-related biases.
- Inconsistent outcome definitions.
- Variability in metformin dose and exposure definitions.
- Funnel plot asymmetry suggests publication bias.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blandino, G.; Valerio, M.; Cioce, M.; Mori, F.; Casadei, L.; Pulito, C.; Sacconi, A.; Biagioni, F.; Cortese, G.; Galanti, S. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat. Commun. 2012, 3, 865. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Karube, Y.; Tanaka, H.; Osada, H.; Tomida, S.; Tatematsu, Y.; Yanagisawa, K.; Yatabe, Y.; Takamizawa, J.; Miyoshi, S.; Mitsudomi, T. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005, 96, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Essa, N.; Elgendy, M.; Gabr, A.; Alharbi, A.A.; Tashkandi, H.; Salem, H.; Harakeh, S.; Boshra, M. The efficacy of metformin as adjuvant to chemotherapy on IGF levels in non-diabetic female patients with progressive and non-progressive metastatic breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5200–5210. [Google Scholar]
- O’connor, L.; Bailey-Whyte, M.; Bhattacharya, M.; Buttera, G.; Hardell, K.N.L.; Seidenberg, A.B.; Castle, P.E.; Loomans-Kropp, H.A. Association of metformin use and cancer incidence: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2024, 116, 518–529. [Google Scholar] [CrossRef]
- Orchard, S.G.; Lockery, J.E.; Broder, J.C.; Ernst, M.E.; Espinoza, S.; Gibbs, P.; Wolfe, R.; Polekhina, G.; Zoungas, S.; Loomans-Kropp, H.A. Association of metformin, aspirin, and cancer incidence with mortality risk in adults with diabetes. JNCI Cancer Spectr. 2023, 7, pkad017. [Google Scholar] [CrossRef]
- Yoon, W.S.; Chang, J.H.; Kim, J.H.; Kim, Y.J.; Jung, T.Y.; Yoo, H.; Kim, S.H.; Ko, Y.C.; Nam, D.H.; Kim, T.M.; et al. Efficacy and safety of metformin plus low-dose temozolomide in patients with recurrent or refractory glioblastoma: A randomized, prospective, multicenter, double-blind, controlled, phase 2 trial (KNOG-1501 study). Discov. Oncol. 2023, 14, 90. [Google Scholar] [CrossRef]
- Serageldin, M.A.; Kassem, A.B.; El-Kerm, Y.; Helmy, M.W.; El-Mas, M.M.; El-Bassiouny, N.A. The Effect of Metformin on Chemotherapy-Induced Toxicities in Non-diabetic Breast Cancer Patients: A Randomised Controlled Study. Drug Saf. 2023, 46, 587–599. [Google Scholar] [CrossRef]
- Kemnade, J.O.; Florez, M.; Sabichi, A.; Zhang, J.; Jhaveri, P.; Chen, G.; Chen, A.; Miller-Chism, C.; Shaun, B.; Hilsenbeck, S.G.; et al. Phase I/II trial of metformin as a chemo-radiosensitizer in a head and neck cancer patient population. Oral. Oncol. 2023, 145, 106536. [Google Scholar] [CrossRef]
- Hu, J.; Fan, H.-D.; Gong, J.-P.; Mao, Q.-S. The relationship between the use of metformin and the risk of pancreatic cancer in patients with diabetes: A systematic review and meta-analysis. BMC Gastroenterol. 2023, 23, 50. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J.; et al. Effect of Metformin vs Placebo on Invasive Disease-Free Survival in Patients with Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Bakry, H.M.; Mansour, N.O.; ElKhodary, T.R.; Soliman, M.M. Efficacy of metformin in prevention of paclitaxel-induced peripheral neuropathy in breast cancer patients: A randomized controlled trial. Front. Pharmacol. 2023, 14, 1181312. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bae, Y.-J.; Kang, H.-T. Metformin Use May Increase Risk of Pancreatic Cancer in Diabetic Women: An Analysis of the Korean National Health Insurance Service-National Health Screening Cohort Database. Korean J. Fam. Med. 2022, 43, 327. [Google Scholar] [CrossRef] [PubMed]
- Rabea, H.; Hassan, A.; Elberry, A.A. Metformin as an Adjuvant Treatment in Non-Diabetic Metastatic Breast Cancer. Bahrain Med. Bull. 2021, 43, 477–481. [Google Scholar]
- Pujalte Martin, M.; Borchiellini, D.; Thamphya, B.; Guillot, A.; Paoli, J.B.; Besson, D.; Hilgers, W.; Priou, F.; El Kouri, C.; Hoch, B.; et al. TAXOMET: A French Prospective Multicentric Randomized Phase II Study of Docetaxel Plus Metformin Versus Docetaxel Plus Placebo in Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2021, 19, 501–509. [Google Scholar] [CrossRef]
- Bever, K.M.; Borazanci, E.H.; Thompson, E.A.; Durham, J.N.; Pinero, K.; Jameson, G.S.; Vrana, A.; Liu, M.; Wilt, C.; Wu, A.A.; et al. An exploratory study of metformin with or without rapamycin as maintenance therapy after induction chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncotarget 2020, 11, 1929–1941. [Google Scholar] [CrossRef]
- Hosio, M.; Urpilainen, E.; Marttila, M.; Hautakoski, A.; Arffman, M.; Sund, R.; Puistola, U.; Läärä, E.; Jukkola, A.; Karihtala, P. Association of antidiabetic medication and statins with breast cancer incidence in women with type 2 diabetes. Breast Cancer Res. Treat. 2019, 175, 741–748. [Google Scholar] [CrossRef]
- Kuo, Y.J.; Sung, F.C.; Hsieh, P.F.; Chang, H.P.; Wu, K.L.; Wu, H.C. Metformin reduces prostate cancer risk among men with benign prostatic hyperplasia: A nationwide population-based cohort study. Cancer Med. 2019, 8, 2514–2523. [Google Scholar] [CrossRef]
