Comparative Efficacy and Tolerability of Treatments for Erythromelalgia: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection of Articles and Data Extraction
2.4. Quality Assessment
3. Results
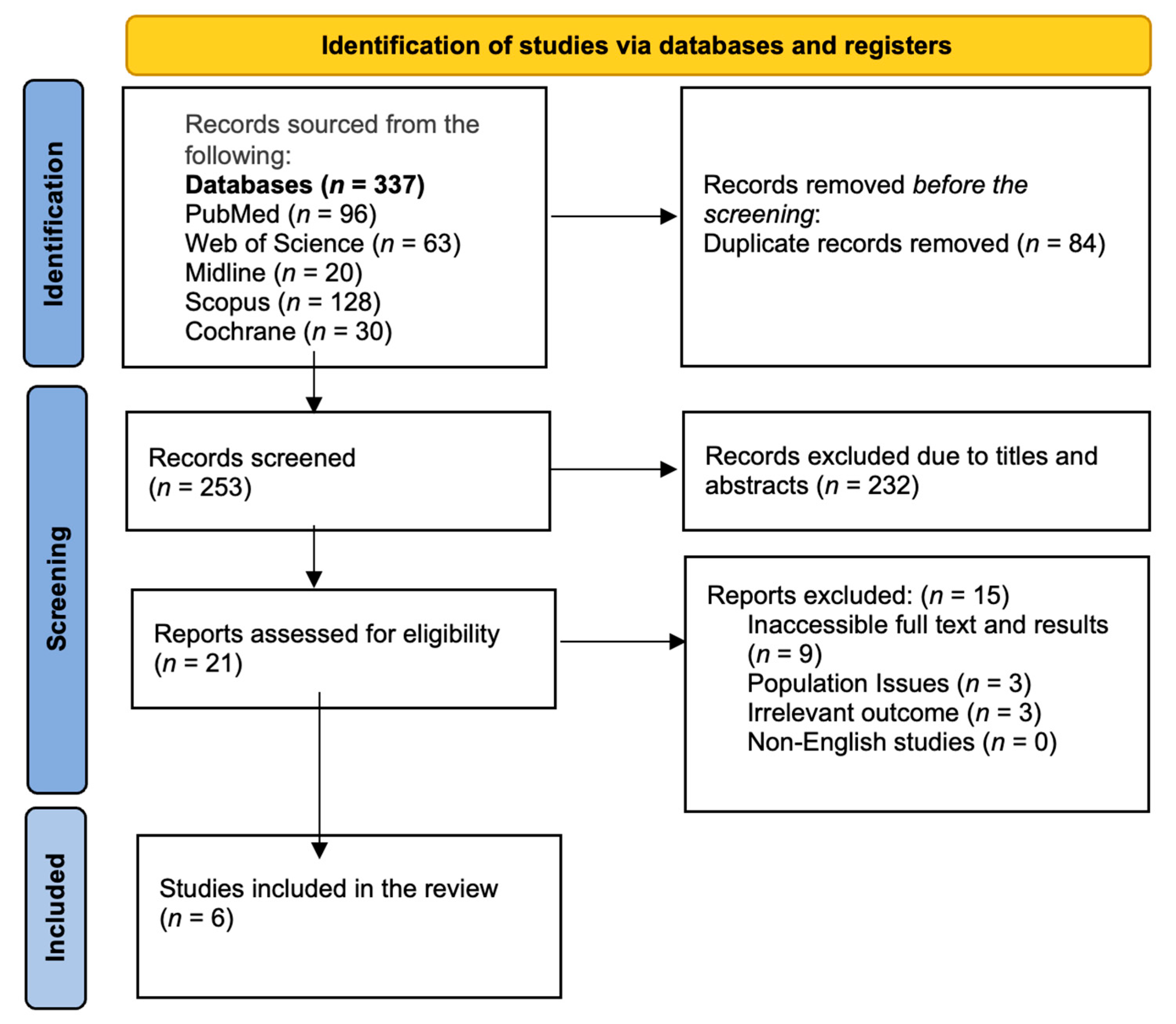
3.1. PRISMA Diagram
3.2. Overview of Studies Included
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CLS | Chemical lumbar sympathectomy |
| EM | Erythromelalgia |
| RCT | Randomized control trial |
| ROB | Risk of bias |
| ROBINS-I | Risk of Bias In Non-randomised Studies—of Interventions |
References
- Davis, M.D.; O’Fallon, W.M.; Rogers, R.S.; Rooke, T.W. Natural History of Erythromelalgia: Presentation and Outcome in 168 Patients. Arch. Dermatol. 2000, 136, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Kalgaard, O.M.; Seem, E.; Kvernebo, K. Erythromelalgia: A Clinical Study of 87 Cases. J. Intern. Med. 1997, 242, 191–197. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Li, S.; Xu, Z.; Li, H.; Ma, L.; Fan, J.; Bu, D.; Liu, B.; Fan, Z.; et al. Mutations in SCN9A, Encoding a Sodium Channel Alpha Subunit, in Patients with Primary Erythermalgia. J. Med. Genet. 2004, 41, 171–174. [Google Scholar] [CrossRef]
- Rush, A.M.; Dib-Hajj, S.D.; Liu, S.; Cummins, T.R.; Black, J.A.; Waxman, S.G. A Single Sodium Channel Mutation Produces Hyper- or Hypoexcitability in Different Types of Neurons. Proc. Natl. Acad. Sci. USA 2006, 103, 8245–8250. [Google Scholar] [CrossRef]
- McDonnell, A.; Schulman, B.; Ali, Z.; Dib-Hajj, S.D.; Brock, F.; Cobain, S.; Mainka, T.; Vollert, J.; Tarabar, S.; Waxman, S.G. Inherited Erythromelalgia Due to Mutations in SCN9A: Natural History, Clinical Phenotype and Somatosensory Profile. Brain J. Neurol. 2016, 139, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G.; Dib-Hajj, S. Erythermalgia: Molecular Basis for an Inherited Pain Syndrome. Trends Mol. Med. 2005, 11, 555–562. [Google Scholar] [CrossRef]
- Burns, T.M.; Te Morsche, R.H.M.; Jansen, J.B.M.J.; Drenth, J.P.H. Genetic Heterogeneity and Exclusion of a Modifying Locus at 2q in a Family with Autosomal Dominant Primary Erythermalgia. Br. J. Dermatol. 2005, 153, 174–177. [Google Scholar] [CrossRef]
- Mann, N.; King, T.; Murphy, R. Review of Primary and Secondary Erythromelalgia. Clin. Exp. Dermatol. 2019, 44, 477–482. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Yang, Y.; Waxman, S.G. Genetics and Molecular Pathophysiology of Na(v)1.7-Related Pain Syndromes. Adv. Genet. 2008, 63, 85–110. [Google Scholar] [CrossRef]
- Cheng, X.; Dib-Hajj, S.D.; Tyrrell, L.; Te Morsche, R.H.; Drenth, J.P.H.; Waxman, S.G. Deletion Mutation of Sodium Channel Na(V)1.7 in Inherited Erythromelalgia: Enhanced Slow Inactivation Modulates Dorsal Root Ganglion Neuron Hyperexcitability. Brain J. Neurol. 2011, 134, 1972–1986. [Google Scholar] [CrossRef]
