Strategies to Reduce Hospital Length of Stay: Evidence and Challenges
Abstract
1. Introduction
1.1. Importance of HLOS as a Metric
1.2. Ethical Considerations and Predictive Models
1.3. Historical and Global Perspectives
1.4. Objective of the Review
1.5. Methods
2. Factors Influencing HLOS
2.1. Impacts of Extended HLOS
2.2. Quality of Life and Hospital Readmissions
2.3. Economic Burden of Prolonged Hospitalization
2.4. Predictive Models for HLOS
3. Factors for Reducing HLOS
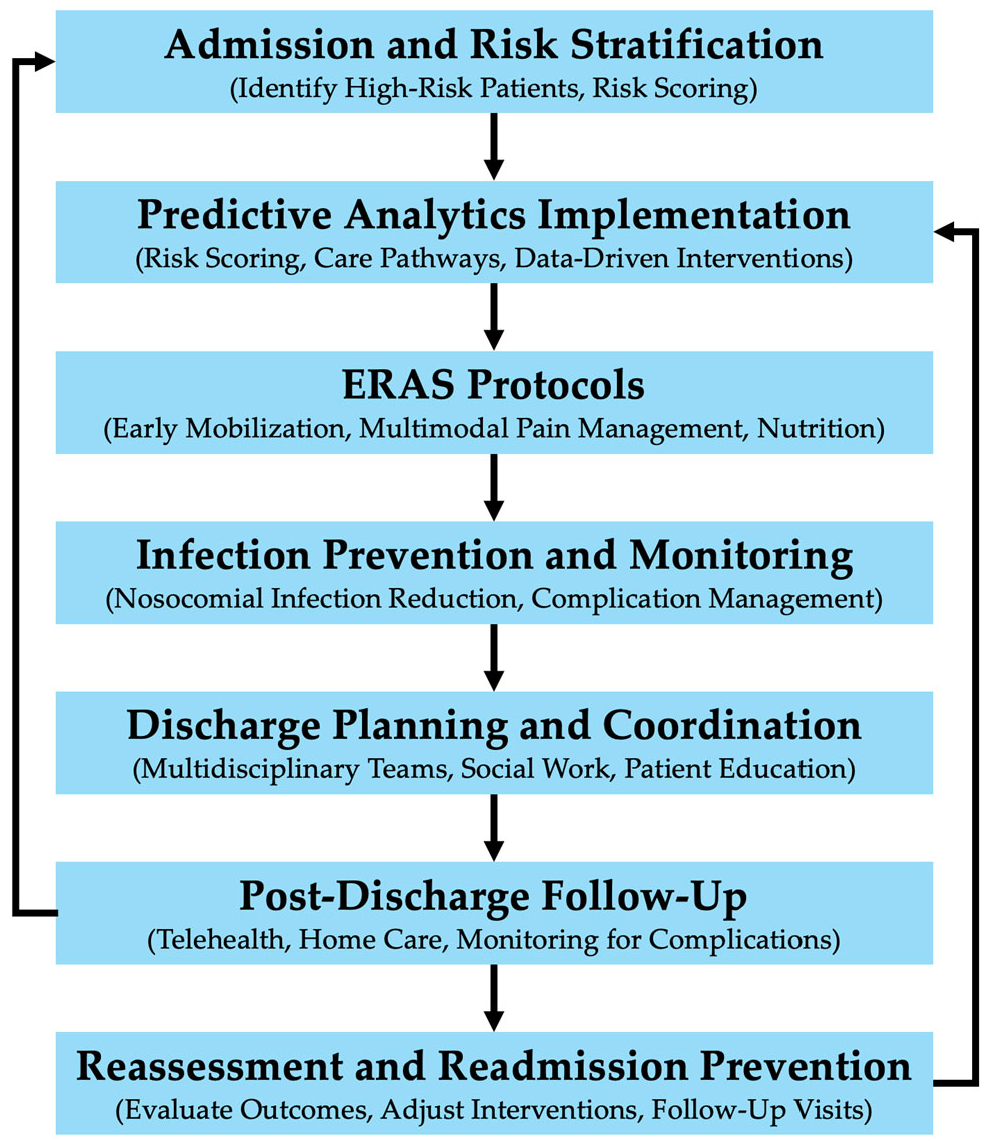
3.1. Enhanced Recovery After Surgery (ERAS) Protocols
3.2. Multidisciplinary Teams
3.3. Outpatient vs. Inpatient Decision-Making
3.4. Discharge Planning and Post-Discharge Care
3.5. Streamlining Patient Intake and Placement
4. Optimizing Hospital Length of Stay: Effective Strategies and Considerations
5. Challenges and Limitations in HLOS Research
5.1. Time-Dependent Bias in HLOS Estimation
5.2. Risk Adjustment Model Limitations
5.3. Challenges in HLOS Prediction Methods
5.4. Data Standardization and Cross-Setting Compatibility
5.5. Review Limitations
6. Future Directions and Research Gaps
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yue, J.K.; Ramesh, R.; Krishnan, N.; Chyall, L.; Halabi, C.; Huang, M.C.; Manley, G.T.; Tarapore, P.E.; DiGiorgio, A.M. Medicaid Insurance is a Predictor of Prolonged Hospital Length of Stay After Traumatic Brain Injury: A Stratified National Trauma Data Bank Cohort Analysis of 552 949 Patients. Neurosurgery 2024, 95, 186. [Google Scholar] [CrossRef] [PubMed]
- Lingsma, H.; Bottle, A.; Middleton, S.; Kievit, J.; Steyerberg, E.; Marang-van de Mheen, P. Evaluation of hospital outcomes: The relation between length-of-stay, readmission, and mortality in a large international administrative database. BMC Health Serv. Res. 2018, 18, 116. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Geisler, B.P.; Ibrahim, S. Racial/ethnic and socioeconomic variations in hospital length of stay: A state-based analysis. Medicine 2021, 100, e25976. [Google Scholar] [CrossRef]
- Chesley, C.F.; Chowdhury, M.; Small, D.S.; Schaubel, D.; Liu, V.X.; Lane-Fall, M.B.; Halpern, S.D.; Anesi, G.L. Racial Disparities in Length of Stay Among Severely Ill Patients Presenting with Sepsis and Acute Respiratory Failure. JAMA Netw. Open 2023, 6, e239739. [Google Scholar] [CrossRef] [PubMed]
- Philbin, E.; Roerden, J. Longer hospital length of stay is not related to better clinical outcomes in congestive heart failure. Am. J. Manag. Care 1997, 3, 1285–1291. [Google Scholar] [PubMed]
- Doherty, Z.; Kippen, R.; Bevan, D.; Duke, G.; Williams, S.; Wilson, A.; Pilcher, D. Long-term outcomes of hospital survivors following an ICU stay: A multi-centre retrospective cohort study. PLoS ONE 2022, 17, e0266038. [Google Scholar] [CrossRef]
- Han, T.S.; Murray, P.; Robin, J.; Wilkinson, P.; Fluck, D.; Fry, C.H. Evaluation of the association of length of stay in hospital and outcomes. Int. J. Qual. Health Care 2022, 34, mzab160. [Google Scholar] [CrossRef]
- Awad, A.; Bader-El-Den, M.; McNicholas, J. Patient length of stay and mortality prediction: A survey. Health Serv. Manag. Res. 2017, 30, 105–120. [Google Scholar] [CrossRef]
- Tessier, L.; Guilcher, S.; Bai, Y.; Ng, R.; Wodchis, W. The impact of hospital harm on length of stay, costs of care and length of person-centred episodes of care: A retrospective cohort study. CMAJ 2019, 191, E879–E885. [Google Scholar] [CrossRef]
- Rahmqvist, M.; Samuelsson, A.; Bastami, S.; Rutberg, H. Direct health care costs and length of hospital stay related to health care-acquired infections in adult patients based on point prevalence measurements. Am. J. Infect. Control 2016, 44, 500–506. [Google Scholar] [CrossRef]
- Doyle, C.; Lennox, L.; Bell, D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013, 3, e001570. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tisdale, R.L.; Heidenreich, P.A.; Sandhu, A.T. Disparities in Hospital Length of Stay Across Race and Ethnicity Among Patients with Heart Failure. Circ. Heart Fail. 2022, 15, e009362. [Google Scholar] [CrossRef]
- Regenbogen, S.E.; Cain-Nielsen, A.H.; Norton, E.C.; Chen, L.M.; Birkmeyer, J.D.; Skinner, J.S. Costs and Consequences of Early Hospital Discharge After Major Inpatient Surgery in Older Adults. JAMA Surg. 2017, 152, e170123. [Google Scholar] [CrossRef]
- Zhu, J.M.; Patel, V.; Shea, J.A.; Neuman, M.D.; Werner, R.M. Hospitals Using Bundled Payment Report Reducing Skilled Nursing Facility Use and Improving Care Integration. Health Aff. 2018, 37, 1282–1289. [Google Scholar] [CrossRef]