- Pimentel, I.; Lohmann, A.E.; Ennis, M.; Dowling, R.J.O.; Cescon, D.; Elser, C.; Potvin, K.R.; Haq, R.; Hamm, C.; Chang, M.C.; et al. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast 2019, 48, 17–23. [Google Scholar] [CrossRef]
- Kim, J.; Han, W.; Kim, E.-K.; Jung, Y.; Kim, H.-A.; Chae, S.M.; Lee, E.S.; Ahn, S.-H.; Kim, T.H.; Jeong, J.; et al. Phase II randomized study of neoadjuvant metformin plus letrozole versus placebo plus letrozole for ER-positive postmenopausal breast cancer [METEOR Study]. J. Clin. Oncol. 2019, 37 (Suppl. S15), 576. [Google Scholar] [CrossRef]
- Ramos-Penafiel, C.; Olarte-Carrillo, I.; Ceron-Maldonado, R.; Rozen-Fuller, E.; Kassack-Ipina, J.J.; Melendez-Mier, G.; Collazo-Jaloma, J.; Martinez-Tovar, A. Effect of metformin on the survival of patients with ALL who express high levels of the ABCB1 drug resistance gene. J. Transl. Med. 2018, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Cheng, W.C.; Liu, D.; Gaude, E.; Haider, S.; Metcalf, T.; Patel, N.; Teoh, E.J.; Gleeson, F.; Bradley, K.; et al. Integrated Pharmacodynamic Analysis Identifies Two Metabolic Adaption Pathways to Metformin in Breast Cancer. Cell Metab. 2018, 28, 679–688.e4. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.; Satkunam, M.; Pond, G.R.; Steinberg, G.R.; Blandino, G.; Schünemann, H.J.; Muti, P. Association of metformin with breast cancer incidence and mortality in patients with type II diabetes. Cancer Epidemiol. Biomark. Prev. 2018, 27, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Tsai, H.-L.; Kung, Y.-T.; Yeh, Y.-S.; Huang, C.-W.; Ma, C.-J.; Chiu, H.-C.; Wang, J.-Y. Dose-dependent relationship between metformin and colorectal cancer occurrence among patients with Type 2 Diabetes—A nationwide cohort study. Transl. Oncol. 2018, 11, 535–541. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.; Chun, K.H.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kim, Y.S.; Woo, J.-T.; Nam, M.-S.; Baik, S.H. Metformin reduces the risk of cancer in patients with type 2 diabetes: An analysis based on the Korean National Diabetes Program Cohort. Medicine 2018, 97, e0036. [Google Scholar] [CrossRef]
- Galsky, M.D.; Shahin, M.; Olson, A.; Shaffer, D.R.; Gimpel-Tetra, K.; Tsao, C.-K.; Baker, C.; Leiter, A.; Holland, J.; Sablinski, T.; et al. Telemedicine-enabled clinical trial of metformin in patients (pts) with biochemically-recurrent prostate cancer (PCa). J. Clin. Oncol. 2017, 35 (Suppl. S6), 243. [Google Scholar] [CrossRef]
- Franchi, M.; Asciutto, R.; Nicotra, F.; Merlino, L.; La Vecchia, C.; Corrao, G.; Bosetti, C. Metformin, other antidiabetic drugs, and endometrial cancer risk: A nested case–control study within Italian healthcare utilization databases. Eur. J. Cancer Prev. 2017, 26, 225–231. [Google Scholar] [CrossRef]
- Sonnenblick, A.; Agbor-Tarh, D.; Bradbury, I.; Di Cosimo, S.; Azim, H.A.; Fumagalli, D., Jr.; Sarp, S.; Wolff, A.C.; Andersson, M.; Kroep, J.; et al. Impact of Diabetes, Insulin, and Metformin Use on the Outcome of Patients with Human Epidermal Growth Factor Receptor 2-Positive Primary Breast Cancer: Analysis From the ALTTO Phase III Randomized Trial. J. Clin. Oncol. 2017, 35, 1421–1429. [Google Scholar] [CrossRef]
- Häggström, C.; Van Hemelrijck, M.; Zethelius, B.; Robinson, D.; Grundmark, B.; Holmberg, L.; Gudbjörnsdottir, S.; Garmo, H.; Stattin, P. Prospective study of Type 2 diabetes mellitus, anti-diabetic drugs and risk of prostate cancer. Int. J. Cancer 2017, 140, 611–617. [Google Scholar] [CrossRef]
- Calip, G.S.; Yu, O.; Elmore, J.G.; Boudreau, D.M. Comparative safety of diabetes medications and risk of incident invasive breast cancer: A population-based cohort study. Cancer Causes Control 2016, 27, 709–720. [Google Scholar] [CrossRef]
- Chak, A.; Buttar, N.S.; Foster, N.R.; Seisler, D.K.; Marcon, N.; Schoen, R.E.; Cruz-Correa, M.; Falk, G.W.; Sharma, P.; Hur, C.; et al. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 2015, 13, 665–672.e4. [Google Scholar] [CrossRef] [PubMed]
- Kordes, S.; Pollak, M.N.; Zwinderman, A.H.; Mathot, R.A.; Weterman, M.J.; Beeker, A.; Punt, C.J.; Richel, D.J.; Wilmink, J.W. Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015, 16, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Kok, V.C.; Chien, C.-H.; Horng, J.-T.; Tsai, J.J. Cancer risk in patients aged 30 years and above with type 2 diabetes receiving antidiabetic monotherapy: A cohort study using metformin as the comparator. Ther. Clin. Risk Manag. 2015, 11, 1315–1323. [Google Scholar] [PubMed]
- Braghiroli, M.I.; de Celis Ferrari, A.C.; Pfiffer, T.E.; Alex, A.K.; Nebuloni, D.; Carneiro, A.S.; Caparelli, F.; Senna, L.; Lobo, J.; Hoff, P.M.; et al. Phase II trial of metformin and paclitaxel for patients with gemcitabine-refractory advanced adenocarcinoma of the pancreas. Ecancermedicalscience 2015, 9, 563. [Google Scholar] [CrossRef]