- Tham, S.W.; Giles, M. Current Pain Management Strategies for Patients with Erythromelalgia: A Critical Review. J. Pain Res. 2018, 11, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, P.; Davis, M.D.P. Combination Gel of 1% Amitriptyline and 0.5% Ketamine to Treat Refractory Erythromelalgia Pain: A New Treatment Option? Arch. Dermatol. 2006, 142, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Al-Minshawy, S.M.; El-Mazary, A.-A.M. An Egyptian Child with Erythromelalgia Responding to a New Line of Treatment: A Case Report and Review of the Literature. J. Med. Case Rep. 2014, 8, 69. [Google Scholar] [CrossRef][Green Version]
- Page, M.J.; Moher, D. Evaluations of the Uptake and Impact of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and Extensions: A Scoping Review. Syst. Rev. 2017, 6, 263. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Mørk, C.; Salerud, E.G.; Asker, C.L.; Kvernebo, K. The Prostaglandin E1 Analog Misoprostol Reduces Symptoms and Microvascular Arteriovenous Shunting in Erythromelalgia-a Double-Blind, Crossover, Placebo-Compared Study. J. Investig. Dermatol. 2004, 122, 587–593. [Google Scholar] [CrossRef]
- Kalgaard, O.M.; Mørk, C.; Kvernebo, K. Prostacyclin Reduces Symptoms and Sympathetic Dysfunction in Erythromelalgia in a Double-Blind Randomized Pilot Study. Acta Derm. Venereol. 2003, 83, 442–444. [Google Scholar] [CrossRef]
- Poterucha, T.J.; Weiss, W.T.; Warndahl, R.A.; Rho, R.H.; Sandroni, P.; Davis, M.D.P.; Murphy, S.L. Topical Amitriptyline Combined with Ketamine for the Treatment of Erythromelalgia: A Retrospective Study of 36 Patients at Mayo Clinic. J. Drugs Dermatol. JDD 2013, 12, 308–310. [Google Scholar]
- Helås, T.; Sagafos, D.; Kleggetveit, I.P.; Quiding, H.; Jönsson, B.; Segerdahl, M.; Zhang, Z.; Salter, H.; Schmelz, M.; Jørum, E. Pain Thresholds, Supra-Threshold Pain and Lidocaine Sensitivity in Patients with Erythromelalgia, Including the I848Tmutation in NaV 1.7. Eur. J. Pain Lond. Engl. 2017, 21, 1316–1325. [Google Scholar] [CrossRef]
- Wang, W.-H.; Zhang, L.; Dong, G.-X.; Sun, T.-T.; Lin, Z.-M.; Yang, Y.; Li, X. Chemical Lumbar Sympathectomy in the Treatment of Recalcitrant Erythromelalgia. J. Vasc. Surg. 2018, 68, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Michelerio, A.; Tomasini, C.; Arbustini, E.; Vassallo, C. Clinical Challenges in Primary Erythromelalgia: A Real-Life Experience from a Single Center and a Diagnostic-Therapeutic Flow-Chart Proposal. Dermatol. Pract. Concept. 2023, 13, e2023191. [Google Scholar] [CrossRef] [PubMed]
