Prognostic Role of Circulating miR-141 in Early Diagnosis of Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Inclusion and Exclusion Criteria
2.3. RNA Extraction
2.4. Real-Time PCR
2.5. Statistical Analysis
3. Results
Patient Characteristics
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| miR-141 | MicroRNA-141 |
| miRNAs | microRNAs |
| CLL | Chronic Lymphocytic Leukemia |
| PDCD4 | programmed cell death-4 |
| AUC | under the curve |
| CEA | Carcinoembryonic Antigen |
| qPCR | quantitative polymerase chain reaction |
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Ichim, C.; Sasu, S.-M.; Todor, S.B. Key Insights into Gut Alterations in Metabolic Syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef]
- Gmeiner, W.H. Recent Advances in Therapeutic Strategies to Improve Colorectal Cancer Treatment. Cancers 2024, 16, 1029. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, G.; Garofalo, M.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 287–314. [Google Scholar] [CrossRef]
- Ellakwa, D.E.; Mushtaq, N.; Khan, S.; Jabbar, A.; Abdelmalek, M.A.; Wadan, A.S.; Ellakwa, T.E.; Raza, A. Molecular functions of microRNAs in colorectal cancer: Recent roles in proliferation, angiogenesis, apoptosis, and chemoresistance. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 5617–5630. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Santos, D.A.R.; Gaiteiro, C.; Santos, M.; Santos, L.; Dinis-Ribeiro, M.; Lima, L. MicroRNA Biomarkers as Promising Tools for Early Colorectal Cancer Screening-A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 11023. [Google Scholar] [CrossRef]
- Min, L.; Shengtao, Z.; Lei, C.; Xiang, L.; Rui, W.; Libo, Z.; Yuqing, Y.; Zheng, Z.; Guanyi, K.; Peng, L.; et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: A comparison with plasma total miRNAs. J. Extracell. Vesicles 2019, 8, 1643670. [Google Scholar] [CrossRef]
- Jung, G.; Hernández-Illán, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef]
- Wu, X.; Yan, F.; Wang, L.; Sun, G.; Liu, J.; Qu, M.; Wang, Y.; Li, T. MicroRNA: Another Pharmacological Avenue for Colorectal Cancer? Front. Cell Dev. Biol. 2020, 8, 812. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, I.; Ciccarese, F.; Sharova, E.; Urso, L.; Raimondi, V.; Silic-Benussi, M.; D’Agostino, D.M.; Ciminale, V. The miR-200 Family of microRNAs: Fine Tuners of Epithelial-Mesenchymal Transition and Circulating Cancer Biomarkers. Cancers 2021, 13, 5874. [Google Scholar] [CrossRef] [PubMed]