- Kalinsky, K.; Crew, K.D.; Refice, S.; Xiao, T.; Wang, A.; Feldman, S.M.; Taback, B.; Ahmad, A.; Cremers, S.; Hibshoosh, H.; et al. Presurgical trial of metformin in overweight and obese patients with newly diagnosed breast cancer. Cancer Investig. 2014, 32, 150–157. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, S.; Cho, S.J.; Park, J.H.; Choi, I.J.; Lee, Y.J.; Lee, E.; Kook, M.C.; Kim, C.; Ryu, K. Long-term metformin use reduces gastric cancer risk in type 2 diabetics without insulin treatment: A nationwide cohort study. Aliment. Pharmacol. Ther. 2014, 39, 854–863. [Google Scholar] [CrossRef]
- Preston, M.A.; Riis, A.H.; Ehrenstein, V.; Breau, R.H.; Batista, J.L.; Olumi, A.F.; Mucci, L.A.; Adami, H.O.; Sørensen, H.T. Metformin use and prostate cancer risk. Eur. Urol. 2014, 66, 1012–1020. [Google Scholar] [CrossRef]
- Miranda, V.C.; Faria, L.D.; Braghiroli, M.I.F.M.; Jacobs, M.; Sabbaga, J.; Hoff, P.M.; Riechelmann, R.P. A phase II trial of metformin and fluorouracil (MetFU) for patients (pts) with metastatic colorectal cancer (mCRC) refractory to standard treatment. J. Clin. Oncol. 2014, 32 (Suppl. S3), 601. [Google Scholar] [CrossRef]
- Bodmer, M.; Becker, C.; Meier, C.; Jick, S.S.; Meier, C.R. Use of metformin is not associated with a decreased risk of colorectal cancer: A case–control analysis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 280–286. [Google Scholar] [CrossRef]
- Romero, I.L.; McCormick, A.; McEwen, K.A.; Park, S.; Karrison, T.; Yamada, S.D.; Pannain, S.; Lengyel, E. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet. Gynecol. 2012, 119, 61. [Google Scholar] [CrossRef]
- He, X.; Esteva, F.; Ensor, J.; Hortobagyi, G.; Lee, M.-H.; Yeung, S.-C. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann. Oncol. 2012, 23, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- He, X.-X.; Tu, S.; Lee, M.-H.; Yeung, S.-C. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann. Oncol. 2012, 22, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-W.; Liao, K.-F.; Chen, P.-C.; Tsai, P.-Y.; Hsieh, D.P.H.; Chen, C.-C. Antidiabetes drugs correlate with decreased risk of lung cancer: A population-based observation in Taiwan. Clin. Lung Cancer 2012, 13, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Geraldine, N.; Marc, A.; Carla, T.; Chantal, M.; Stefaan, B.; Welcome, W.; Frank, B. Relation between diabetes, metformin treatment and the occurrence of malignancies in a Belgian primary care setting. Diabetes Res. Clin. Pract. 2012, 97, 331–336. [Google Scholar] [CrossRef]
- Bosco, J.L.F.; Antonsen, S.; Sørensen, H.T.; Pedersen, L.; Lash, T.L. Metformin and incident breast cancer among diabetic women: A population-based case–control study in Denmark. Cancer Epidemiol. Biomark. Prev. 2011, 20, 101–111. [Google Scholar] [CrossRef]
- Monami, M.; Colombi, C.; Balzi, D.; Dicembrini, I.; Giannini, S.; Melani, C.; Vitale, V.; Romano, D.; Barchielli, A.; Marchionni, N. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 2011, 34, 129–131. [Google Scholar] [CrossRef]
- Chen, T.M.; Lin, C.C.; Huang, P.T.; Wen, C.F. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J. Gastroenterol. Hepatol. 2011, 26, 858–865. [Google Scholar] [CrossRef]
- Lee, M.-S.; Hsu, C.-C.; Wahlqvist, M.L.; Tsai, H.-N.; Chang, Y.-H.; Huang, Y.-C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011, 11, 20. [Google Scholar] [CrossRef]
- Bodmer, M.; Meier, C.; Krähenbühl, S.; Jick, S.S.; Meier, C.R. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 2010, 33, 1304–1308. [Google Scholar] [CrossRef]
- Donadon, V.; Balbi, M.; Mas, M.D.; Casarin, P.; Zanette, G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010, 30, 750–758. [Google Scholar] [CrossRef]
- Bowker, S.; Yasui, Y.; Veugelers, P.; Johnson, J. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: Assessing effects of time-varying exposure. Diabetologia 2010, 53, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Libby, G.; Donnelly, L.A.; Donnan, P.T.; Alessi, D.R.; Morris, A.D.; Evans, J.M. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care 2009, 32, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Stanford, J.L. Metformin use and prostate cancer in Caucasian men: Results from a population-based case–control study. Cancer Causes Control 2009, 20, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.J.; Poole, C.D.; Gale, E. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009, 52, 1766–1777. [Google Scholar] [CrossRef]
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006, 29, 254–258. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, P.; Dai, H.; Deng, Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim. Care Diabetes 2021, 15, 52–58. [Google Scholar] [CrossRef]
- Galal, M.A.; Al-Rimawi, M.; Hajeer, A.; Dahman, H.; Alouch, S.; Aljada, A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. Int. J. Mol. Sci. 2024, 25, 4083. [Google Scholar] [CrossRef]