- Dabby, R. Pain Disorders and Erythromelalgia Caused by Voltage-Gated Sodium Channel Mutations. Curr. Neurol. Neurosci. Rep. 2012, 12, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, B.S.; Nguyen, P.T.; Zhou, E.Y.; Yang, Y.; Yarov-Yarovoy, V.; Dib-Hajj, S.D.; Waxman, S.G. Gain-of-Function Mutation of a Voltage-Gated Sodium Channel NaV1.7 Associated with Peripheral Pain and Impaired Limb Development. J. Biol. Chem. 2017, 292, 9262–9272. [Google Scholar] [CrossRef]
- Elgueta, F.; de la Cuadra-Fontaine, J.C.; Clede, L.; Fierro, C.; Valderrama, A. Erythromelagia: A Rare and Hard-to-Treat Condition: A 9-Year-Old Boy Responsive to Intravenous Lidocaine and Oral Mexilitene. Pain Med. 2013, 14, 311–312. [Google Scholar] [CrossRef][Green Version]
- Jakob, A.; Creutzfeldt, R.; Staszewski, O.; Winterpacht, A.; Berner, R.; Hufnagel, M. Primary Erythromelalgia in a 12-Year-Old Boy: Positive Response to Sodium Channel Blockers despite Negative SCN9A Mutations. Klin. Padiatr. 2012, 224, 309–312. [Google Scholar] [CrossRef]
- Cregg, R.; Cox, J.J.; Bennett, D.L.H.; Wood, J.N.; Werdehausen, R. Mexiletine as a Treatment for Primary Erythromelalgia: Normalization of Biophysical Properties of Mutant L858F NaV 1.7 Sodium Channels. Br. J. Pharmacol. 2014, 171, 4455–4463. [Google Scholar] [CrossRef]
- Han, C.; Lampert, A.; Rush, A.M.; Dib-Hajj, S.D.; Wang, X.; Yang, Y.; Waxman, S.G. Temperature Dependence of Erythromelalgia Mutation L858F in Sodium Channel Nav1.7. Mol. Pain 2007, 3, 3. [Google Scholar] [CrossRef]
- Faber, C.G.; Hoeijmakers, J.G.J.; Ahn, H.-S.; Cheng, X.; Han, C.; Choi, J.-S.; Estacion, M.; Lauria, G.; Vanhoutte, E.K.; Gerrits, M.M.; et al. Gain of Function Naν1.7 Mutations in Idiopathic Small Fiber Neuropathy. Ann. Neurol. 2012, 71, 26–39. [Google Scholar] [CrossRef]
- Parker, L.K.; Ponte, C.; Howell, K.J.; Ong, V.H.; Denton, C.P.; Schreiber, B.E. Clinical Features and Management of Erythromelalgia: Long Term Follow-up of 46 Cases. Clin. Exp. Rheumatol. 2017, 35, 80–84. [Google Scholar]
- Cohen, J.S. Erythromelalgia: New Theories and New Therapies. J. Am. Acad. Dermatol. 2000, 43, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Li, L.; Dong, G.; Zhao, J.; Luan, J.; Sun, T. Long-Term Remission of Primary Erythermalgia with R1150W Polymorphism in SCN9A after Chemical Lumbar Sympathectomy. Eur. J. Dermatol. EJD 2010, 20, 763–767. [Google Scholar] [PubMed]
- Davis, M.D.P.; Sandroni, P. Lidocaine Patch for Pain of Erythromelalgia: Follow-up of 34 Patients. Arch. Dermatol. 2005, 141, 1320–1321. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year | Study Design and Follow-Up | No. of Exp. Patients/ Control | Median (Range) Age (Years) | Gender Male/Female (n) | Median (Range) Duration of Disease (Years) | Comorbidities and Genetic Factors | Symptoms, Triggers, and Relievers | Intervention |