- Klicka, K.; Grzywa, T.M.; Mielniczuk, A.; Klinke, A.; Włodarski, P.K. The role of miR-200 family in the regulation of hallmarks of cancer. Front. Oncol. 2022, 12, 965231. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.-q.; Tian, Y.; Qu, G.-j.; Kun, H.; Luo, S.-s. Functional mechanism and clinical implications of miR-141 in human cancers. Cell. Signal. 2022, 95, 110354. [Google Scholar] [CrossRef]
- Zedan, A.H.; Osther, P.J.S.; Assenholt, J.; Madsen, J.S.; Hansen, T.F. Circulating miR-141 and miR-375 are associated with treatment outcome in metastatic castration resistant prostate cancer. Sci. Rep. 2020, 10, 227. [Google Scholar] [CrossRef]
- Guz, M.; Jeleniewicz, W.; Cybulski, M. Interactions between circRNAs and miR-141 in Cancer: From Pathogenesis to Diagnosis and Therapy. Int. J. Mol. Sci. 2023, 24, 11861. [Google Scholar] [CrossRef]
- Ding, L.; Yu, L.L.; Han, N.; Zhang, B.T. miR-141 promotes colon cancer cell proliferation by inhibiting MAP2K4. Oncol. Lett. 2017, 13, 1665–1671. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, L.; Cogdell, D.E.; Zheng, H.; Schetter, A.J.; Nykter, M.; Harris, C.C.; Chen, K.; Hamilton, S.R.; Zhang, W. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS ONE 2011, 6, e17745. [Google Scholar] [CrossRef]