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.; Pellegrini, F.; Nicolucci, A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS ONE 2013, 8, e71583. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Zheng, Z.-J.; Kan, H.; Song, Y.; Cui, W.; Zhao, G.; Kip, K.E. Reduced Risk of Colorectal Cancer with Metformin Therapy in Patients with Type 2 Diabetes: A meta-analysis. Diabetes Care 2011, 34, 2323–2328. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, F.; Liu, J.; Xu, J.; Wu, Q.; Li, X. The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: A meta-analysis. J. Clin. Pharm. Ther. 2020, 45, 783–792. [Google Scholar] [CrossRef]
- Farmer, R.E.; Ford, D.; Forbes, H.J.; Chaturvedi, N.; Kaplan, R.; Smeeth, L.; Bhaskaran, K. Metformin and cancer in type 2 diabetes: A systematic review and comprehensive bias evaluation. Int. J. Epidemiol. 2017, 46, 728–744. [Google Scholar] [CrossRef]

| Authors with Year | Study Design | Sample Size | Country | Comparison Groups | Outcome | Conclusion | Relative Risk Or Odds Ratio (95 Percent Confidence Interval) |
|---|---|---|---|---|---|---|---|
| Orchard S.G. and associates in 2023 [6] | Cohort | 2045 | Australia USA | Metformin users Metformin aspirin users | Any cancer/cancer mortality | Metformin consumption was related to reduced risk of cancer among older persons having diabetes who lived in community. | 0.70 (0.53–0.92) |
| Yoon W.S. and associates in 2023 [7] | Clinical trial phase II | 92 | South Korea. | Metformin + temozolomide versus placebo | Glioblastoma (GBM) treatment | No therapeutic advantage in refractory GBM, although it was tolerated effectively. | 0.98 (0.86–1.10) |
| Serageldin M.A. and associates in 2023 [8] | RCT Phase II | 70 | Egypt | Metformin + AC-T versus placebo | Breast cancer treatment | Better control of chemotherapy-induced toxicities. | 1.23 (0.90–1.46) |
| Kemnade J.O. and associates in 2023 [9] | Clinical trial phase I/II | 26 | USA | Metformin + cisplatin-based chemoradiation anti-cancer treatment | Head and neck cancer treatment | The efficacy of metformin for use as a chemo-radiosensitizer is questionable due to the small number of participants. | 1.34 (1.13–1.54) |
| Hu J. and associates in 2023 [10] | Cohort | 2367 | China | Metformin non-users Metformin users | Pancreatic cancer | Metformin consumers who suffer from diabetes can lower their chances of pancreatic cancer in comparison to those who do not take the medication. | 0.83 (0.70–0.99) |
| Goodwin P.J. and associates in 2023 [11] | Phase III clinical trial | 3649 | USA, Canada, Switzerland | Metformin monotherapy for cancer treatment | Carcinoma of breast treatment | The DFS was not improved by metformin in vulnerable surgically manageable BC. | 0.99 (0.90–1.00) |
| Bakry H.M. and associates in 2023 [12] | Phase II clinical trial | 76 | Egypt | Metformin + paclitaxel | Treatment of carcinoma of breast | Outstanding defense against PN caused by paclitaxel. | 1.45 (1.23–1.65) |
| Kim J. and associates in 2022 [13] | Cohort | 2432 | Republic of Korea | DM patients taking metformin DM patients not taking metformin Non-DM patients | Pancreatic cancer | Compared to women who have diabetes who do not take metformin, women having diabetes who take metformin are more likely to develop pancreatic cancer. On the other hand, men with diabetes mellitus (DM) who employ metformin are just as likely to acquire cancer of the pancreas as those who do not. | 1.127 (0.660–1.934) and 2.870 (1.014–7.753) |
| Rabea H. and associates in 2021 [14] | RCT phase II | 50 | Bahrain | Metformin + gemcitabine | Metastatic breast carcinoma treatment | Superior radiologic RR and, more importantly, negligible OS as well as PFS. | 1.34 (1.13–1.54) |
| Pujalte Martin M. and associates in 2021 [15] | Phase II clinical trial | 99 | France | Metformin + docetaxel | Prostate cancer treatment | Failed to improve the outcome. | 0.99 (0.90–1.10) |
| Bever K.M. and associates in 2020 [16] | 22 | USA | Metformin + rapamycin versus placebo | Pancreatic adenocarcinoma treatment | A sound, acceptable illness with a remarkably extended lifespan was attained. | 1.56 (1.36–1.76) | |
| Hoiso M. and associates in 2019 [17] | Cohort | 2300 | Finland | Metformin users, other forms of antidiabetic medication users (ADM) and statins users | Breast cancer prevention | There was no proof that the frequency of breast cancer in females suffering from T2D was correlated with the administration of statins or metformin. A marginally higher frequency of breast cancer among insulin users was there. | 0.98 (0.90–1.06) |
| Kuo Y.J. and associates in 2019 [18] | Cohort | 5812 | China | Metformin users Non-metformin users | Prostate cancer prevention | For men who have diabetes and BPH, taking metformin may lower their risk of acquiring prostate cancer. | 0.70 (0.50–0.97) |
| Pimentel I. and associates in 2019 [19] | Clinical trial phase II | 40 | Canada | Metformin + anthracycline versus placebo | Metastatic breast cancer Treatment | There was negligible impact on survival. | 1.01 (0.91–1.21) |