- Nazzani, S.; Preisser, F.; Mazzone, E.; Tian, Z.; Mistretta, F.A.; Shariat, S.F.; Saad, F.; Graefen, M.; Tilki, D.; Montanari, E.; et al. In-hospital length of stay after major surgical oncological procedures. Eur. J. Surg. Oncol. 2018, 44, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Molloy, I.B.; Martin, B.I.; Moschetti, W.E.; Jevsevar, D.S. Effects of the Length of Stay on the Cost of Total Knee and Total Hip Arthroplasty from 2002 to 2013. J. Bone Jt. Surg. Am. 2017, 99, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, P.J.; Go, J.T.; Hockenberry, J.; Glasgow, J.M.; Johnson, S.R.; Rosenthal, G.E.; Jones, M.P.; Vaughan-Sarrazin, M. Associations between reduced hospital length of stay and 30-day readmission rate and mortality: 14-year experience in 129 Veterans Affairs hospitals. Ann. Intern. Med. 2012, 157, 837–845. [Google Scholar] [CrossRef]
- Kalra, A.; Fisher, R.; Axelrod, P. Decreased Length of Stay and Cumulative Hospitalized Days Despite Increased Patient Admissions and Readmissions in an Area of Urban Poverty. J. Gen. Intern. Med. 2010, 25, 930–935. [Google Scholar] [CrossRef]
- Darzi, S.A.; Munz, Y. The impact of minimally invasive surgical techniques. Annu. Rev. Med. 2004, 55, 223–237. [Google Scholar] [CrossRef]
- Lequertier, V.; Wang, T.; Fondrevelle, J.; Augusto, V.; Duclos, A. Hospital Length of Stay Prediction Methods: A Systematic Review. Med. Care 2021, 59, 929–938. [Google Scholar] [CrossRef]
- Siddique, S.M.; Tipton, K.; Leas, B.; Greysen, S.R.; Mull, N.K.; Lane-Fall, M.; McShea, K.; Tsou, A.Y. Interventions to Reduce Hospital Length of Stay in High-risk Populations: A Systematic Review. JAMA Netw. Open 2021, 4, e2125846. [Google Scholar] [CrossRef]
- Fernandez, J.-L.; McGuire, A.; Raikou, M. Hospital coordination and integration with social care in England: The effect on post-operative length of stay. J. Health Econ. 2018, 61, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Kutz, A.; Koch, D.; Haubitz, S.; Conca, A.; Baechli, C.; Regez, K.; Gregoriano, C.; Ebrahimi, F.; Bassetti, S.; Eckstein, J.; et al. Association of Interprofessional Discharge Planning Using an Electronic Health Record Tool with Hospital Length of Stay Among Patients with Multimorbidity: A Nonrandomized Controlled Trial. JAMA Netw. Open 2022, 5, e2233667. [Google Scholar] [CrossRef]
- El-Sokkary, R.; Asaad, A. Hospital antibiotic stewardship interventions in low and middle income countries: A systematic review and meta-analysis. Microbes Infect. Dis. 2020, 1, 153–167. [Google Scholar] [CrossRef]
- Mohamedsharif, A.; Gemperli, A. Healthcare interventions to improve transitions from hospital to home in low-income countries: A scoping review of systematic reviews. Int. J. Integr. Care 2023, 23, 49. [Google Scholar] [CrossRef]
- Khosravizadeh, O.; Vatankhah, S.; Bastani, P.; Kalhor, R.; Alirezaei, S.; Doosty, F. Factors affecting length of stay in teaching hospitals of a middle-income country. Electron. Physician 2016, 8, 3042–3047. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-Y.; Lin, K.-T.R.; Hsiao, Y.-L.; Huang, H.-C.; Chang, S.-N.; Hung, C.-H.; Chang, Y.; Wang, Y.-C.; Kuo, C.-C. Association Between Preoperative Blood Glucose Level and Hospital Length of Stay for Patients Undergoing Appendectomy or Laparoscopic Cholecystectomy. Diabetes Care 2020, 44, 107–115. [Google Scholar] [CrossRef]
- Arias-Fernández, P.; Romero-Martin, M.; Gómez-Salgado, J.; Fernández-García, D. Rehabilitation and early mobilization in the critical patient: Systematic review. J. Phys. Ther. Sci. 2018, 30, 1193–1201. [Google Scholar] [CrossRef]
- Haghparast-Bidgoli, H.; Saadat, S.; Bogg, L.; Yarmohammadian, M.; Hasselberg, M. Factors affecting hospital length of stay and hospital charges associated with road traffic-related injuries in Iran. BMC Health Serv. Res. 2013, 13, 281. [Google Scholar] [CrossRef]
- Walsh, B.; Smith, S.; Wren, M.; Eighan, J.; Lyons, S. The impact of inpatient bed capacity on length of stay. Eur. J. Health Econ. 2022, 23, 499–510. [Google Scholar] [CrossRef]
- Hirani, R.; Smiley, A.; Latifi, L.; Latifi, R. The Risk of Mortality in Geriatric Patients with Emergent Gastroparesis is 7-fold Greater than that in Adult Patients: An Analysis of 27,000 Patients. Surg. Technol. Int. 2022, 40, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Elgar, G.; Smiley, A.; Latifi, R. Major Risk Factors for Mortality in Elderly and Non-Elderly Adult Patients Emergently Admitted for Blunt Chest Wall Trauma: Hospital Length of Stay as an Independent Predictor. Int. J. Environ. Res. Public Health 2022, 19, 8729. [Google Scholar] [CrossRef]