|---|---|---|---|---|---|---|---|---|
| Kalgaard et al., 2003 [18] | Double-blind RCT in Norway | 12 EM primary bilateral patients [feet] I (iloprost): 8 C (placebo): 4 | 52 (17–74) I: 39 (17–60) C: 56 (50–74) | 4/8 I: 2/6 C: 2/2 | 9.9 (3.6–23.4) I: 10.5 (5.7–14.9) C: 8.9 (3.6–23.4) | NA |
| Iloprost IV (3 days) vs. placebo |
| Mørk et al., 2004 [17] | Double-blind RCT in Norway and Sweden with follow-up for 3 months | 21 EM [left toe] patients 11 healthy controls (for physiological evaluation) | EM: 47.8 (21.2–60.6) | EM: 7/14 | 11.2 (0.1–34.7) |
|
| Misoprostol (oral, 6 weeks) vs. placebo (crossover) |
| Poterucha et al., 2013 [19] | Retrospective study in New York | 36 EM in different parts of body [35 lower extremities, 22 hands or upper extremities, 6 face, 5 ears, 1 trunk, and 1 neck] | Mean (SD): 44.7 (15.8) Median (range): 47.8 (5–74) | 4/32 | NA | NA | NA | Amitriptyline-ketamine (topical) with varying concentrations |
| Helås et al., 2017 [20] | Double-blind RCT in Norway and Sweden | 52 EM patients: 27 (primary and secondary bilateral erythomelal [hands and/or feet]) Healthy controls: 25 | Patient group: 55 (29–73) Control group: 26.5 (23–48) | Patient group: 3/24 Control group: 11/14 | NA |
|
| Lidocaine (intradermal) with varying doses vs. placebo |
| Wang et al., 2018 [21] | Prospective study in China with average six-year follow-up. | 13 primary recalcitrant bilateral EM [lower extremities] | 15 (11–52) | 4/9 | 36 (2–120) months |
|
| Chemical lumbar sympathectomy (5% phenol topical) |
| Michelerio et al., 2023 [22] | Retrospective study in Italy with average five-year follow-up. | 11 primary bilateral EM [limbs] | Mean (range): 36 (16–57) | Females: 11 | NA |
|
|
|
| First Author, Year | Outcomes | Adverse Events | Conclusion |
|---|---|---|---|
| Kalgaard et al., 2003 [18] | Change from pre-treatment: Iloprost (n = 8)
|
| Iloprost significantly reduced cooling scores in erythromelalgia patients compared to the baseline, showing improvements in symptoms and sympathetic dysfunction. |
| Mørk et al., 2004 [17] | Change from pre-treatment: EM Severity (Pain VAS, mm)
|
| Significant improvements in all clinical outcomes after treatment with misoprostol compared to a placebo, even after a three-month follow-up. |
| Poterucha et al., 2013 [19] | Relief Using Amitriptyline-Ketamine: Presence of small fiber neuropathy: 50% improved Involvement of hands or face: 11% improved Efficacy: Completely improved: 3% Significant improvement: 39% Some improvement: 33% |