| Kim J. and associates in 2019 [20] | Clinical trial phase II | 23 | South Korea | Metformin + letrozole versus placebo | ER-positive breast cancer treatment | Acceptable response among patients. | 1.10 (0.90–1.30) |
| Ramos-Penafiel C. and associates in 2018 [21] | Phase II clinical trial | 102 | Mexico | Metformin monotherapy for cancer treatment | All types of cancer treatment | Metformin + chemotherapy is beneficial for those with elevated ABCB1 gene activity. | 1.21 (1.00–1.41) |
| Lord S.R. and associates in 2018 [22] | Phase II clinical trial | 2342 | USA | Metformin monotherapy for cancer treatment | Breast cancer treatment | Reduced risk of BC. | 1.14 (0.94–1.24) |
| Tang G.S. and associates in 2018 [23] | Cohort | 2013 | Canada | Metformin users Other antidiabetic drug users Non-metformin users Insulin users | Breast cancer/BC all-cause mortality | Individuals with T2D experiencing breast cancer may have a higher cumulative survival rate if they take metformin. There was no discernible influence of metformin on incidence of carcinoma of the breast. | 0.975 (0.772–1.232) / 0.653 (0.489–0.874) |
| Chang Y.T. and associates in 2018 [24] | Cohort | 47,597 | Taiwan | Metformin users Non-metformin users | Colorectal cancer | According to this research, Taiwanese individuals suffering from T2D who employed metformin had a dose-sensitive, substantially lower colorectal cancer risk. | 0.37 (0.30–0.45) 0.62 (0.50–0.76) |
| Kim H.J. and associates in 2018 [25] | Cohort | 1918 | South Korea | Metformin users Non-metformin users | Any cancer | According to the results of this research, persons who are metformin users who have T2D had a reduced likelihood of cancer. | 0.524 (0.319–0.827) |
| Galsky M.D. and associates in 2017 [26] | Clinical trial | 15 | USA | Metformin monotherapy for cancer treatment versus placebo | Prostate cancer treatment | Metformin showed little anti-PCa action and seemed tp be tolerated satisfactorily. | 1.46 (1.26–1.66) |
| Franchi M. and associates in 2017 [27] | Nested case-control | 376 | Italy | Metformin users, sulfonylurea users, and insulin users Other antidiabetic drugs | Endometrial cancer | Research shows that danger of endometrial cancer was not significantly impacted by metformin usage, insulin usage, or other antidiabetic medications. | 0.97 (0.81–1.34) |
| Sonnenblick A. and associates in 2017 [28] | Clinical trial phase III | 8381 | Israel | Metformin + trastuzumab versus placebo | Breast cancer treatment | Reduced the poor prognosis, primarily for carcinoma of the breast that is HR-positive and HER2-positive. | 1.38 (1.17–1.56) |
| Häggström C. and associates in 2016 [29] | Cohort | 6128 | Sweden | Metformin users, sulfonylurea users, and insulin users | Prostate cancer | The findings provide no evidence in favor of the recently proposed theory that metformin prevents prostate cancer. The findings do, however, provide some evidence for negative correlation among risk of prostate cancer as well as level of severity of T2DM. | 0.97 (0.78–1.20) |
| Calip G.S. and associates in 2016 [30] | Cohort | 2050 | USA | Metformin users, sulfonylureas users, and insulin users | Breast cancer | The results of the research are inconsistent with the formerly postulated reduced likelihood of breast cancer associated with taking metformin or the higher risk associated with insulin utilization. | 0.96 (0.52–1.78) |
| Chak A. and associates in 2015 [31] | Clinical trial | 74 | USA | Metformin monotherapy for cancer treatment versus Placebo | Barret’s esophagus treatment | No significant change in pS6K levels. | 9.99 (0.90–1.11) |
| Kordes S. and associates in 2015 [32] | Clinical trial phase II | 121 | Netherland | Metformin + gemcitabine erlotinib versus placebo | Pancreatic cancer treatment | No additional outcome improvement. | 0.97 (0.87–1.07) |
| Chen Y.C. and associates in 2015 [33] | Cohort | 7325 | Taiwan | Metformin monotherapy Sulfonylurea monotherapy | Any cancer | In contrast to sulfonylurea monotherapy, metformin monotherapy could have been associated with lower risk of developing cancer. | 1.37 (1.14–1.68) |
| Braghiroli M.I. and associates in 2015 [34] | Clinical trial phase II | 24 | Brazil | Metformin + paclitaxel versus placebo | Metastatic pancreatic cancer treatment | Patients’ insufficient tolerance and lack of prognostic significance. | 0.67 (0.44–0.88) |
| Kalinsky K. and associates in 2014 [35] | Clinical trial | 35 | USA | Metformin monotherapy for cancer treatment | Breast cancer | Although there were no alterations in proliferating activity, there was a decrease in pertinent biomarkers. | 1.23 (1.13–1.34) |
| Kim Y.I. and associates in 2014 [36] | Cohort | 100,000 | South Korea | Metformin users and non-metformin users in patients not taking regular insulin Individuals on regular diabetes medications who use metformin and those who do not | Cancer of stomach | In people having T2D without insulin, taking metformin for more than three years is linked to markedly lower incidence of stomach cancer. | 0.58 (0.38–0.88) |