- Hirani, R.; Smiley, A.; Latifi, R. Odds of Mortality in Geriatric Patients with Appendicitis is 22 Times Higher than that in Non-Geriatrics: An Analysis of 336,880 Patients from NIS Database. Surg. Technol. Int. 2023, 43, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Latifi, R.; Levy, L.; Reddy, M.; Okumura, K.; Smiley, A. Delayed Operation as a Major Risk Factor for Mortality Among Elderly Patients with Ventral Hernia Admitted Emergently: An Analysis of 33,700 Elderly Patients. Surg. Technol. Int. 2021, 39, 206–213. [Google Scholar] [CrossRef]
- Levy, L.; Smiley, A.; Latifi, R. Independent Predictors of In-Hospital Mortality in Elderly and Non-elderly Adult Patients Undergoing Emergency Admission for Hemorrhoids. Am. Surg. 2022, 88, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Attrill, S.; White, S.; Murray, J.; Hammond, S.; Doeltgen, S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: A systematic review. BMC Health Serv. Res. 2018, 18, 594. [Google Scholar] [CrossRef]
- Lin, N.; Smiley, A.; Goud, M.; Lin, C.; Latifi, R. Risk Factors of Mortality in Patients Hospitalized with Chronic Duodenal Ulcers. Am. Surg. 2022, 88, 764–769. [Google Scholar] [CrossRef]
- Murni, I.; Duke, T.; Kinney, S.; Daley, A.; Wirawan, M.; Soenarto, Y. Risk factors for healthcare-associated infection among children in a low-and middle-income country. BMC Infect. Dis. 2022, 22, 406. [Google Scholar] [CrossRef]
- Morgan, D.; Lomotan, L.; Agnes, K.; McGrail, L.; Roghmann, M. Characteristics of Healthcare-Associated Infections Contributing to Unexpected In-Hospital Deaths. Infect. Control Hosp. Epidemiol. 2010, 31, 864–866. [Google Scholar] [CrossRef]
- Goiburu, M.E.; Jure Goiburu, M.M.; Bianco, H.; Ruiz Díaz, J.; Alderete, F.; Palacios, M.C.; Cabral, V.; Escobar, D.; López, R.; Waitzberg, D.L. The impact of malnutrition on morbidity, mortality and length of hospital stay in trauma patients. Nutr. Hosp. 2006, 21, 604–610. [Google Scholar]
- Nigatu, Y.; Gebreyesus, S.; Allard, J.; Endris, B. The effect of malnutrition at admission on length of hospital stay among adult patients in developing country: A prospective cohort study. Clin. Nutr. ESPEN 2021, 41, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Vong, T.; Yanek, L.R.; Wang, L.; Yu, H.; Fan, C.; Zhou, E.; Oh, S.J.; Szvarca, D.; Kim, A.; Potter, J.J.; et al. Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19. Nutrients 2022, 14, 1310. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shi, J.; He, S.; Luo, X.; Zhong, W.; Yang, F. Understanding variations and influencing factors on length of stay for T2DM patients based on a multilevel model. PLoS ONE 2021, 16, e0248157. [Google Scholar] [CrossRef]
- Hindi, M.N.; Dandurand, C.; Ailon, T.; Boyd, M.; Charest-Morin, R.; Dea, N.; Dvorak, M.F.; Fisher, C.; Kwon, B.K.; Paquette, S.; et al. Factors contributing to a longer length of stay in adults admitted to a quaternary spinal care center. Eur. Spine J. 2023, 32, 824–830. [Google Scholar] [CrossRef]
- Lim, J. Big Data-Driven Determinants of Length of Stay for Patients with Hip Fracture. Int. J. Environ. Res. Public Health 2020, 17, 4949. [Google Scholar] [CrossRef] [PubMed]
- Hurisa Dadi, H.; Habte, N.; Mulu, Y. Length of hospital stay and associated factors among adult surgical patients admitted to surgical wards in Amhara Regional State Comprehensive Specialized Hospitals, Ethiopia. PLoS ONE 2024, 19, e0296143. [Google Scholar] [CrossRef]
- Proaño-Zamudio, J.A.; Argandykov, D.; Renne, A.; Gebran, A.; Ouwerkerk, J.J.J.; Dorken-Gallastegi, A.; de Roulet, A.; Velmahos, G.C.; Kaafarani, H.M.A.; Hwabejire, J.O. Timing of regional analgesia in elderly patients with blunt chest-wall injury. Surgery 2023, 174, 901–906. [Google Scholar] [CrossRef]
- Anil, U.; Robitsek, R.J.; Kingery, M.T.; Lin, C.C.; McKenzie, K.; Konda, S.R.; Egol, K.A. Orthopedic pelvic and extremity injuries increase overall hospital length of stay but not in-hospital complications or mortality in trauma ICU patients: Orthopedic Injuries in Trauma ICU Patients. Injury 2024, 55, 111843. [Google Scholar] [CrossRef]
- Emmett, C.J.; Lim, W.K.; Griffiths, A.; Aitken, R.; Read, D.J.; Gregorevic, K. Low falls and inpatient complications increase risk for longer length of stay in older persons admitted following trauma. BMC Geriatr. 2025, 25, 98. [Google Scholar] [CrossRef]