- Das, V.; Kalita, J.; Pal, M. Predictive and prognostic biomarkers in colorectal cancer: A systematic review of recent advances and challenges. Biomed. Pharmacother. 2017, 87, 8–19. [Google Scholar] [CrossRef]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Popa, M.L.; Ichim, C.; Anderco, P.; Todor, S.B.; Pop-Lodromanean, D. MicroRNAs in the Diagnosis of Digestive Diseases: A Comprehensive Review. J. Clin. Med. 2025, 14, 2054. [Google Scholar] [CrossRef] [PubMed]
- Sur, D.; Advani, S.; Braithwaite, D. MicroRNA panels as diagnostic biomarkers for colorectal cancer: A systematic review and meta-analysis. Front. Med. 2022, 9, 915226. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, Z.; Hou, Y.; Yuan, T.; Gao, C.; Jia, W.; Yi, X.; Liu, M. MicroRNA-92a as a potential biomarker in diagnosis of colorectal cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e88745. [Google Scholar] [CrossRef]
- Hirao, A.; Sato, Y.; Tanaka, H.; Nishida, K.; Tomonari, T.; Hirata, M.; Bando, M.; Kida, Y.; Tanaka, T.; Kawaguchi, T.; et al. MiR-125b-5p Is Involved in Sorafenib Resistance through Ataxin-1-Mediated Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. Cancers 2021, 13, 4917. [Google Scholar] [CrossRef] [PubMed]
- Strubberg, A.M.; Madison, B.B. MicroRNAs in the etiology of colorectal cancer: Pathways and clinical implications. Dis. Models Mech. 2017, 10, 197–214. [Google Scholar] [CrossRef]