| Preston M.A. and associates in 2014 [37] | Nested case-control | 6013 | Denmark | Metformin users Other hypoglycaemic users Insulin users | Prostate cancer | Although people with diabetes taking alternative oral hypoglycemics did not exhibit lower chance of PCa development, usage of metformin was linked to a lower risk. | 0.86 (0.76–0.98) |
| Miranda V.C. and associates in 2014 [38] | Clinical trial phase III | 50 | Brazil | Metformin + fluorouracil, leucovorin versus placebo | Refractory metastatic colorectal cancer | Anticancer activity and better response to treatments. | 1.46 |
| Bodmer M. and associates in 2012 [39] | Nested case-control | 1920 | UK (United Kingdom) | Metformin users Non-metformin users | Colorectal cancer and pancreatic cancer | Consumption of metformin was connected to an elevated likelihood of developing colorectal cancer among men. Insulin or sulfonylurea use was not linked to a changed likelihood of developing colorectal cancer. | 1.44 (1.09–2.0) |
| Romero I.L. and associates in 2012 [40] | Cohort | 341 | USA | Metformin users Non-metformin users | Ovarian cancer progression/all-cause mortality | Individuals suffering from ovarian cancer who have T2D who took metformin had a prolonged survival rate without progression in this group of individuals having ovarian cancer. | 0.39 (0.17–0.91) / 0.44 (0.17–1.20) |
| He X., Esteva F. and associates, 2012 [41] | Cohort | 1983 | USA | Metformin users and thiazolidinedione users | Breast: all-cause mortality/cancer mortality | In people with diabetes with stage 2 HER2+ breast cancer, those who utilize metformin, as well as thiazolidinediones, had more favorable clinical results than those who do not. The long-term outcome of these individuals may be impacted by the antidiabetic medication selection. | 0.53 (0.29–0.98) / 0.48 (0.25–0.91) |
| He X.-X., Tu S. and associates, 2011 [42] | Cohort | 250 | USA | Metformin users Non-metformin users | Colorectal cancer: all-cause mortality/cancer mortality | Diabetes-suffering CRC patients who take metformin have decreased risk of both general along with CRC-specific mortality. | 0.67 (0.46–0.99) / 0.67 (0.49–0.93) |
| Lai S.W. and associates in 2012 [43] | Cohort | 98,120 | Taiwan | Metformin users, thiazolidinediones users, or alpha-glucosidase inhibitors users | Lung cancer | Although probability of lung cancer is not elevated in those with DM, the use of ADM would significantly reduce it. | 0.56 (0.33–0.95) |
| Geraldine N. and associates in 2012 [44] | Cohort | 90 | Belgium | Metformin and other antidiabetic drugs users and non-antidiabetic drugs users | Any cancer | Compared to people lacking diabetes, those who have the disease have a higher risk of acquiring cancer. Furthermore, metformin and other ADMs were linked to decreased likelihood of cancer in males with diabetes. | 0.21 (0.04–0.83) |
| Bosco J.L.F. and associates in 2011 [45] | Nested case-control | 2323 | Denmark | Metformin users versus hormone replacement therapy | Breast cancer | For women having T2D who are perimenopausal or postmenopausal, metformin may offer safeguards against breast cancer. | 0.82 (0.64–0.97) |
| Monami M. and associates in 2011 [46] | Nested case-control | 1340 | Italy | Metformin users and sulfonylureas users | Any cancer | Another compelling argument for continuing metformin therapy among individuals receiving insulin treatment may be a reduced likelihood of cancer. | 0.29 (0.14–0.58) |
| Chen T.M. and associates in 2011 [47] | Cohort | 135 | Taiwan | Metformin users Non-metformin users | Liver cancer | Contrary to patients not receiving metformin medication, diabetic individuals who have HCC receiving RFA who took the metformin medication had a better life expectancy rate. | 0.25 (0.08–0.9) |
| Lee M.S. and associates in 2011 [48] | Cohort | 800,000 | Taiwan | Metformin users Non-metformin users | Any cancer | When diabetes is controlled, metformin can lower the frequency of a number of GIT cancers. | 0.13 (0.09–0.20) |
| Bodmer M. and associates in 2010 [49] | Nested case-control | 2621 | United Kingdom (UK) | Metformin users Non-metformin users | Breast cancer | Long-term use of metformin by women who have T2D was linked to a decreased chance of developing breast cancer. | 0.45 (0.23–0.83) |
| Dandon V. and associates in 2010 [50] | Case-control | 1236 | Italy | Metformin users, sulfonylurea users, and insulin users | Liver cancer | Metformin medication appears to demonstrate an inhibitory impact on the onset of HCC and is linked to decreased risk of HCC among DM2 individuals. | 0.16 (0.05–0.52) |
| Bowker S. and associates in 2010 [51] | Cohort | 10,309 | Canada | Metformin users, sulfonylurea users, and insulin usedrs | Cancer mortality | Metformin treatment is linked to a lower likelihood of cancer events than sulfonylurea treatment. | 0.81 (0.66–0.99) |