- Qattea, I.; Ejaz, S.; Kattea, M.O.; Kteish, R.; Chaudry, S.; Aly, H. The effect of demographic, financial and hospital factors on the length of stay of preterm infants: A US national database study. J. Perinatol. 2024, 44, 173–178. [Google Scholar] [CrossRef]
- Marfil-Garza, B.A.; Belaunzarán-Zamudio, P.F.; Gulias-Herrero, A.; Zuñiga, A.C.; Caro-Vega, Y.; Kershenobich-Stalnikowitz, D.; Sifuentes-Osornio, J. Risk factors associated with prolonged hospital length-of-stay: 18-year retrospective study of hospitalizations in a tertiary healthcare center in Mexico. PLoS ONE 2018, 13, e0207203. [Google Scholar] [CrossRef] [PubMed]
- Peres, I.T.; Hamacher, S.; Oliveira, F.L.C.; Thomé, A.M.T.; Bozza, F.A. What factors predict length of stay in the intensive care unit? Systematic review and meta-analysis. J. Crit. Care 2020, 60, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J. Relationships Among Comorbidities, Disease Severity, and Hospitalization Duration in the United States Using the Healthcare Cost and Utilization Project (HCUP) Database. J. Clin. Med. 2025, 14, 680. [Google Scholar] [CrossRef] [PubMed]
- Sedrakyan, A.; Kamel, H.; Mao, J.; Ting, H.; Paul, S. Hospital Readmission and Length of Stay Over Time in Patients Undergoing Major Cardiovascular and Orthopedic Surgery: A Tale of 2 States. Med. Care 2016, 54, 592–599. [Google Scholar] [CrossRef]
- Castorina, S.; Guglielmino, C.; Castrogiovanni, P.; Szychlinska, M.; Ioppolo, F.; Massimino, P.; Leonardi, P.; Maci, C.; Iannuzzi, M.; Di Giunta, A.; et al. Clinical evidence of traditional vs fast track recovery methodologies after total arthroplasty for osteoarthritic knee treatment. A retrospective observational study. Muscles Ligaments Tendons J. 2018, 7, 504–513. [Google Scholar] [CrossRef]
- Kontos, A.P.; Jorgensen-Wagers, K.; Trbovich, A.M.; Ernst, N.; Emami, K.; Gillie, B.; French, J.; Holland, C.; Elbin, R.J.; Collins, M.W. Association of Time Since Injury to the First Clinic Visit With Recovery Following Concussion. JAMA Neurol. 2020, 77, 435–440. [Google Scholar] [CrossRef]
- Glied, S.; Cohen, B.; Liu, J.; Neidell, M.; Larson, E. Trends in mortality, length of stay, and hospital charges associated with health care-associated infections, 2006-2012. Am. J. Infect. Control 2016, 44, 983–989. [Google Scholar] [CrossRef]
- Pittet, D.; Tarara, D.; Wenzel, R.P. Nosocomial Bloodstream Infection in Critically Ill Adults: Excess Length of Stay, Extra Costs, and Attributable Mortality. Surv. Anesthesiol. 1994, 38, 361. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chou, Y.-C.; Chou, P. Impact of nosocomial infection on cost of illness and length of stay in intensive care units. Infect. Control Hosp. Epidemiol. 2005, 26, 281–287. [Google Scholar] [CrossRef]
- Jia, H.; Li, L.; Li, W.; Hou, T.; Ma, H.; Yang, Y.; Wu, A.; Liu, Y.; Wen, J.; Yang, H.; et al. Impact of Healthcare-Associated Infections on Length of Stay: A Study in 68 Hospitals in China. Biomed. Res. Int. 2019, 2019, 2590563. [Google Scholar] [CrossRef]
- Lagoe, R.; Johnson, P.; Murphy, M. Inpatient hospital complications and lengths of stay: A short report. BMC Res. Notes 2011, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Chen, I.; Tang, Y.; Lee, J.; Liu, J. Increased financial burdens and lengths of stay in patients with healthcare-associated infections due to multidrug-resistant bacteria in intensive care units: A propensity-matched case-control study. PLoS ONE 2020, 15, e0233265. [Google Scholar] [CrossRef] [PubMed]
- Valent, F.; Tonutti, L.; Grimaldi, F. Does diabetes mellitus comorbidity affect in-hospital mortality and length of stay? Analysis of administrative data in an Italian Academic Hospital. Acta Diabetol. 2017, 54, 1081–1090. [Google Scholar] [CrossRef]
- Baek, H.; Cho, M.; Kim, S.; Hwang, H.; Song, M.; Yoo, S. Analysis of length of hospital stay using electronic health records: A statistical and data mining approach. PLoS ONE 2018, 13, e0195901. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.; Lim, Z.; Yap, P.; Tang, T. Factors associated with prolonged length of stay in older patients. Singap. Med. J. 2017, 58, 134–138. [Google Scholar] [CrossRef]
- Hauck, K.; Zhao, X. How dangerous is a day in hospital? A model of adverse events and length of stay for medical inpatients. Med. Care 2011, 49, 1068–1075. [Google Scholar] [CrossRef]
