Biochemical Recurrence in High-Risk Localized Prostate Cancer: A Comparison of Laparoscopic Prostatectomy and External Radiotherapy
Abstract
1. Introduction
2. Subjects and Methods
2.1. Selection Criteria
2.2. Treatment Groups
- LRP Group
- a.
- Underwent transperitoneal laparoscopic radical prostatectomy (TLRP);
- b.
- Extended pelvic lymphadenectomy using a modified Montsouris technique (Guillonneau et al., 2000) [14].
- EBRT Group
- a.
- Received intensity-modulated radiation therapy (IMRT) to a total dose of 76 Gy;
- b.
- EBRT was initiated 4–6 months after ADT, and it was maintained for two years, keeping levels of circulating testosterone under 50 ng/L.
2.3. Monitored Parameters
2.4. Tumor Stage and Gleason Score Concordance
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaccarella, S.; Li, M.; Bray, F.; Kvale, R.; Serraino, D.; Lorenzoni, V.; Auvinen, A.; Dal Maso, L. Prostate Cancer Incidence and Mortality in Europe and Implications for Screening Activities: Population Based Study. BMJ 2024, 386, e077738. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2024; American Cancer Society: Atlanta, GA, USA, 2024. [Google Scholar]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Chen, R.C.; Basak, R.; Meyer, A.-M.; Kuo, T.-M.; Carpenter, W.R.; Agans, R.P.; Broughman, J.R.; Reeve, B.B.; Nielsen, M.E.; Usinger, D.S.; et al. Association Between Choice of Radical Prostatectomy, External Beam Radiotherapy, Brachytherapy, or Active Surveillance and Patient-Reported Quality of Life Among Men with Localized Prostate Cancer. JAMA 2017, 317, 1141–1150. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Wang, Y.; Zhang, Y.; Ma, X. Comparison on Efficacy of Radical Prostatectomy versus External Beam Radiotherapy for the Treatment of Localized Prostate Cancer. Oncotarget 2017, 8, 79854–79863. [Google Scholar] [CrossRef]
- Chen, C.-H.; Tsai, C.-Y.; Pu, Y.-S. Primary Total Prostate Cryoablation for Localized High-Risk Prostate Cancer: 10-Year Outcomes and Nomograms. Cancers 2023, 15, 3873. [Google Scholar] [CrossRef]
- Van den Broeck, T.; van den Bergh, R.C.N.; Briers, E.; Cornford, P.; Cumberbatch, M.; Tilki, D.; De Santis, M.; Fanti, S.; Fossati, N.; Gillessen, S.; et al. Biochemical Recurrence in Prostate Cancer: The European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur. Urol. Focus 2020, 6, 231–234. [Google Scholar] [CrossRef]
- Bejrananda, T.; Pliensiri, P. Prediction of Biochemical Recurrence after Laparoscopic Radical Prostatectomy. BMC Urol. 2023, 23, 183. [Google Scholar] [CrossRef]
- Guerra, A.; Alves, F.C.; Maes, K.; Maio, R.; Villeirs, G.; Mouriño, H. Risk Biomarkers for Biochemical Recurrence after Radical Prostatectomy for Prostate Cancer Using Clinical and MRI-Derived Semantic Features. Cancers 2023, 15, 5296. [Google Scholar] [CrossRef]