| Libby G. and associates in 2009 [52] | Cohort | 8170 | United Kingdom | Metformin users and metformin users | Any cancer | Using metformin may lower likelihood of acquiring cancer. | 0.64 (0.54–0.76) |
| Wright J.L. and associates in 2009 [53] | Case-control | 6101 | USA | Metformin users and non-metformin users | Prostate cancer | In Caucasians, taking metformin was associated with a somewhat substantial reduction in the general likelihood of PCa. | 0.57 (0.33–1.11) |
| Currie C.J. and associates in 2009 [54] | Cohort | 62,809 | UK | Monotherapy with metformin or sulfonylurea, combined therapy (metformin plus sulfonylurea) or insulin | Any cancer | Malignancies were more common in people taking insulin or secretagogues of insulin than in people on metformin; the majority of this increased risk was eliminated when metformin was used in conjunction with insulin. | 0.56 (0.44–0.67) |
| Bowker S.L. and associates in 2006 [55] | Cohort | 10,309 | Canada | Metformin users and sulfonylurea monotherapy users | Cancer mortality | Patients experiencing T2D who were subjected to exogenous insulin and sulfonylureas were at considerably higher risk of dying from cancer than those who were administered metformin. | 0.82 (0.61–0.91) |
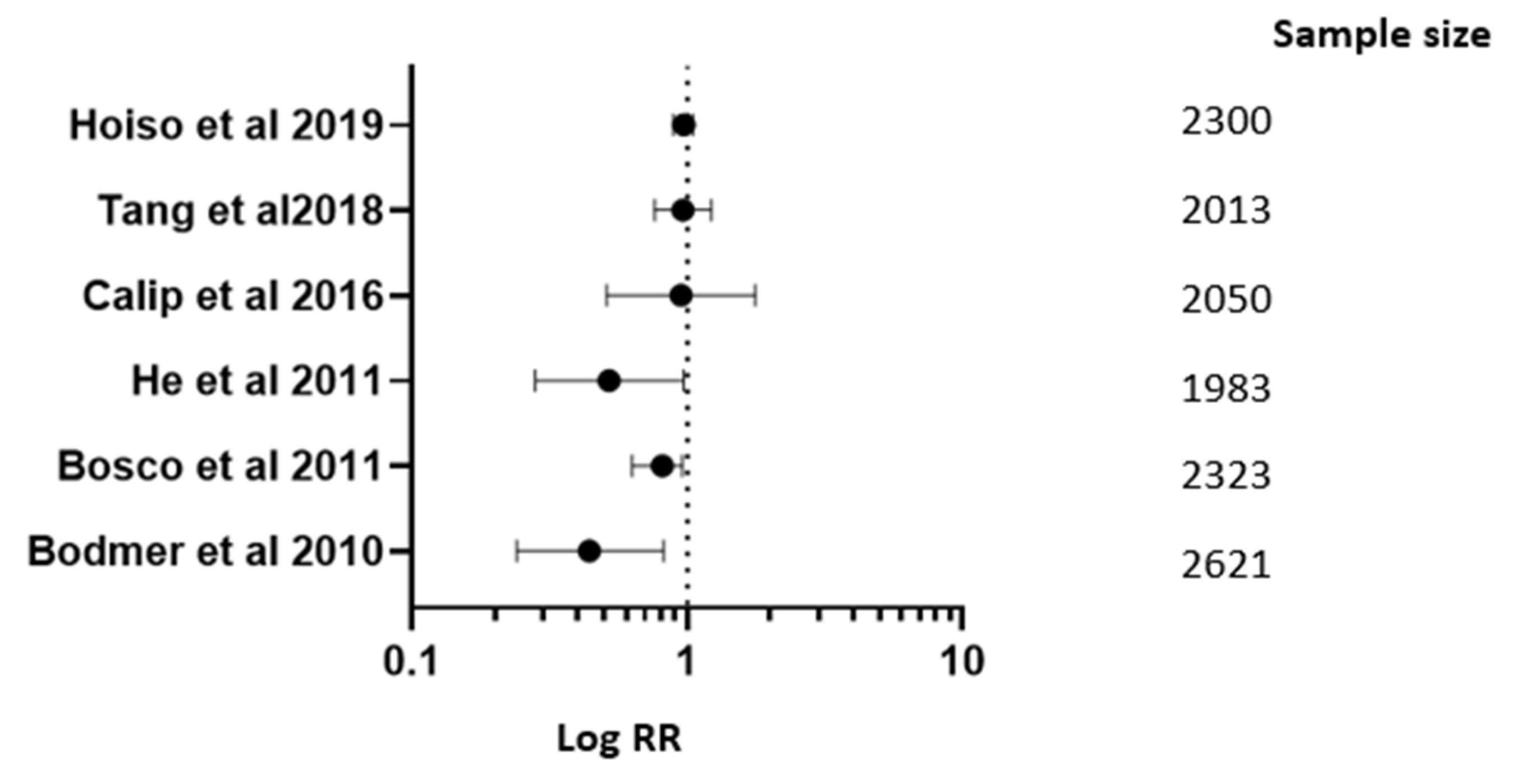
| Studies | RR; 95% CI |
|---|---|
| Hoiso M. and associates in 2019 [17] | RR 0.98; 95% CI (0.90–1.06) |
| Tang G.S. and associates in 2018 [23] | RR 0.975; 95% CI (0.772–1.232) |
| Calip G.S. and associates in 2016 [30] | RR 0.96; 95% CI (0.52–1.78) |
| Bosco J.L.F. and associates in 2011 [45] | RR 0.82; 95% CI (0.64–0.97) |
| Bodmer M. and associates in 2010 [49] | RR 0.45; 95% CI (0.23–0.83) |
| He and associates in 2012 [41] | RR 0.53; 95% CI (0.29–0.98) |
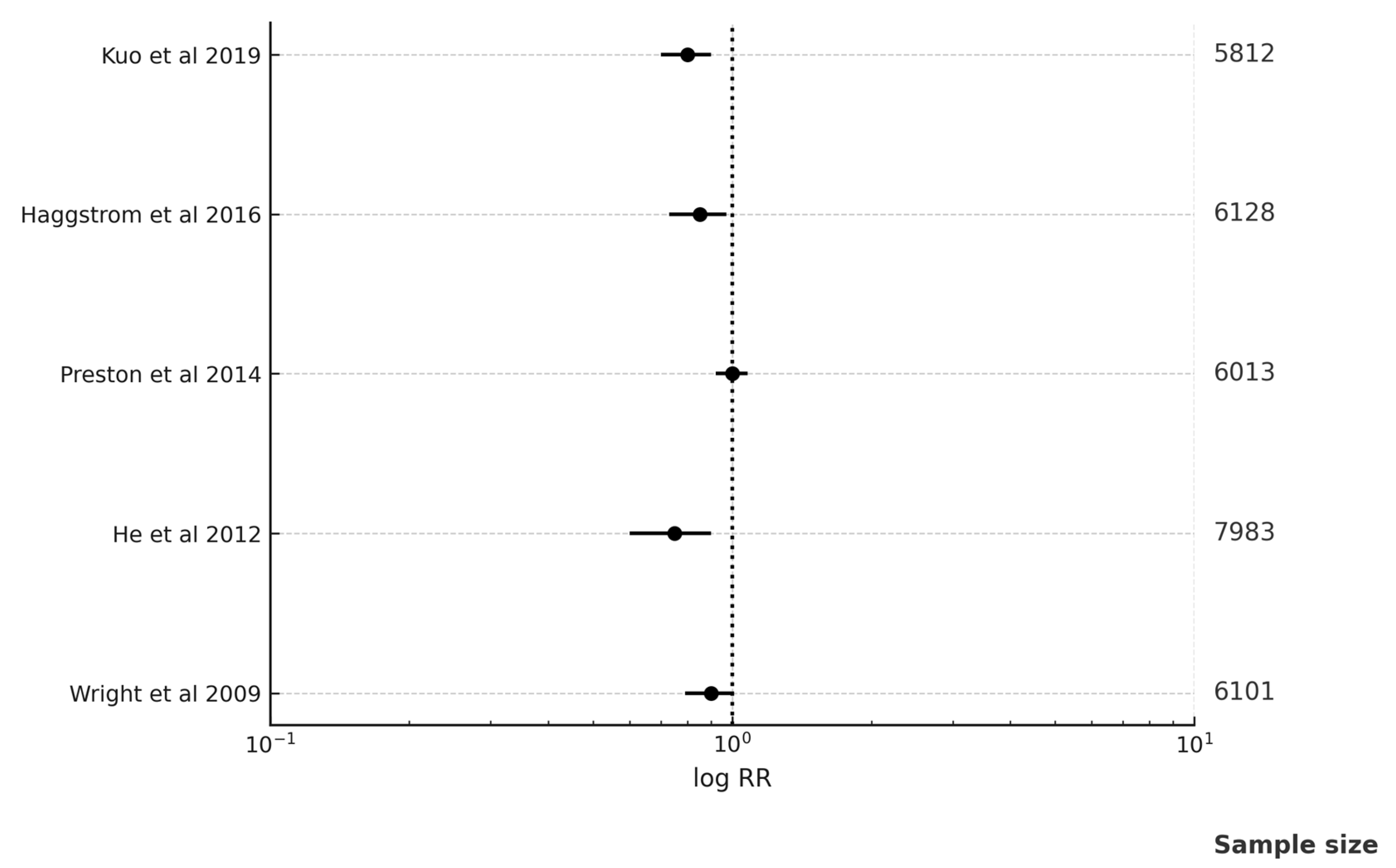
| Studies | RR; 95% CI |
|---|---|
| Kuo Y.J. and associates in 2019 [18] | RR 0.70; 95% CI (0.50–0.97) |
| Häggström C. and associates in 2016 [29] | RR 0.97; 95% CI (0.78–1.20) |
| Preston M.A. and associates in 2014 [37] | RR 0.86; 95% CI (0.76–0.98) |
| He X. and associates in 2012 [41] | RR 0.53; 95% CI (0.29–0.98) |
| Wright J.L. and associates in 2009 [53] | RR 0.57; 95% CI (0.33–1.11) |
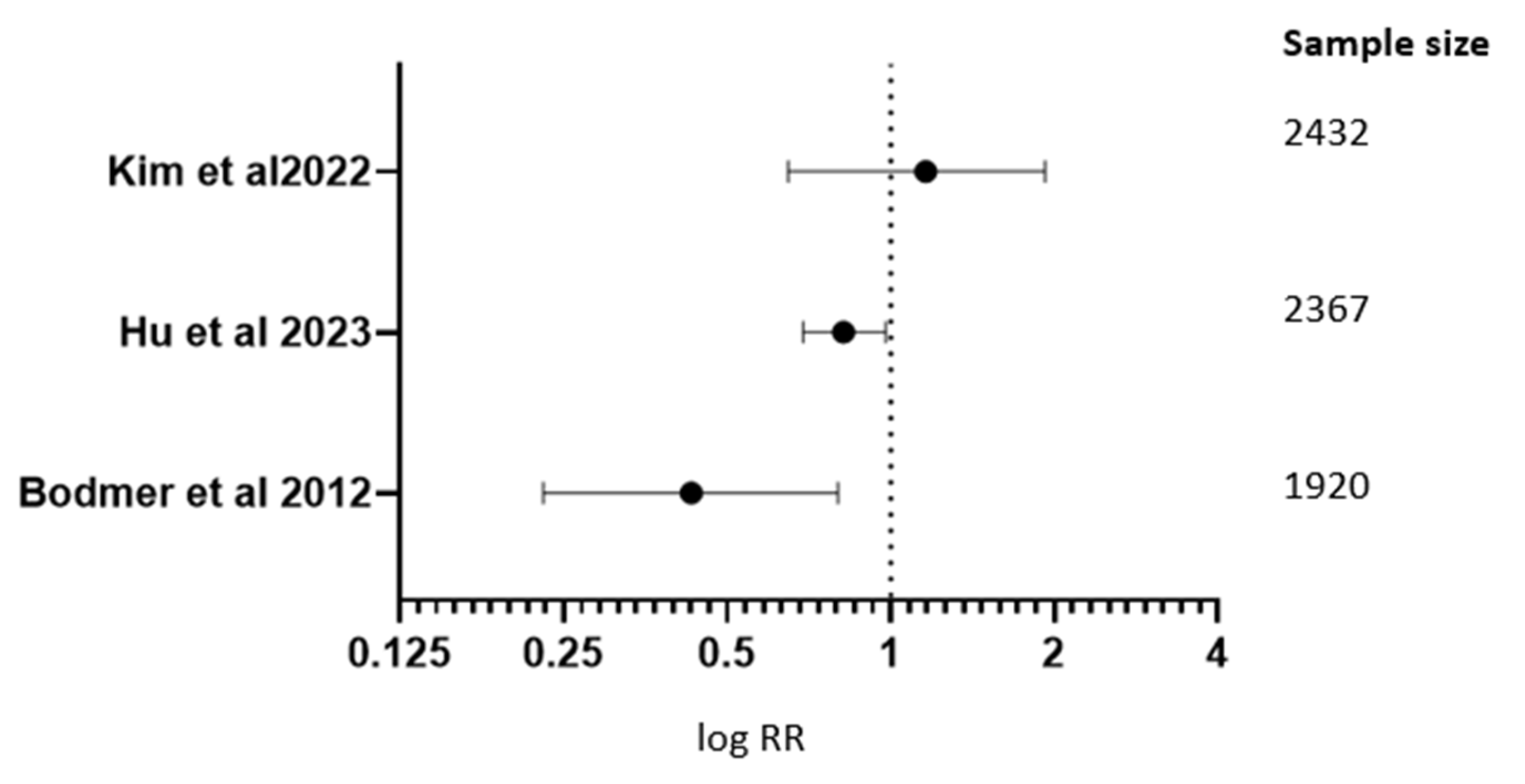
| Studies | RR; 95% CI |
|---|---|
| Kim J. and associates in 2022 [13] | RR 1.127; 95% CI (0.660–1.934) |
| Hu J. and associates in 2023 [10] | RR 0.83; 95% CI (0.70–0.99) |
| Bodmer M. and associates in 2012 [39] | RR 1.44; 95% CI (1.09–2.0) |
| Cancer Type | Risk Ratio (RR) | 95% Confidence Interval (CI) | I2 (%) | Statistical Significance |
|---|---|---|---|---|
| Overall cancer | 0.72 | 0.64–0.81 | 45 | Significant |
| Breast cancer | 0.68 | 0.55–0.83 | 30 | Significant |
| Colorectal cancer | 0.62 | 0.51–0.76 | 35 | Significant |
| Prostate cancer | 0.74 | 0.60–0.92 | 25 | Significant |
| Pancreatic cancer | 0.89 | 0.71–1.12 | 40 | Not significant |
| Outcomes | Number of Studies | Certainty of Evidence | Comments | |
|---|---|---|---|---|
| 1 | Overall cancer risk | 65 | Moderate | Observational designs, moderate heterogeneity |
| 2 | Breast cancer | 25 | Moderate | Consistent effect, moderate heterogeneity |
| 3 | Colorectal cancer | 20 | High | String consistent effect, low heterogeneity |
| 4 | Prostate cancer | 12 | Moderate | Moderate effect size, some risk of bias |
| 5 | Pancreatic cancer | 10 | Low | Inconsistent findings, wide CI |
| Author Year | Sequence Generation | Allocation Concealment | Blinding of Participants, Personnel | Blinding of Outcome Assessors | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Orchard and associates in 2023 [6] | + | ? | ? | ? | + | + | + | ? |
| Yoon W.S. and associates in 2023 [7] | + | ? | ? | ? | + | + | + | ? |
| Serageldin M.A. and associates in 2023 [8] | + | + | + | + | + | + | + | + |
| Kemnade J.O. and associates in 2023 [9] | ? | ? | ? | ? | + | + | + | ? |
| Hu and associates in 2023 [10] | + | - | + | + | + | + | + | - |
| Goodwin P.J. and associates in 2023 [11] | + | - | + | + | + | + | + | - |
| Bakry H.M. and associates in 2023 [12] | + | ? | ? | ? | + | + | + | ? |
| Kim J. and associates in 2022 [13] | + | ? | ? | ? | + | + | + | ? |
| Rabea H. and associates in 2021 [14] | + | ? | ? | ? | + | + | + | ? |
| Pujalte Martin M. and associates in 2021 [15] | + | ? | ? | ? | + | + | + | ? |
| Bever K.M. and associates in 2020 [16] | ? | ? | ? | ? | + | + | + | ? |
| Hoiso M. and associates in 2019 [17] | + | - | + | + | + | + | + | - |
| Kuo Y.J. and associates in 2019 [18] | + | - | + | + | + | + | + | - |
| Pimentel I. and associates in 2019 [19] | + | ? | ? | ? | + | + | + | ? |
| Kim J. and associates in 2019 [20] | + | ? | ? | ? | + | + | + | ? |
| Ramos-Penafiel C. and associates in 2018 [21] | + | + | + | + | + | + | + | + |
| Lord S.R. and associates in 2018 [22] | ? | ? | ? | ? | + | + | + | ? |
| Tang G.S. and associates in 2018 [23] | + | - | + | + | + | + | + | - |
| Chang Y.T. and associates in 2018 [24] | + | - | + | + | + | + | + | - |