- Rachoin, J.-S.; Aplin, K.S.; Gandhi, S.; Kupersmith, E.; Cerceo, E. Impact of Length of Stay on Readmission in Hospitalized Patients. Cureus 2020, 12, e10669. [Google Scholar] [CrossRef]
- Ward, C.; Patel, V.; Elsaid, M.I.; Jaisinghani, P.; Sharma, R. A case-control study of length of stay outliers. Am. J. Manag. Care 2021, 27, e66–e71. [Google Scholar] [CrossRef]
- Chen, F.; Day, S.L.; Metcalfe, R.A.; Sethi, G.; Kapembwa, M.S.; Brook, M.G.; Churchill, D.; De Ruiter, A.; Robinson, S.; Lacey, C.J.; et al. Characteristics of Autoimmune Thyroid Disease Occurring as a Late Complication of Immune Reconstitution in Patients With Advanced Human Immunodeficiency Virus (HIV) Disease. Medicine 2005, 84, 98–106. [Google Scholar] [CrossRef]
- Gidey, K.; Gidey, M.T.; Hailu, B.Y.; Gebreamlak, Z.B.; Niriayo, Y.L. Clinical and economic burden of healthcare-associated infections: A prospective cohort study. PLoS ONE 2023, 18, e0282141. [Google Scholar] [CrossRef]
- Taliwal, N.; Pandya, A.; Dixon, A.; Tibrewal, A.; Kumar, R.J.; Doshi, K.; Warady, B.A.; Raina, R. Economic burden of inpatient hospitalizations for pediatric chronic kidney disease in the US. Pediatr. Nephrol. 2025, 40, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Monard, C.; Rimmelé, T.; Blanc, E.; Goguillot, M.; Bénard, S.; Textoris, J. Economic burden of in-hospital AKI: A one-year analysis of the nationwide French hospital discharge database. BMC Nephrol. 2023, 24, 343. [Google Scholar] [CrossRef] [PubMed]
- Osenenko, K.M.; Kuti, E.; Deighton, A.M.; Pimple, P.; Szabo, S.M. Burden of hospitalization for heart failure in the United States: A systematic literature review. J. Manag. Care Spec. Pharm. 2022, 28, 157–167. [Google Scholar] [CrossRef]
- Asegu, L.M.; Kitschen, A.; Neuwirth, M.M.; Sauerland, D. The economic burden of nosocomial infections for hospitals: Evidence from Germany. BMC Infect. Dis. 2024, 24, 1294. [Google Scholar] [CrossRef]
- Wakil, A.; Wu, Y.-C.; Mazzaferro, N.; Greenberg, P.; Pyrsopoulos, N.T.M. Trends of Hepatocellular Carcinoma (HCC) Inpatients Mortality and Financial Burden From 2011 to 2017: A Nationwide Analysis. J. Clin. Gastroenterol. 2024, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Nie, P.; Zhang, J.; Zhou, D.; Zhang, J.; Chen, J. Clinical and Economic Insights into Parkinson’s Disease Hospitalization: A Comprehensive Study of 19,719 Inpatient Cases in Hubei Province, China. Neuroepidemiology 2024, 58, 237–246. [Google Scholar] [CrossRef]
- Dai, X.; Gao, M.; Liu, Y.; Lv, R.; Chen, H.; Miao, H.; Zhang, Y. Analysis of inpatient cost burden and influencing factors of seniors’ patients with mental illness in Dalian, China. BMC Geriatr. 2023, 23, 739. [Google Scholar] [CrossRef]
- Liu, X.; Spencer, A.; Long, Y.; Greenhalgh, C.; Steeg, S.; Verma, A. A systematic review and meta-analysis of disease burden of healthcare-associated infections in China: An economic burden perspective from general hospitals. J. Hosp. Infect. 2022, 123, 1–11. [Google Scholar] [CrossRef]
- Chu, X.; Liu, S.; Dai, X.; Chen, W.; Qi, G.; Jiang, X.; Wu, Z.; Zhou, Y.; Shi, X. Systematic analysis of the occurrence characteristics and impact on hospitalization costs of trauma complications. Am. J. Surg. 2024, 237, 115936. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, X.; Wu, J.; Xiao, X.; Ma, C.; Jiang, X.; Zhou, P.; Liu, L.; Jiang, Y.; Zou, A.; et al. Economic burden attributable to healthcare-associated infections at western China hospitals: 6 Year, prospective cohort study. J. Infect. 2024, 88, 112–122. [Google Scholar] [CrossRef]
- Gholipour, K.; Behpaie, S.; Iezadi, S.; Ghiasi, A.; Tabrizi, J.S. Costs of inpatient care and out-of-pocket payments for COVID-19 patients: A systematic review. PLoS ONE 2023, 18, e0283651. [Google Scholar] [CrossRef]
- Turgeman, L.; May, J.; Sciulli, R. Insights from a machine learning model for predicting the hospital Length of Stay (LOS) at the time of admission. Expert. Syst. Appl. 2017, 78, 376–385. [Google Scholar] [CrossRef]
- Daghistani, T.; Elshawi, R.; Sakr, S.; Ahmed, A.; Al-Thwayee, A.; Al-Mallah, M. Predictors of in-hospital length of stay among cardiac patients: A machine learning approach. Int. J. Cardiol. 2019, 288, 140–147. [Google Scholar] [CrossRef]
- Cummings, D. Predicting Hospital Length-of-Stay at Time of Admission. Available online: https://medium.com/data-science/predicting-hospital-length-of-stay-at-time-of-admission-55dfdfe69598 (accessed on 20 February 2025).