| About 75% of erythromelalgia patients found pain relief with a topical combination of amitriptyline and ketamine, and the treatment was well accepted. |
| Helås et al., 2017 [20] | Warmth Detection Threshold (WD °C)
| NA | Although lidocaine reduced nociceptive feelings in a dose-dependent manner, no patients showed heightened sensitivity to it, limiting insights for potential treatments. |
| Wang et al., 2018 [21] | The VAS value from the baseline:
|
| CLS shows promise for refractory erythromelalgia, with notable improvements but possible relapses, especially in mutation carriers. |
| Michelerio et al., 2023 [22] |
|
| The most effective therapies were antihistamines, venlafaxine, and mexiletine. |
| Study ID | D1 | D2 | D3 | D4 | D5 | Overall ROB-2 |
|---|---|---|---|---|---|---|
| Kalgaard et al., 2003 [18] |  |  |  |  |  |  |
| Mørk et al., 2004 [17] |  |  |  |  |  |  |
| Helås et al., 2017 [20] |  |  |  |  |  |  |
 : Low bias;
: Low bias;  : High bias;
: High bias;  : Unclear bias.
: Unclear bias.| Study ID | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall RoB |
|---|---|---|---|---|---|---|---|---|
| Poterucha et al., 2013 [19] |  |  |  |  |  |  |  |  |
| Wang et al., 2018 [21] |  |  |  |  |  |  |  |  |
| Michelerio et al., 2023 [22] |  |  |  |  |  |  |  |  |
 : Low bias;
: Low bias;  : High bias;
: High bias;  : Unclear bias.
: Unclear bias.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algarni, A.S.; Alharthi, R.M.; Alqurashi, S.O.; Alghanmi, R.M.; Aldawsari, R.R.; Alghamdi, M.A.; Samargandi, R. Comparative Efficacy and Tolerability of Treatments for Erythromelalgia: A Systematic Review. Medicina 2025, 61, 920. https://doi.org/10.3390/medicina61050920
Algarni AS, Alharthi RM, Alqurashi SO, Alghanmi RM, Aldawsari RR, Alghamdi MA, Samargandi R. Comparative Efficacy and Tolerability of Treatments for Erythromelalgia: A Systematic Review. Medicina. 2025; 61(5):920. https://doi.org/10.3390/medicina61050920
Chicago/Turabian StyleAlgarni, Abdullah S., Reem M. Alharthi, Shaden O. Alqurashi, Ruba M. Alghanmi, Rimaz R. Aldawsari, Maysaa A. Alghamdi, and Ramy Samargandi. 2025. "Comparative Efficacy and Tolerability of Treatments for Erythromelalgia: A Systematic Review" Medicina 61, no. 5: 920. https://doi.org/10.3390/medicina61050920
APA StyleAlgarni, A. S., Alharthi, R. M., Alqurashi, S. O., Alghanmi, R. M., Aldawsari, R. R., Alghamdi, M. A., & Samargandi, R. (2025). Comparative Efficacy and Tolerability of Treatments for Erythromelalgia: A Systematic Review. Medicina, 61(5), 920. https://doi.org/10.3390/medicina61050920