- Towler, B.P.; Jones, C.I.; Newbury, S.F. Mechanisms of regulation of mature miRNAs. Biochem. Soc. Trans. 2015, 43, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, C.G.; Karlsson, W.K.; Pommergaard, H.-C.; Burcharth, J.; Rosenberg, J. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence—A systematic review. Int. J. Surg. 2016, 25, 134–144. [Google Scholar] [CrossRef]
- Chen, K.; Collins, G.; Wang, H.; Toh, J.W.T. Pathological Features and Prognostication in Colorectal Cancer. Curr. Oncol. 2021, 28, 5356–5383. [Google Scholar] [CrossRef]
- Fujii, T.; Sutoh, T.; Morita, H.; Yajima, R.; Yamaguchi, S.; Tsutsumi, S.; Asao, T.; Kuwano, H. Vascular invasion, but not lymphatic invasion, of the primary tumor is a strong prognostic factor in patients with colorectal cancer. Anticancer Res. 2014, 34, 3147–3151. [Google Scholar]
- Huang, X.; Liu, H.; Liao, X.; Xiao, Z.; Huang, Z.; Li, G. Prognostic factors for T1-2 colorectal cancer after radical resection: Lymph node distribution is a valuable predictor of its survival. Asian J. Surg. 2021, 44, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Zuhan, A.; Riwanto, I.; Listiana, D.E.; Djannah, F.; Rosyidi, R.M. The extent of distal intramural spread of colorectal cancer cell study of it’s relationship with histological grading, stage of disease and CEA level. Ann. Med. Surg. 2021, 64, 102227. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Feng, B.; Han, S.; Zhang, K.; Chen, J.; Li, C.; Wang, R.; Chen, L. The Roles of MicroRNA-141 in Human Cancers: From Diagnosis to Treatment. Cell. Physiol. Biochem. 2016, 38, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Leung, L.L.; Mak, C.S.L.; Wang, X.; Chan, W.-S.; Hui, L.M.N.; Tang, H.W.M.; Siu, M.K.Y.; Sharma, R.; Xu, D.; et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Mol. Cancer 2023, 22, 4. [Google Scholar] [CrossRef]



| Variable | Category | Patients (n = 40) | Controls (n = 20) | Total (n = 60) |
|---|---|---|---|---|
| Gender | Female | 20 (50.0%) | 9 (45.0%) | 29 (48.3%) |
| Male | 20 (50.0%) | 11 (55.0%) | 31 (51.7%) | |
| Age (years) | Mean ± SD | 61.15 ± 14.1 | 59.10 ± 13.3 | 60.47 ± 13.8 |
| Tumor Stage | Stage I | 7 (17.5%) | - | 7 (11.7%) |
| Stage II | 9 (22.5%) | - | 9 (15.0%) | |
| Stage III | 12 (30.0%) | - | 12 (20.0%) | |
| Stage IV | 12 (30.0%) | - | 12 (20.0%) |
| Number | % | Percentage of Patients | Cumulative Percentage | ||
|---|---|---|---|---|---|
| Patient | Colon | 26 | 43.3 | 65.0 | 65.0 |
| Rectum | 14 | 23.3 | 35.0 | 100.0 | |
| Total | 40 | 66.7 | 100.0 | ||
| Control | 20 | 33.3 | |||
| Total | 60 | 100 | |||
| miR-141 Median (Min–Max) | CEA Median (Min–Max) | p | |
|---|---|---|---|