- LaFaro, R.J.; Pothula, S.; Kubal, K.P.; Inchiosa, M.E.; Pothula, V.M.; Yuan, S.C.; Maerz, D.A.; Montes, L.; Oleszkiewicz, S.M.; Yusupov, A.; et al. Neural Network Prediction of ICU Length of Stay Following Cardiac Surgery Based on Pre-Incision Variables. PLoS ONE 2015, 10, e0145395. [Google Scholar] [CrossRef] [PubMed]
- Greer, N.L.; Gunnar, W.P.; Dahm, P.; Lee, A.E.; MacDonald, R.; Shaukat, A.; Sultan, S.; Wilt, T.J. Enhanced Recovery Protocols for Adults Undergoing Colorectal Surgery: A Systematic Review and Meta-analysis. Dis. Colon. Rectum 2018, 61, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Changjun, C.; Jingkun, L.; Yun, Y.; Yingguang, W.; Yanjun, R.; Debo, Z.; Kaining, Z.; Pengde, K. Enhanced Recovery after Total Joint Arthroplasty (TJA): A Contemporary Systematic Review of Clinical Outcomes and Usage of Key Elements. Orthop. Surg. 2023, 15, 1228–1240. [Google Scholar] [CrossRef]
- De Roo, A.C.; Vu, J.V.; Regenbogen, S.E. Statewide Utilization of Multimodal Analgesia and Length of Stay After Colectomy. J. Surg. Res. 2020, 247, 264–270. [Google Scholar] [CrossRef]
- Ward, C.T.; Moll, V.; Boorman, D.W.; Ooroth, L.; Groff, R.F.; Gillingham, T.D.; Pyronneau, L.; Prabhakar, A. The impact of a postoperative multimodal analgesia pathway on opioid use and outcomes after cardiothoracic surgery. J. Cardiothorac. Surg. 2022, 17, 342. [Google Scholar] [CrossRef]
- O’Mahony, S.; Mazur, E.; Charney, P.; Wang, Y.; Fine, J. Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J. Gen. Intern. Med. 2007, 22, 1073–1079. [Google Scholar] [CrossRef]
- Chava, R.; Karki, N.; Ketlogetswe, K.; Ayala, T. Multidisciplinary rounds in prevention of 30-day readmissions and decreasing length of stay in heart failure patients. Medicine 2019, 98, e16233. [Google Scholar] [CrossRef]
- Suarez, J.I.; Zaidat, O.O.; Suri, M.F.; Feen, E.S.; Lynch, G.; Hickman, J.; Georgiadis, A.; Selman, W.R. Length of stay and mortality in neurocritically ill patients: Impact of a specialized neurocritical care team. Crit. Care Med. 2004, 32, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Mercedes, A.; Fairman, P.; Hogan, L.; Thomas, R.; Slyer, J. Effectiveness of structured multidisciplinary rounding in acute care units on length of stay and satisfaction of patients and staff: A quantitative systematic review. JBI Evid. Synth. 2016, 14, 131–168. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Schultz, T.; Halligan, P.; Sheridan, A.; Kinsman, L.; Rotter, T.; Beaumier, J.; Kelly, R.G.; Drennan, J. Hospital nurse-staffing models and patient- and staff-related outcomes. Cochrane Database Syst. Rev. 2019, 4, 1465–1858. [Google Scholar] [CrossRef]
- Pollard, J.; Garnerin, P.; Dalman, R. Use of Outpatient Preoperative Evaluation to Decrease Length of Stay for Vascular Surgery. Anesth. Analg. 1997, 85, 1307. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Bradley, D.C.; Lannin, N.A.; Clemson, L.; Cameron, I.D.; Shepperd, S. Discharge planning from hospital. Cochrane Database Syst. Rev. 2022, 2, CD000313. [Google Scholar] [CrossRef]
- Rachoin, J.; Aplin, K.; Kupersmith, E.; Gandhi, S.; Travis, K.; Stefaniak, M.; Cerceo, E. Discharge Before Noon: Is the Sun Half Up or Half Down? Am. J. Manag. Care 2020, 26, e246–e251. [Google Scholar]
- Hyslop, B. Should older people ever be discharged from hospital at night? Bioethical Inq. 2022, 19, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, J.; Hu, H.; Wang, S. Effectiveness of nurse-led early discharge planning programmes for hospital inpatients with chronic disease or rehabilitation needs: A systematic review and meta-analysis. J. Clin. Nurs. 2015, 24, 2993–3005. [Google Scholar] [CrossRef]
- Rathlev, N.; Bryson, C.; Samra, P.; Garreffi, L.; Li, H.; Geld, B.; Wu, R.Y.; Visintainer, P. Reducing Patient Placement Errors in Emergency Department Admissions: Right Patient, Right Bed. West. J. Emerg. Med. 2014, 15, 687–692. [Google Scholar] [CrossRef]
- Weingarten, S.; Riedinger, M.; Sandhu, M.; Bowers, C.; Ellrodt, A.; Nunn, C.; Hobson, P.; Greengold, N. Can practice guidelines safely reduce hospital length of stay? Results from a multicenter interventional study. Am. J. Med. 1998, 105, 33–40. [Google Scholar] [CrossRef]
- Delgadillo, J.; Moreea, O.; Lutz, W. Different people respond differently to therapy: A demonstration using patient profiling and risk stratification. Behav. Res. Ther. 2016, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sacristán, J.; Avendaño-Solá, C. On heterogeneity of treatment effects and clinical freedom. Int. J. Clin. Pract. 2015, 69, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Weiland, D. Why use clinical pathways rather than practice guidelines? Am. J. Surg. 1997, 174, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Miani, C.; Ball, S.; Pitchforth, E.; Exley, J.; King, S.; Roland, M.; Fuld, J.; Nolte, E. Organisational interventions to reduce length of stay in hospital: A rapid evidence assessment. Health Serv. Deliv. Res. 2014, 2, 1–178. [Google Scholar] [CrossRef]
- Lee, Y.; Yu, J.; Doumouras, A.; Li, J.; Hong, D. Enhanced recovery after surgery (ERAS) versus standard recovery for elective gastric cancer surgery: A meta-analysis of randomized controlled trials. Surg. Oncol. 2020, 32, 75–87. [Google Scholar] [CrossRef]
- Fu, V.; Oomens, P.; Klimek, M.; Verhofstad, M.; Jeekel, J. The Effect of Perioperative Music on Medication Requirement and Hospital Length of Stay. Ann. Surg. 2020, 272, 961–972. [Google Scholar] [CrossRef]
- Pannick, S.; Davis, R.; Ashrafian, H.; Byrne, B.; Beveridge, I.; Athanasiou, T.; Wachter, R.M.; Sevdalis, N. Effects of Interdisciplinary Team Care Interventions on General Medical Wards: A Systematic Review. JAMA Intern. Med. 2015, 175, 1288–1298. [Google Scholar] [CrossRef]