- Siech, C.; Wenzel, M.; Lange, C.; Cano Garcia, C.; Humke, C.; Tian, Z.; Karakiewicz, P.I.; Traumann, M.; Kluth, L.A.; Chun, F.K.H.; et al. The Association between Patient Characteristics and Biochemical Recurrence after Radical Prostatectomy. Medicina 2024, 60, 1119. [Google Scholar] [CrossRef]
- Shore, N.D.; Moul, J.W.; Pienta, K.J.; Czernin, J.; King, M.T.; Freedland, S.J. Biochemical Recurrence in Patients with Prostate Cancer after Primary Definitive Therapy: Treatment Based on Risk Stratification. Prostate Cancer Prostatic Dis. 2024, 27, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Suzuki, G.; Masui, K.; Yamada, K.; Ueda, T.; Shiraishi, T.; Fujihara, A.; Kato, T.; Hashimoto, Y.; Okabe, H. Ultra-High Prostate-Specific Antigen Level: A Potential Very-High-Risk Factor for Localized High-Risk Prostate Cancer. Cancers 2023, 15, 5644. [Google Scholar] [CrossRef]
- Guillonneau, B.; Vallancien, G. Laparoscopic Radical Prostatectomy: The Montsouris Experience. J. Urol. 2000, 163, 418–422. [Google Scholar] [CrossRef]
- Liesenfeld, L.; Kron, M.; Gschwend, J.E.; Herkommer, K. Prognostic Factors for Biochemical Recurrence More than 10 Years after Radical Prostatectomy. J. Urol. 2017, 197, 143–148. [Google Scholar] [CrossRef]
- Spratt, D.E.; McKay, R.R.; Khan, N.; Constantinovici, N.; Chen, G.; Ortiz, J.A.; Srinivasan, S.; Vassilev, Z.; Xu, J.; Shore, N.D. Biochemical Recurrence (BCR) among Patients (Pts) with Prostate Cancer (PC) after Radiation Therapy (RT). J. Clin. Oncol. 2023, 41, e17111. [Google Scholar] [CrossRef]
- Tourinho-Barbosa, R.; Srougi, V.; Nunes-Silva, I.; Baghdadi, M.; Rembeyo, G.; Eiffel, S.S.; Barret, E.; Rozet, F.; Galiano, M.; Cathelineau, X.; et al. Biochemical Recurrence after Radical Prostatectomy: What Does It Mean? Int. Braz. J. Urol. 2018, 44, 14–21. [Google Scholar] [CrossRef]
- Beauval, J.-B.; Roumiguié, M.; Filleron, T.; Benoit, T.; de la Taille, A.; Malavaud, B.; Salomon, L.; Soulié, M.; Ploussard, G. Biochemical Recurrence-Free Survival and Pathological Outcomes after Radical Prostatectomy for High-Risk Prostate Cancer. BMC Urol. 2016, 16, 26. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Yumioka, T.; Iwamoto, H.; Masago, T.; Morizane, S.; Honda, M.; Sejima, T.; Takenaka, A. Biochemical Recurrence Prediction in High-Risk Prostate Cancer Patients, Following Robot-Assisted Radical Prostatectomy. Yonago Acta Medica 2016, 59, 288–295. [Google Scholar]
- Tanimoto, R.; Fashola, Y.; Scotland, K.B.; Calvaresi, A.E.; Gomella, L.G.; Trabulsi, E.J.; Lallas, C.D. Risk Factors for Biochemical Recurrence after Robotic Assisted Radical Prostatectomy: A Single Surgeon Experience. BMC Urol. 2015, 15, 27. [Google Scholar] [CrossRef][Green Version]
- Aoun, F.; Albisinni, S.; Henriet, B.; Tombal, B.; Van Velthoven, R.; Roumeguère, T. Predictive Factors Associated with Biochemical Recurrence Following Radical Prostatectomy for Pathological T2 Prostate Cancer with Negative Surgical Margins. Scand. J. Urol. 2017, 51, 20–26. [Google Scholar] [CrossRef]