| Patient | 0.00016 (0.000000–0.001460) | 11.23 (0.49–92.47) | |
| Control | 0.000057 (0.000000–0.000382) | 1.85 (0.30–3.30) | 0.612 |
| Variable | Comparison | Result | p Value | Interpretation |
|---|---|---|---|---|
| CEA | Patients vs. Controls | 11.2 ± 19.4 vs. 1.8 ± 0.88 | 0.132 | Not significant |
| CEA | By Disease Stage | Higher CEA in advanced stages | 0.009 | Significant |
| miR-141 | Patients vs. Controls | 0.00016 vs. 0.000057 | 0.612 | Not significant |
| miR-141 | By Disease Stage | No significant difference | 0.219 | Not significant |
| CEA | With Vascular Invasion | Higher in invasive cases | 0.087 | Not significant |
| miR-141 | With Vascular Invasion | No significant difference | 0.292 | Not significant |
| CEA | With Perineural Invasion | No significant difference | 0.650 | Not significant |
| miR-141 | With Perineural Invasion | No significant difference | 0.681 | Not significant |
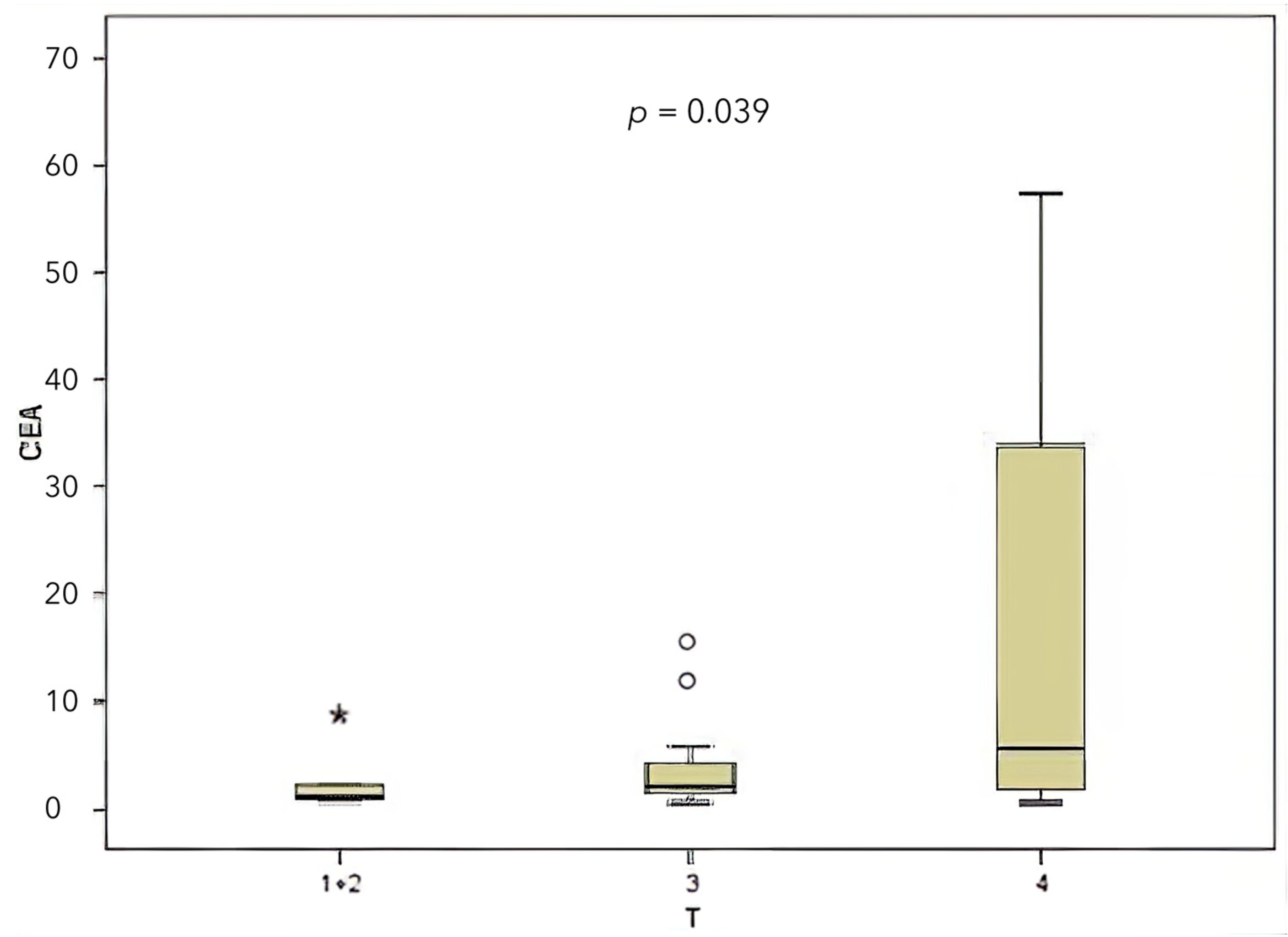
| CEA | T | Higher CEA in T4 | 0.039 | Significant |
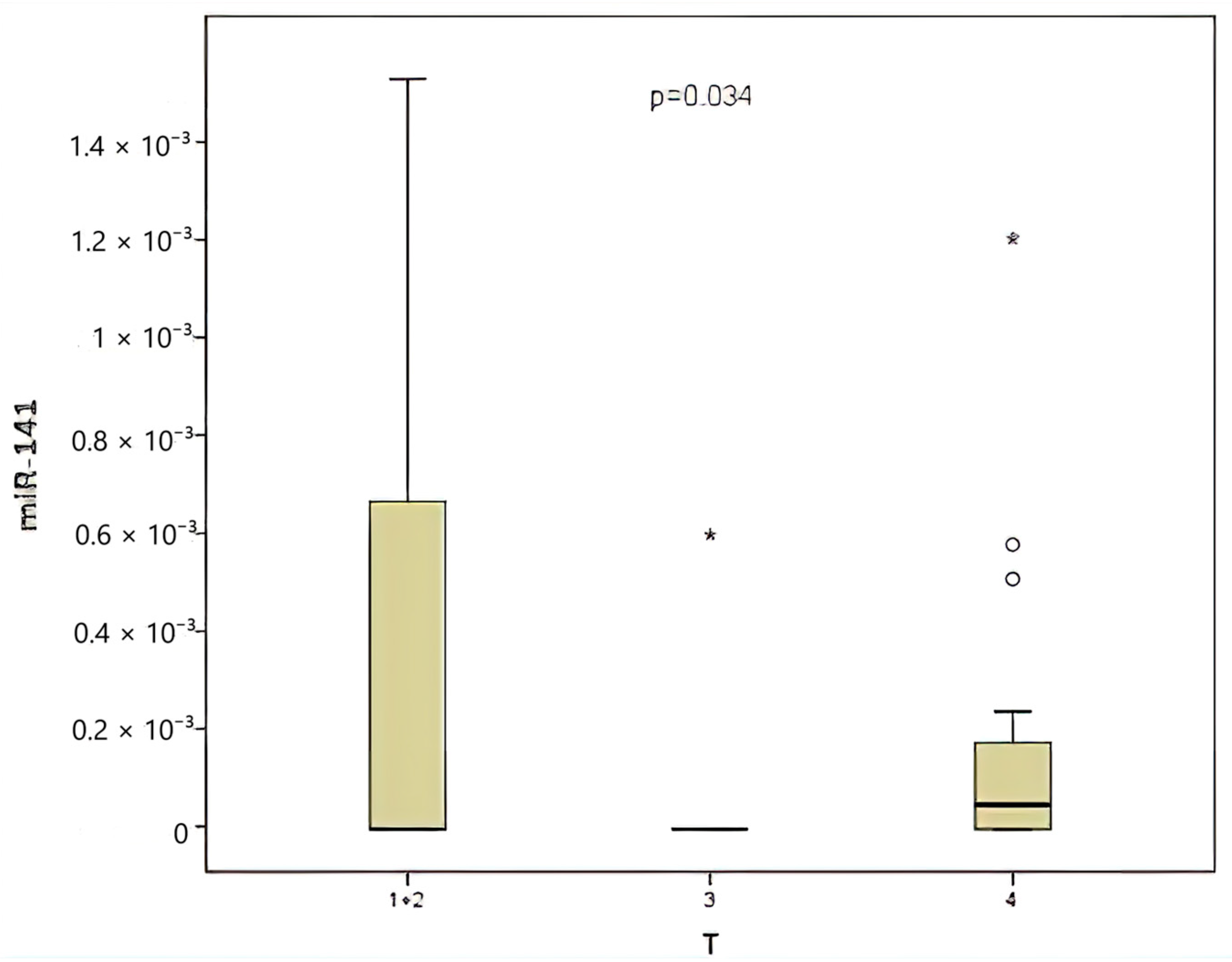
| miR-141 | T | Higher in T4 | 0.034 | Significant |
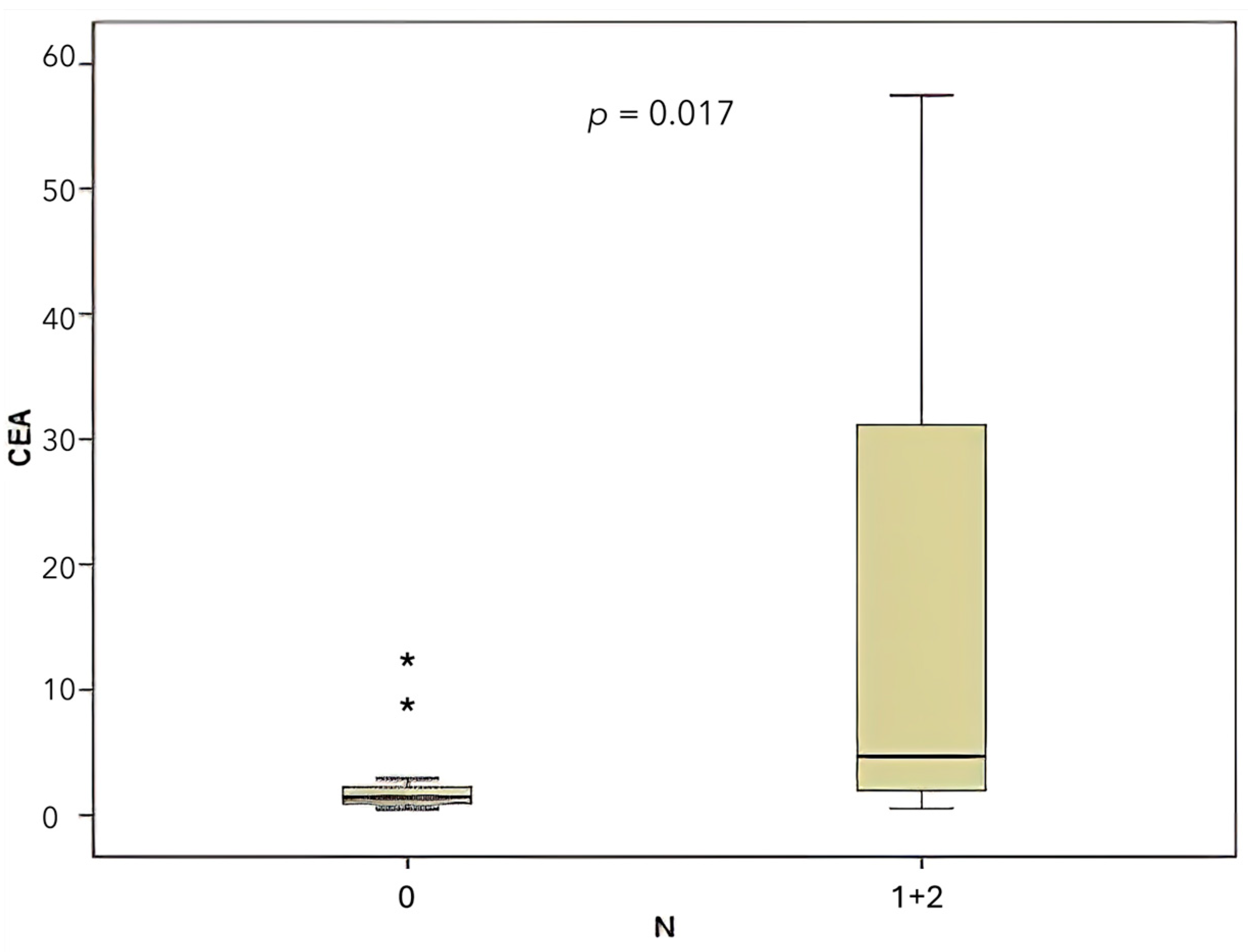
| CEA | N | Higher with N1/N2 | 0.017 | Significant |
| miR-141 | N | No significant difference | 0.221 | Not significant |
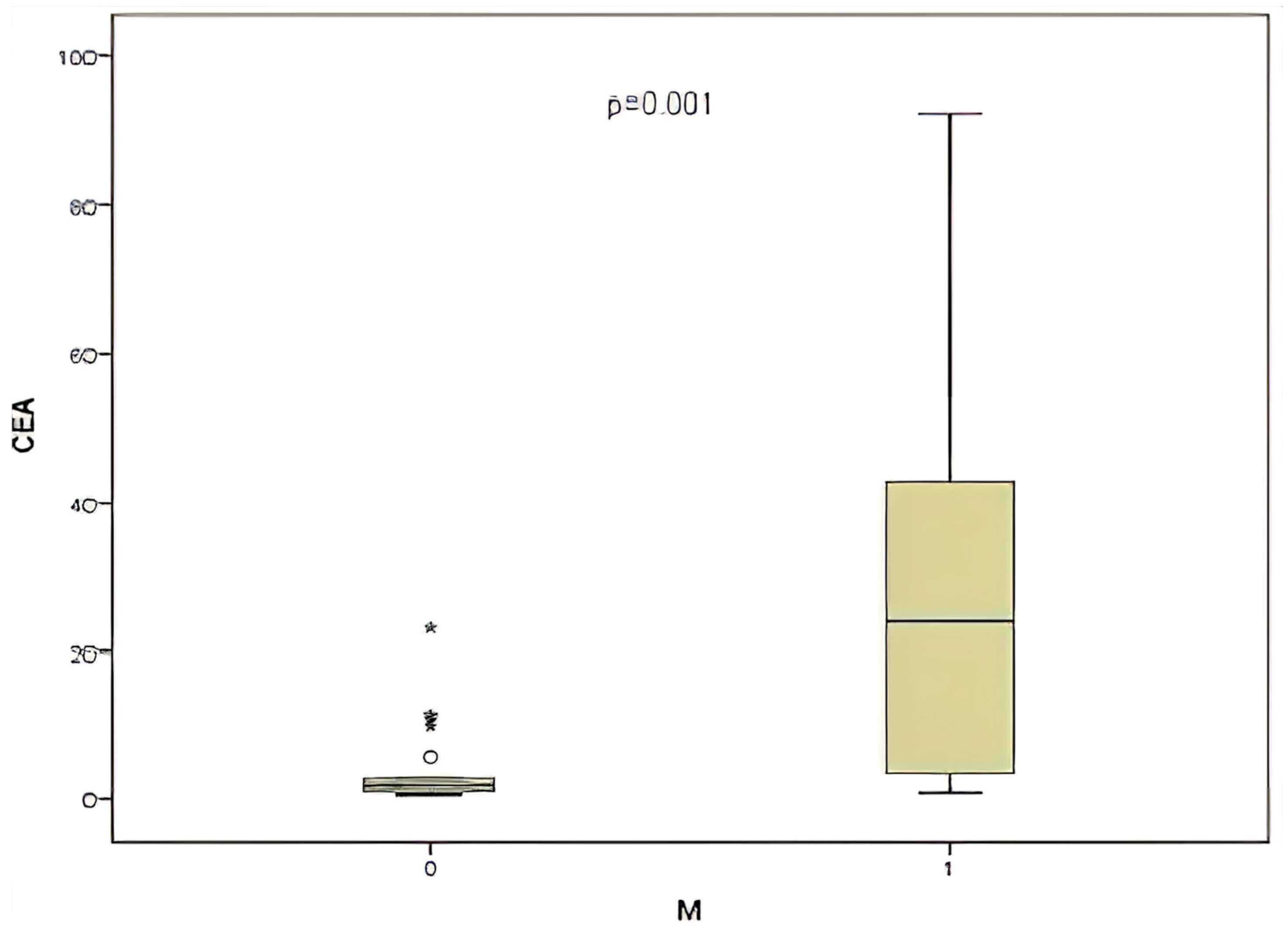