- Nelson, R.E.; Nelson, S.D.; Khader, K.; Perencevich, E.L.; Schweizer, M.L.; Rubin, M.A.; Graves, N.; Harbarth, S.; Stevens, V.W.; Samore, M.H. The Magnitude of Time-Dependent Bias in the Estimation of Excess Length of Stay Attributable to Healthcare-Associated Infections. Infect. Control Hosp. Epidemiol. 2015, 36, 1089–1094. [Google Scholar] [CrossRef]
- Manoukian, S.; Stewart, S.; Dancer, S.; Graves, N.; Mason, H.; McFarland, A.; Robertson, C.; Reilly, J. Estimating excess length of stay due to healthcare-associated infections: A systematic review and meta-analysis of statistical methodology. J. Hosp. Infect. 2018, 100, 222–235. [Google Scholar] [CrossRef]
- De Angelis, G.; Murthy, A.; Beyersmann, J.; Harbarth, S. Estimating the impact of healthcare-associated infections on length of stay and costs. Clin. Microbiol. Infect. 2010, 16, 1729–1735. [Google Scholar] [CrossRef]
- Busby, J.; Purdy, S.; Hollingworth, W. Calculating hospital length of stay using the Hospital Episode Statistics; a comparison of methodologies. BMC Health Serv. Res. 2017, 17, 347. [Google Scholar] [CrossRef]
- Lu, M.; Sajobi, T.; Lucyk, K.; Lorenzetti, D.; Quan, H. Systematic review of risk adjustment models of hospital length of stay (LOS). Med. Care 2015, 53, 355–365. [Google Scholar] [CrossRef]
- Sajobi, T.T.; Wang, M.; Awosoga, O.; Santana, M.; Southern, D.; Liang, Z.; Galbraith, D.; Wilton, S.B.; Quan, H.; Graham, M.M.; et al. Trajectories of Health-Related Quality of Life in Coronary Artery Disease. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e003661. [Google Scholar] [CrossRef]
- Petch, J.; Di, S.; Nelson, W. Opening the Black Box: The Promise and Limitations of Explainable Machine Learning in Cardiology. Can. J. Cardiol. 2022, 38, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.K.; Pollock, B.D.; Kurtz, S.M.; Lau, E. State and Government Administrative Databases: Medicare, National Inpatient Sample (NIS), and State Inpatient Databases (SID) Programs. J. Bone Jt. Surg. Am. 2022, 104, 4–8. [Google Scholar] [CrossRef] [PubMed]
- González-Colom, R.; Herranz, C.; Vela, E.; Monterde, D.; Contel, J.C.; Sisó-Almirall, A.; Piera-Jiménez, J.; Roca, J.; Cano, I. Prevention of Unplanned Hospital Admissions in Multimorbid Patients Using Computational Modeling: Observational Retrospective Cohort Study. J. Med. Internet Res. 2023, 25, e40846. [Google Scholar] [CrossRef]
- Ng, K.; Kartoun, U.; Stavropoulos, H.; Zambrano, J.A.; Tang, P.C. Personalized treatment options for chronic diseases using precision cohort analytics. Sci. Rep. 2021, 11, 1139. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, H.R.; Branquinho, A.; Pinto, J.; Rodrigues, A.M.; Santos, C.P. Digital biomarkers of mobility and quality of life in Parkinson’s disease based on a wearable motion analysis LAB. Comput. Methods Programs Biomed. 2024, 244, 107967. [Google Scholar] [CrossRef]
- Liu, J.-H.; Shih, C.-Y.; Huang, H.-L.; Peng, J.-K.; Cheng, S.-Y.; Tsai, J.-S.; Lai, F. Evaluating the Potential of Machine Learning and Wearable Devices in End-of-Life Care in Predicting 7-Day Death Events Among Patients With Terminal Cancer: Cohort Study. J. Med. Internet Res. 2023, 25, e47366. [Google Scholar] [CrossRef]
- Glicksberg, B.S.; Johnson, K.W.; Dudley, J.T. The next generation of precision medicine: Observational studies, electronic health records, biobanks and continuous monitoring. Hum. Mol. Genet. 2018, 27, R56–R62. [Google Scholar] [CrossRef]
- Schüssler-Fiorenza Rose, S.M.; Contrepois, K.; Moneghetti, K.J.; Zhou, W.; Mishra, T.; Mataraso, S.; Dagan-Rosenfeld, O.; Ganz, A.B.; Dunn, J.; Hornburg, D.; et al. A longitudinal big data approach for precision health. Nat. Med. 2019, 25, 792–804. [Google Scholar] [CrossRef] [PubMed]
- van de Poll-Franse, L.V.; Horevoorts, N.; Schoormans, D.; Beijer, S.; Ezendam, N.P.M.; Husson, O.; Oerlemans, S.; Schagen, S.B.; Hageman, G.J.; Van Deun, K.; et al. Measuring Clinical, Biological, and Behavioral Variables to Elucidate Trajectories of Patient-Reported Outcomes: The PROFILES Registry. J. Natl. Cancer Inst. 2022, 114, 800–807. [Google Scholar] [CrossRef] [PubMed]
| Study Name | Year | Sample Size | Variables Studied | Impact on HLOS |
|---|---|---|---|---|
| Major Risk Factors for Mortality in Elderly and Non-Elderly Adult Patients Emergently Admitted for Blunt Chest Wall Trauma [32] | 2022 | 2158 | HLOS, comorbidities, mortality | Each additional day: 9% increased mortality (OR = 1.09, p = 0.033) |
| Understanding Variations and Influencing Factors on Length of Stay for T2DM Patients Based on a Multilevel Model [43] | 2021 | 12,888 | Gender, insurance type, hospital characteristics, complications | Female: shorter LOS; Elderly: longer LOS (p < 0.001) |
| Factors Contributing to a Longer Length of Stay in Adults Admitted to a Quaternary Spinal Care Center [44] | 2023 | 13,493 | Age, admission status, ASIA grade, operative management, adverse events | Advanced age: 1.011/year; Emergency: 1.615 (p < 0.05) |
| Big Data-Driven Determinants of Length of Stay for Patients With Hip Fracture [45] | 2020 | 2238 | Gender, age, insurance type, admission route, comorbidities | Comorbidities: significant impact (p < 0.05) |
| Length of Hospital Stay and Associated Factors Among Adult Surgical Patients [46] | 2024 | 452 | Referral status, pneumonia, surgery duration, BMI, preoperative anemia | Pneumonia: 3.64; Surgery ≥ 110 min: 2.54 (AOR) |
| Timing of Regional Analgesia in Elderly Patients with Blunt Chest-Wall Injury Fall [47] | 2023 | 2248 | Early vs. late regional analgesia, unplanned intubation, ICU admission, discharge | Early analgesia: 5.5 days vs. 6.5 days (p = 0.002) |
| Orthopedic Pelvic and Extremity Injuries Increase Overall Hospital Length of Stay but Not In-Hospital Complications or Mortality in Trauma ICU Patients [48] | 2024 | 1785 | Orthopedic injuries, ICU LOS, hospital LOS, complications, mortality | Orthopedic injury: 1.23 times longer LOS (p < 0.001) |