- Alexander, G.S.; Krc, R.F.; Assif, J.W.; Sun, K.; Molitoris, J.K.; Tran, P.; Rana, Z.; Bentzen, S.M.; Mishra, M.V. Conditional Risks of Biochemical Failure and Prostate Cancer-Specific Death in Patients Undergoing External Beam Radiotherapy: A Secondary Analysis of 2 Randomized Clinical Trials. JAMA Netw. Open 2023, 6, e2335069. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Tatsugami, K.; Yoshikawa, M.; Hamaguchi, M.; Yamada, S.; Hayakawa, Y.; Ueda, K.; Momosaki, S.; Sakamoto, N. Predictive Factors of Biochemical Recurrence after Radical Prostatectomy for High-Risk Prostate Cancer. Int. J. Urol. 2018, 25, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Kanehira, M.; Takata, R.; Ishii, S.; Ito, A.; Ikarashi, D.; Matsuura, T.; Kato, Y.; Obara, W. Predictive Factors for Short-Term Biochemical Recurrence-Free Survival after Robot-Assisted Laparoscopic Radical Prostatectomy in High-Risk Prostate Cancer Patients. Int. J. Clin. Oncol. 2019, 24, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Shindo, T.; Hashimoto, K.; FumimasaFukuta; Masumori, N. Preoperative Serum Prostate-Specific Antigen Level Is a Predictive Factor for Biochemical Recurrence in High Risk Prostate Cancer after Radical Prostatectomy. ARC J. Urol. 2017, 2, 11–17. [Google Scholar]
- John, A.; Lim, A.; Catterwell, R.; Selth, L.; O’Callaghan, M. Length of Positive Surgical Margins after Radical Prostatectomy: Does Size Matter?—A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2023, 26, 673–680. [Google Scholar] [CrossRef]
- Beckmann, K.R.; O’Callaghan, M.E.; Vincent, A.D.; Moretti, K.L.; Brook, N.R. Clinical Outcomes for Men with Positive Surgical Margins after Radical Prostatectomy—Results from the South Australian Prostate Cancer Clinical Outcomes Collaborative Community-Based Registry. Asian J. Urol. 2023, 10, 502–511. [Google Scholar] [CrossRef]
- Khan, N.; McKay, R.R.; Spratt, D.E.; Constantinovici, N.; Chen, G.; Ortiz, J.A.; Srinivasan, S.; Vassilev, Z.; Xu, J.; Shore, N.D. Biochemical Recurrence (BCR) and Outcomes in Patients (Pts) with Prostate Cancer (PC) Following Radical Prostatectomy (RP). J. Clin. Oncol. 2023, 41, e17112. [Google Scholar] [CrossRef]
- Ongün, Ş.; Sarıkaya, A.E.; Yılmaz, S.H.B.; Sevgi, B.; Çelik, S.; Şen, V.; Tuna, B.; Yörükoğlu, K.; Aslan, G.; Mungan, M.U.; et al. Long-Term Surveillance Outcomes of Prostate Cancer Patients Eligible for Active Surveillance but Who Underwent Radical Prostatectomy. J. Urol. Surg. 2024, 11, 153–158. [Google Scholar] [CrossRef]
- Yamada, Y.; Fujii, Y.; Kakutani, S.; Kimura, N.; Sugimoto, K.; Hakozaki, Y.; Sugihara, T.; Takeshima, Y.; Kawai, T.; Nakamura, M.; et al. Development of Risk-Score Model in Patients with Negative Surgical Margin after Robot-Assisted Radical Prostatectomy. Sci. Rep. 2024, 14, 7607. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Histologically confirmed localized prostate cancer | Locally advanced or metastatic disease (cT3-4, cN1, or cM1) |