| CEA | With Metastasis | Higher in metastatic cases | 0.017 | Significant |
| miR-141 | With Metastasis | No significant difference | 0.149 | Not significant |
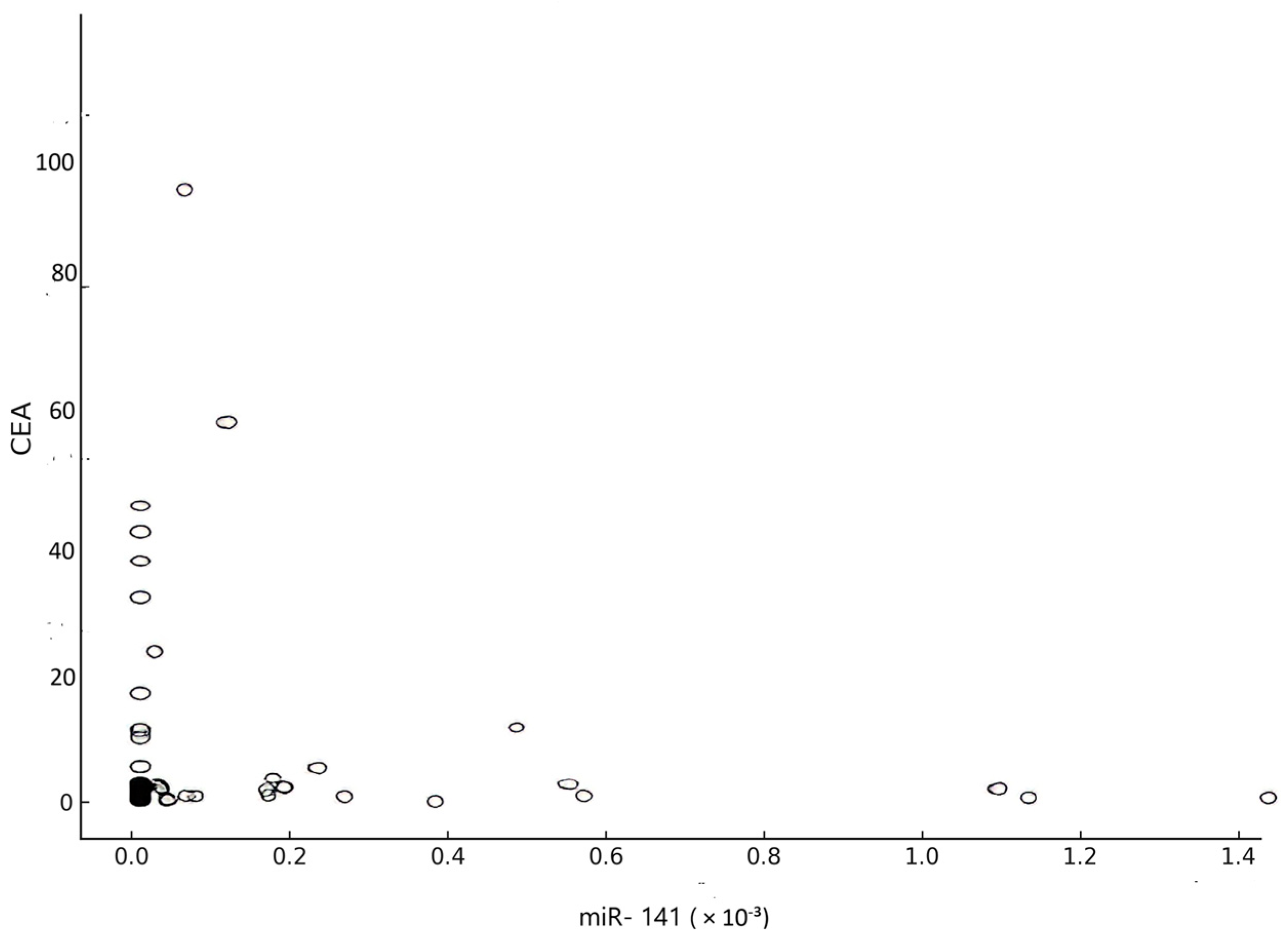
| CEA vs. miR-141 | Correlation | R = −0.05 | 0.71 | Not significant |
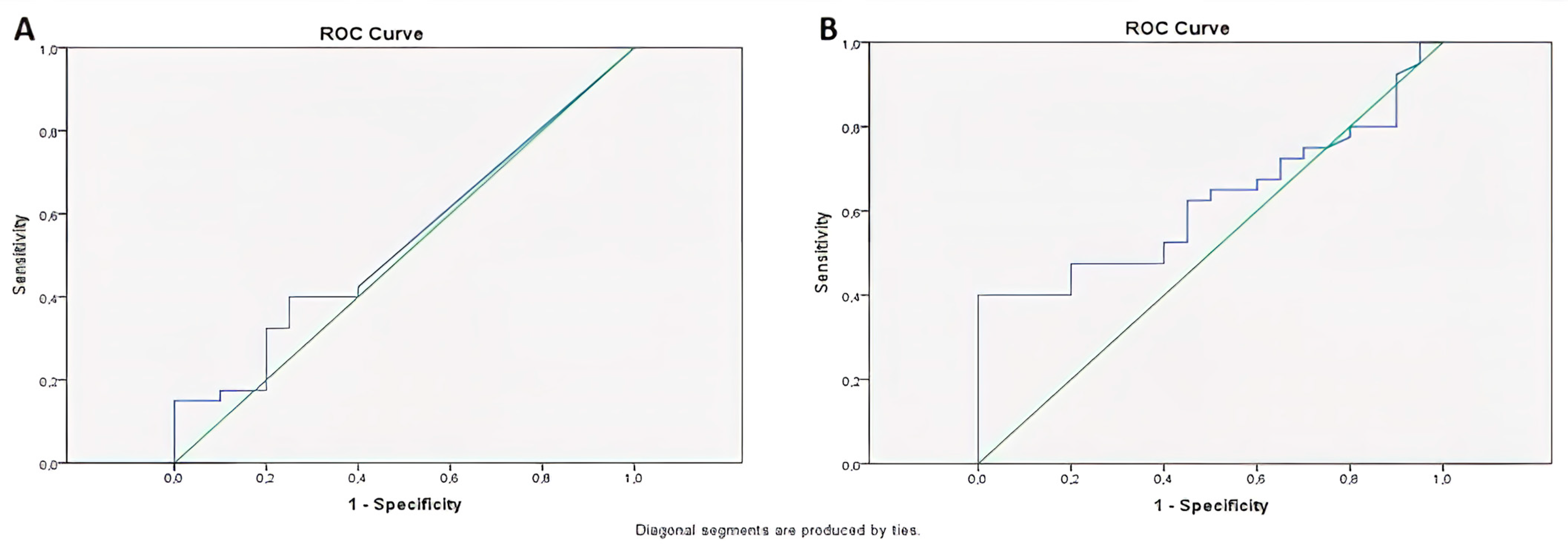
| ROC Analysis | miR-141 | AUC = 0.54 | 0.65 | Low diagnostic value |
| ROC Analysis | CEA | AUC = 0.62 | 0.13 | Moderate diagnostic value |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simsek Dilli, M.; Bayram, E.; Kara, I.O. Prognostic Role of Circulating miR-141 in Early Diagnosis of Colorectal Cancer. Medicina 2025, 61, 1007. https://doi.org/10.3390/medicina61061007
Simsek Dilli M, Bayram E, Kara IO. Prognostic Role of Circulating miR-141 in Early Diagnosis of Colorectal Cancer. Medicina. 2025; 61(6):1007. https://doi.org/10.3390/medicina61061007
Chicago/Turabian StyleSimsek Dilli, Merve, Ertugrul Bayram, and Ismail Oguz Kara. 2025. "Prognostic Role of Circulating miR-141 in Early Diagnosis of Colorectal Cancer" Medicina 61, no. 6: 1007. https://doi.org/10.3390/medicina61061007
APA StyleSimsek Dilli, M., Bayram, E., & Kara, I. O. (2025). Prognostic Role of Circulating miR-141 in Early Diagnosis of Colorectal Cancer. Medicina, 61(6), 1007. https://doi.org/10.3390/medicina61061007