| Low Falls and Inpatient Complications Increase Risk for Longer Length of Stay in Older Persons Admitted Following Trauma [49] | 2025 | 1250 | Delirium, inpatient fall, pneumonia, thromboembolism, blood transfusion, ICU admission | Delirium: IRR 1.41; Inpatient fall: IRR 1.46; Pneumonia: IRR 1.28 (p < 0.05) |
| The Effect of Demographic, Financial and Hospital Factors on the Length of Stay of Preterm Infants [50] | 2024 | 1,359,280 | Gestational age (GA), ethnic group, hospital size, geographic region | GA: significant impact; Ethnic group: significant impact (p < 0.001) |
| Risk Factors Associated With Prolonged Hospital Length-of-Stay: 18-Year Retrospective Study of Hospitalizations in a Tertiary Healthcare Center in Mexico [51] | 2018 | 85,904 | Age, gender, physician-to-patient ratio, emergency admission, comorbidities | Bone marrow transplant: OR 18.39; Infections: OR 4.65 |
| What Factors Predict Length of Stay in the Intensive Care Unit? Systematic Review and Meta-Analysis [52] | 2020 | N/A | Mechanical ventilation, hypomagnesemia, delirium, malnutrition | Mechanical ventilation: longer LOS (p < 0.05) |
| Relationships Among Comorbidities, Disease Severity, and Hospitalization Duration in the United States Using the Healthcare Cost and Utilization Project (HCUP) Database [53] | 2025 | N/A | Age, comorbidities, disease severity | MCCs: >90% elderly; Severe disease: longer LOS |
| Racial/Ethnic and Socioeconomic Variations in Hospital Length of Stay: A State-Based Analysis [3] | 2021 | 1,432,683 | Race/ethnicity, socioeconomic status, median household income by ZIP code | Black patients: 0.25 days longer LOS; Wealthier patients: shorter LOS |
| Hospital Readmission and Length of Stay Over Time in Patients Undergoing Major Cardiovascular and Orthopedic Surgery: A Tale of 2 States [54] | 2016 | 959,446 | CABG, hip and knee replacements, readmission, discharge disposition, mortality | Hip/knee surgery: LOS decreased by 1 day; CABG: unchanged LOS |
| Infection Type | Infection Rate (%) | Increase in HLOS (Days) |
|---|---|---|
| Urinary Tract Infection (UTI) | 12.95 | 3.9 |
| Bloodstream Infection (BSI) | 13.00 | 4.0 |
| Surgical Site Infection (SSI) | 9.00 | 4.7 |
| Pneumonia | 15.00 | 6.6 |
| Study | Condition | Additional Cost per Patient (USD) |
|---|---|---|
| Chen et al. (2005) [69] | Nosocomial Infections | 6369 |
| Glied et al. (2016) [57] | Urinary Tract Infections (UTIs) | 41,715 |
| Bloodstream Infections (BSIs) | 34,394 | |
| Surgical Site Infections (SSIs) | 69,626 | |
| Pneumonia | 78,585 | |
| Ward et al. (2021) [68] | HLOS Outliers | 77,228 |
| Study | Population Studied | Length of Stay (LOS) | Cost Impact |
|---|---|---|---|
| Gidey et al. (2023) [70] | Patients with and without HAIs in Ethiopia | Patients with HAIs stayed 8.3 days longer (18.85 vs. 10.59 days) | Average direct medical costs for HAIs were 3033 ETB higher (4826 vs. 1793 ETB) |
| Taliwal et al. (2025) [71] | Pediatric patients with CKD in the US | LOS for CKD stage 4 and 5 was 56% and 71% longer, respectively | Hospitalization costs were 92% and 147% higher for CKD stage 4 and 5 |
| Monard et al. (2023) [72] | Patients with and without AKI in France | Median LOS for AKI patients was 9 days vs. 0–2 days for non-AKI | Median hospitalization cost was EUR 4719 vs. EUR 735 for non-AKI |
| Osenenko et al. (2022) [73] | Heart failure (HF) patients in the US | LOS ranged from 3–5 days (median) and 4–7 days (mean) | Cost per hospitalization ranged from USD 7094–USD 9769 (median), USD 10,737–USD 17,830 (mean) |
| Asegu et al. (2024) [74] | Patients with and without nosocomial infections in Germany | NI patients had a longer LOS by 10 days | Opportunity cost savings of EUR 1000 per preventable NI case |
| Wakil et al. (2024) [75] | HCC patients in the US | Median LOS increased from 5.79 days (2011) to 6.07 days (2017) | Total charges increased from USD 58,406 (2011) to USD 78,791 (2017) |
| Liu et al. (2024) [76] | PD patients in Hubei Province, China | Average LOS was 9.9 days | Average cost per patient was USD 1759.9 |
| Dai et al. (2023) [77] | Seniors with mental illness in Dalian, China | Average LOS was 127.51 days | Average hospitalization cost was CNY 33,656.07 |
| Liu et al. (2022) [78] | Patients with and without HAIs in China | Patients with HAIs had 13.89 additional hospitalization days | Total medical expenditure for HAIs was CNY 24,881.37 higher |
| Chu et al. (2024) [79] | Trauma patients with complications | Complications resulted in increased LOS | Hospitalization costs increased by 1.32-fold with complications |
| Lv et al. (2023) [80] | Patients with and without HAIs in Western China | Patients with HAIs had significantly higher LOS | Adjusted-discounted costs were significantly higher for HAIs |
| Gholipour et al. (2023) [81] | COVID-19 patients globally | LOS varied by country and care level | Highest ICU cost per patient was USD 100,789 in Germany |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirani, R.; Podder, D.; Stala, O.; Mohebpour, R.; Tiwari, R.K.; Etienne, M. Strategies to Reduce Hospital Length of Stay: Evidence and Challenges. Medicina 2025, 61, 922. https://doi.org/10.3390/medicina61050922
Hirani R, Podder D, Stala O, Mohebpour R, Tiwari RK, Etienne M. Strategies to Reduce Hospital Length of Stay: Evidence and Challenges. Medicina. 2025; 61(5):922. https://doi.org/10.3390/medicina61050922
Chicago/Turabian StyleHirani, Rahim, Dhruba Podder, Olivia Stala, Ryan Mohebpour, Raj K. Tiwari, and Mill Etienne. 2025. "Strategies to Reduce Hospital Length of Stay: Evidence and Challenges" Medicina 61, no. 5: 922. https://doi.org/10.3390/medicina61050922
APA StyleHirani, R., Podder, D., Stala, O., Mohebpour, R., Tiwari, R. K., & Etienne, M. (2025). Strategies to Reduce Hospital Length of Stay: Evidence and Challenges. Medicina, 61(5), 922. https://doi.org/10.3390/medicina61050922