| Male patients eligible for and consenting to curative treatment | PSA > 50 ng/mL [13] |
| Life expectancy > 10 years | Life expectancy < 10 years |
| Disease limited to the prostate (no metastases) | Prior curative treatment or contraindication to curative treatment |
| At least one of the following—clinical stage of up to cT2c, or initial PSA > 20 ng/mL, or Gleason score > 7 |
| Variable | LRP | EBRT | |
|---|---|---|---|
| N (%) | N (%) | ||
| Patients with BCR | 21 (34.4) | 10 (21.7) | |
| 5-year BCR-free survival | 40 (65.6) | 33 (71.7) | |
| Median time to BCR | 30.0 months (IQR: 12.5–60.0) | 48.0 months (IQR: 30.0–60.0) | |
| Neurovascular bundle preservation | none | 25 (40.9) | - |
| unilateral | 32 (52.5) | - | |
| bilateral | 4 (6.6) | - | |
| Lymph node status | negative | 55 (90.2) | - |
| positive | 6 (9.8) | - | |
| Variable | LRP | EBRT | |||
|---|---|---|---|---|---|
| BCR (n) | BCR-Free (n) | BCR (n) | BCR-Free (n) | ||
| ISUP grade | 1 | 1 | 1 | 0 | 1 |
| 2 | 2 | 7 | 0 | 8 | |
| 3 | 2 | 8 | 1 | 4 | |
| 4 | 13 | 22 | 3 | 23 | |
| 5 | 3 | 2 | 6 | 2 | |
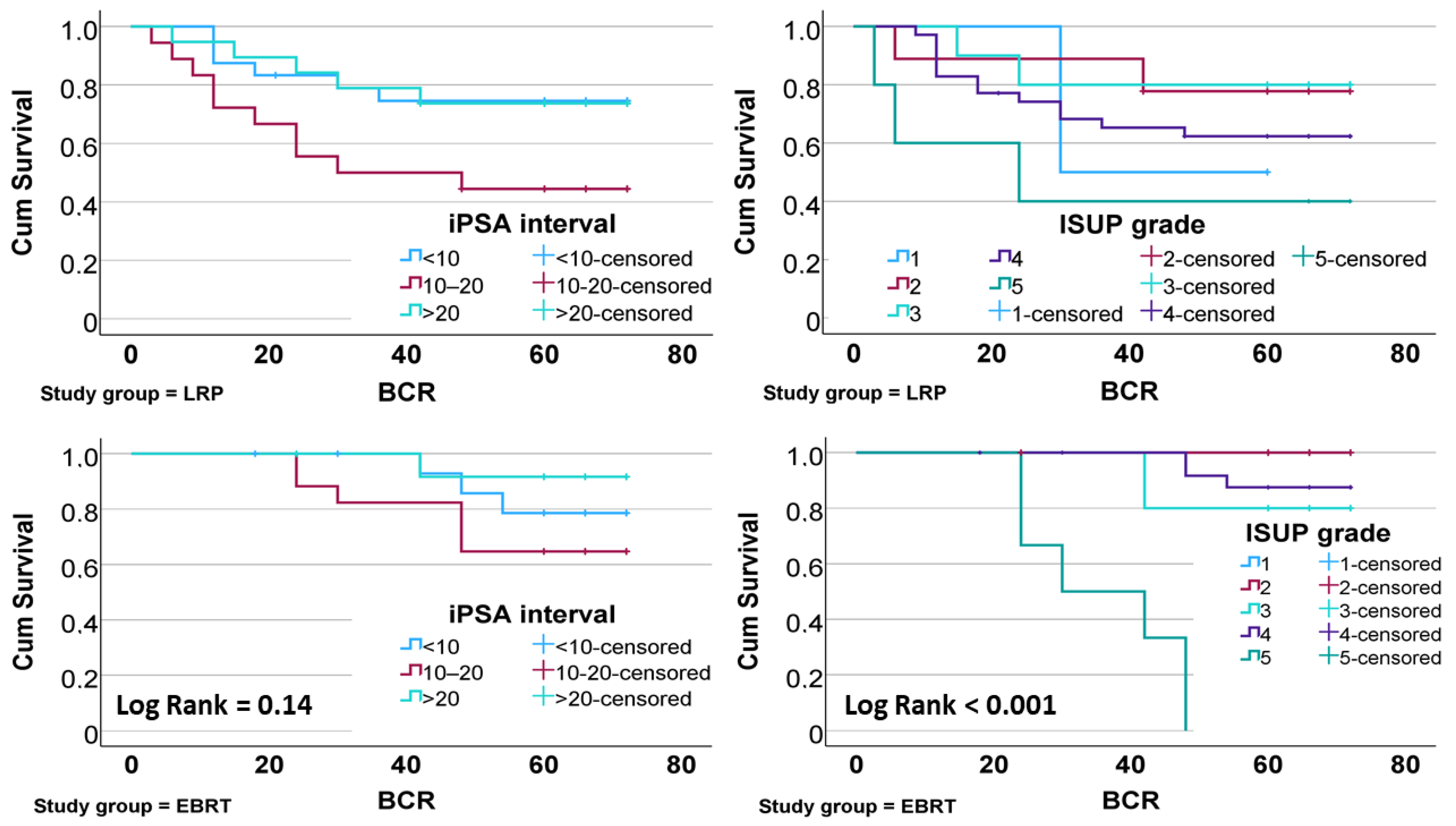
| χ2 p-value | <0.001 | 0.510 | |||
| Pathological stage | pT2 | 12 | 35 | - | - |
| pT3a | 9 | 2 | - | - | |
| pT3b | 0 | 3 | - | - | |
| χ2 p-value | <0.001 | - | |||
| Surgical margins | Negative | 11 | 38 | - | - |
| Positive | 10 | 2 | - | - | |
| χ2 p-value | <0.001 | - | |||
| iPSA interval | <10 | 18 | 6 | 13 | 3 |
| 10–20 | 8 | 10 | 11 | 6 | |
| >20 | 14 | 5 | 12 | 1 | |
| χ2 p-value | 0.080 | 0.180 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybar, L.; Hesko, P.; Miko, M.; Bujdak, P.; Harsanyi, S. Biochemical Recurrence in High-Risk Localized Prostate Cancer: A Comparison of Laparoscopic Prostatectomy and External Radiotherapy. Medicina 2025, 61, 928. https://doi.org/10.3390/medicina61050928
Rybar L, Hesko P, Miko M, Bujdak P, Harsanyi S. Biochemical Recurrence in High-Risk Localized Prostate Cancer: A Comparison of Laparoscopic Prostatectomy and External Radiotherapy. Medicina. 2025; 61(5):928. https://doi.org/10.3390/medicina61050928
Chicago/Turabian StyleRybar, Lubos, Patrik Hesko, Michal Miko, Peter Bujdak, and Stefan Harsanyi. 2025. "Biochemical Recurrence in High-Risk Localized Prostate Cancer: A Comparison of Laparoscopic Prostatectomy and External Radiotherapy" Medicina 61, no. 5: 928. https://doi.org/10.3390/medicina61050928
APA StyleRybar, L., Hesko, P., Miko, M., Bujdak, P., & Harsanyi, S. (2025). Biochemical Recurrence in High-Risk Localized Prostate Cancer: A Comparison of Laparoscopic Prostatectomy and External Radiotherapy. Medicina, 61(5), 928. https://doi.org/10.3390/medicina61050928