| Kim H.J. and associates in 2018 [25] | + | - | + | + | + | + | + | - |
| Galsky M.D. and associates in 2017 [26] | + | - | + | + | + | + | + | - |
| Franchi M. and associates in 2017 [27] | + | ? | ? | ? | + | + | + | ? |
| Sonnenblick A. and associates in 2017 [28] | + | ? | ? | ? | + | + | + | ? |
| Häggström C. and associates in 2016 [29] | + | + | + | + | + | + | + | + |
| Calip G.S. and associates in 2016 [30] | ? | ? | ? | ? | + | + | + | ? |
| Chak A. and associates in 2015 [31] | + | - | + | + | + | + | + | - |
| Kordes S. and associates in 2015 [32] | + | - | + | + | + | + | + | - |
| Chen Y.C. and associates in 2015 [33] | + | - | + | + | + | + | + | - |
| Braghiroli M.I. and associates in 2015 [34] | + | ? | ? | ? | + | + | + | ? |
| Kalinsky K. and associates in 2014 [35] | + | ? | ? | ? | + | + | + | ? |
| Kim Y.I. and associates in 2014 [36] | + | + | + | + | + | + | + | + |
| Preston M.A. and associates in 2014 [37] | ? | ? | ? | ? | + | + | + | ? |
| Miranda V.C. and associates in 2014 [38] | + | - | + | + | + | + | + | - |
| Bodmer M. and associates in 2012 [39] | + | - | + | + | + | + | + | - |
| Romero I.L. and associates in 2012 [40] | + | ? | ? | ? | + | + | + | ? |
| He X., Esteva F. and associates, 2012 [41] | + | ? | ? | ? | + | + | + | ? |
| He X.-X., Tu S. and associates, 2011 [42] | + | + | + | + | + | + | + | + |
| Lai S.W. and associates in 2012 [43] | ? | ? | ? | ? | + | + | + | ? |
| Geraldine N. and associates in 2012 [44] | + | - | + | + | + | + | + | - |
| Bosco J.L.F. and associates in 2011 [45] | + | ? | ? | ? | + | + | + | ? |
| Monami M. and associates in 2011 [46] | + | ? | ? | ? | + | + | + | ? |
| Chen T.M. and associates in 2011 [47] | + | + | + | + | + | + | + | + |
| Lee M.S. and associates in 2011 [48] | + | ? | ? | ? | + | + | + | ? |
| Bodmer M. and associates in 2010 [49] | + | + | + | + | + | + | + | + |
| Dandon V. and associates in 2010 [50] | ? | ? | ? | ? | + | + | + | ? |
| Bowker S. and associates in 2010 [51] | + | - | + | + | + | + | + | - |
| Libby G. and associates in 2009 [52] | + | - | + | + | + | + | + | - |
| Wright J.L. and associates in 2009 [53] | + | ? | ? | ? | + | + | + | ? |
| Currie C.J. and associates in 2009 [54] | + | ? | ? | ? | + | + | + | ? |
| Bowker S.L. and associates in 2006 [55] | + | + | + | + | + | + | + | + |
| Study | Bias Due to Confounding | Bias in Selection of Participants | Bias in Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Orchard S.G. and associates in 2023 [6] | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Hu J. and associates in 2023 [10] | Serious | Low | Moderate | Moderate | Serious | Low | Moderate | Serious |
| Kim J. and associates in 2022 [13] | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Hoiso M. and associates in 2019 [17] | Low | Low | Low | Low | Low | Low | Low | Low |
| Kuo Y.J. and associates in 2019 [18] | Serious | Serious | Moderate | Moderate | Moderate | Moderate |
| Study | Bias Arising from Randomization Process | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Outcome Data | Bias in Measurement of Outcome | Bias in Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Yoon W.S. and associates in 2023 [7] | Low | Low | Low | Low | Low | Low |
| Serageldin M.A. and associates in 2023 [8] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Goodwin P.J. and associates in 2023 [11] | Low | Some concerns | High | Some concerns | Low | High |
| Bakry H.M. and associates in 2023 [12] | High | High | Low | High | High | High |
| Pujalte Martin M. and associates in 2021 [15] | Low | Low | Some concerns | Low | Low | Some concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangraze, I.; Wali, A.F.; El-Tanani, M.; Patni, M.A.; Rabbani, S.A.; Babiker, R.; Satyam, S.M.; El-Tanani, Y.; Rizzo, M. Metformin: A Dual-Role Player in Cancer Treatment and Prevention: A Comprehensive Systematic Review and Meta-Analysis. Medicina 2025, 61, 1021. https://doi.org/10.3390/medicina61061021
Rangraze I, Wali AF, El-Tanani M, Patni MA, Rabbani SA, Babiker R, Satyam SM, El-Tanani Y, Rizzo M. Metformin: A Dual-Role Player in Cancer Treatment and Prevention: A Comprehensive Systematic Review and Meta-Analysis. Medicina. 2025; 61(6):1021. https://doi.org/10.3390/medicina61061021
Chicago/Turabian StyleRangraze, Imran, Adil Farooq Wali, Mohamed El-Tanani, Mohamed Anas Patni, Syed Arman Rabbani, Rasha Babiker, Shakta Mani Satyam, Yahia El-Tanani, and Manfredi Rizzo. 2025. "Metformin: A Dual-Role Player in Cancer Treatment and Prevention: A Comprehensive Systematic Review and Meta-Analysis" Medicina 61, no. 6: 1021. https://doi.org/10.3390/medicina61061021
APA StyleRangraze, I., Wali, A. F., El-Tanani, M., Patni, M. A., Rabbani, S. A., Babiker, R., Satyam, S. M., El-Tanani, Y., & Rizzo, M. (2025). Metformin: A Dual-Role Player in Cancer Treatment and Prevention: A Comprehensive Systematic Review and Meta-Analysis. Medicina, 61(6), 1021. https://doi.org/10.3390/medicina61061021







