The Interplay of Cancer and Hypertension: Rising Mortality and Widening Disparities Across the United States (1999–2023)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
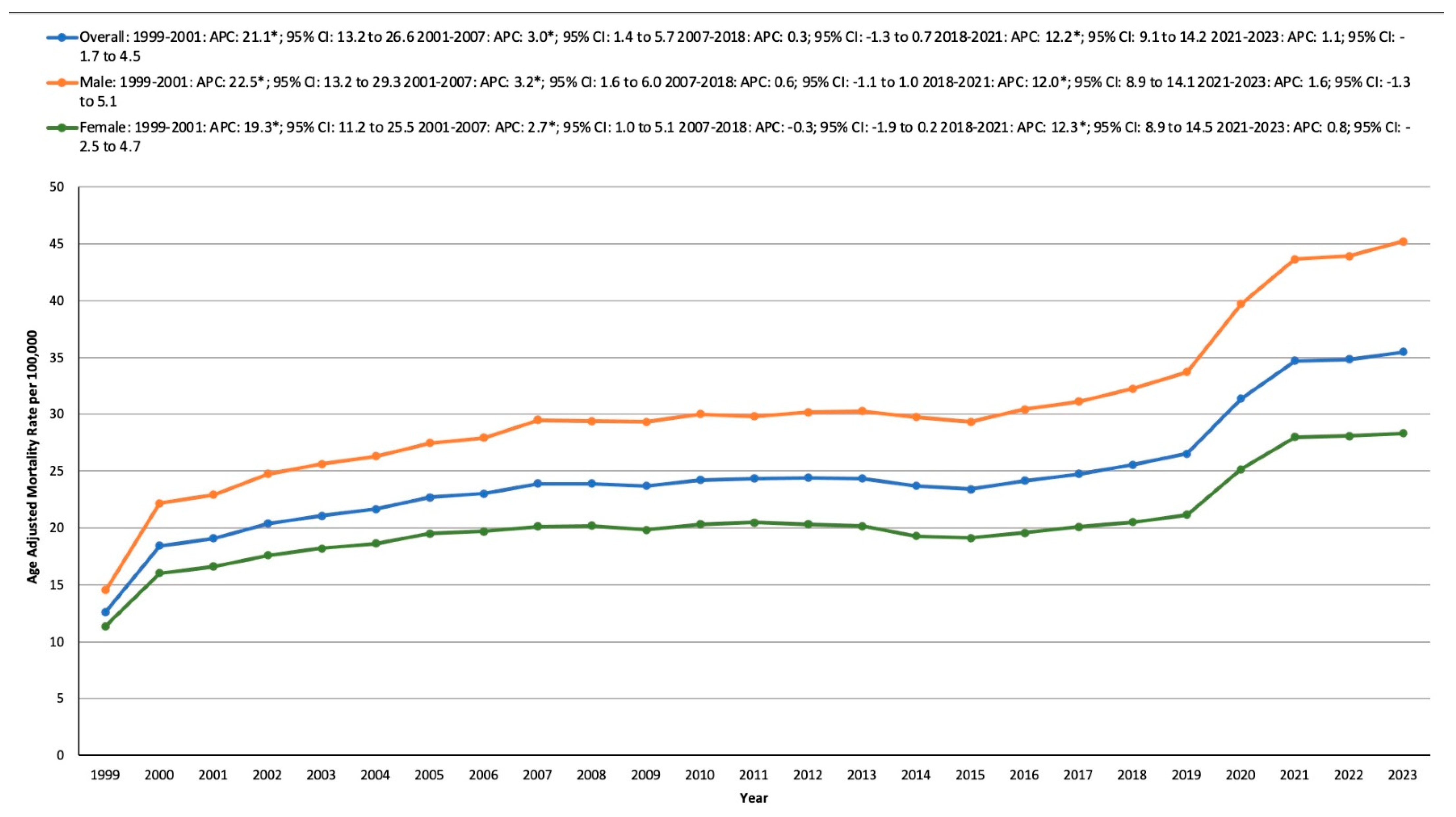
3.1. Overall
3.2. HTN- and Cancer-Related AAMR Stratified by Sex
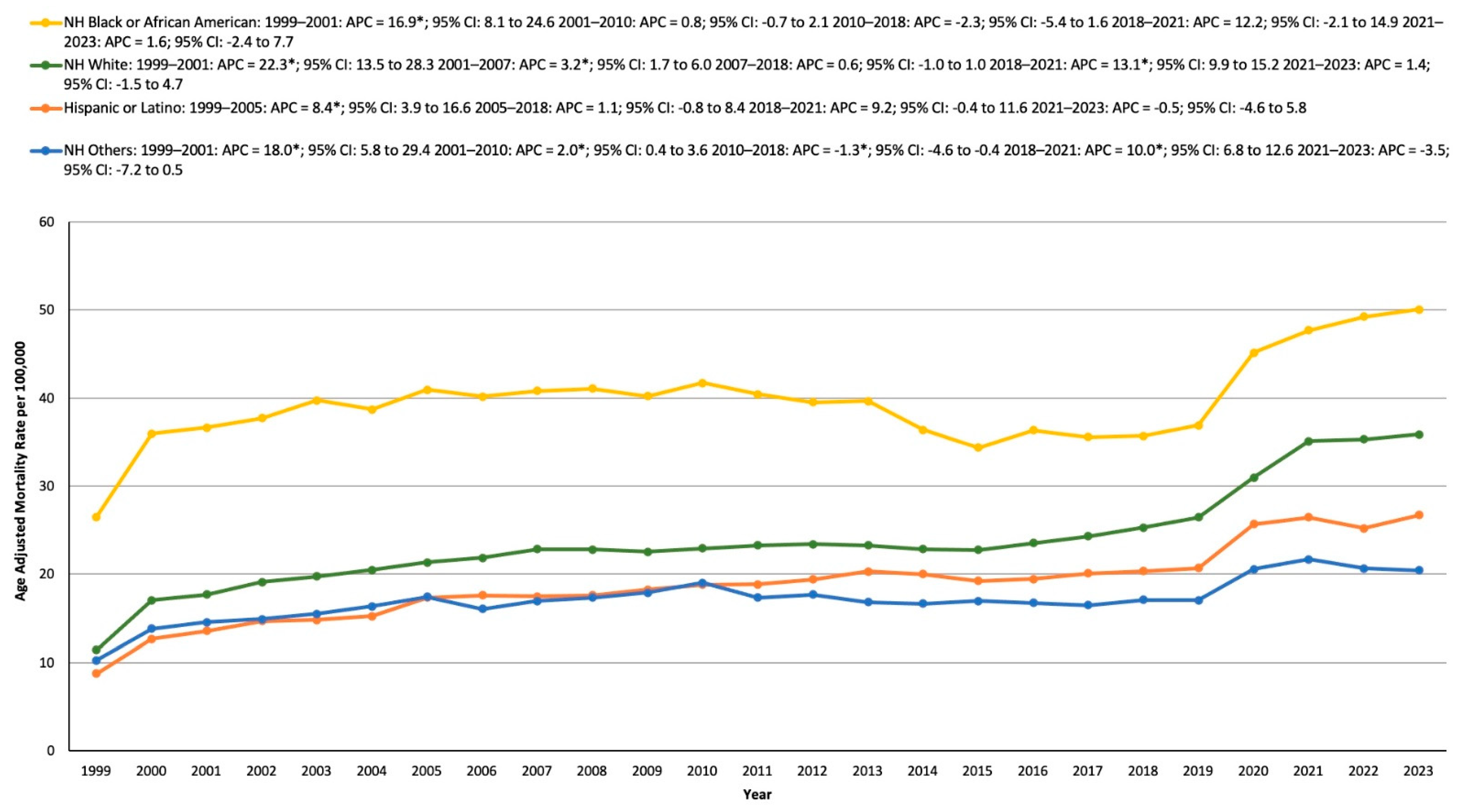
3.3. HTN- and Cancer-Related AAMR Stratified by Race/Ethnicity
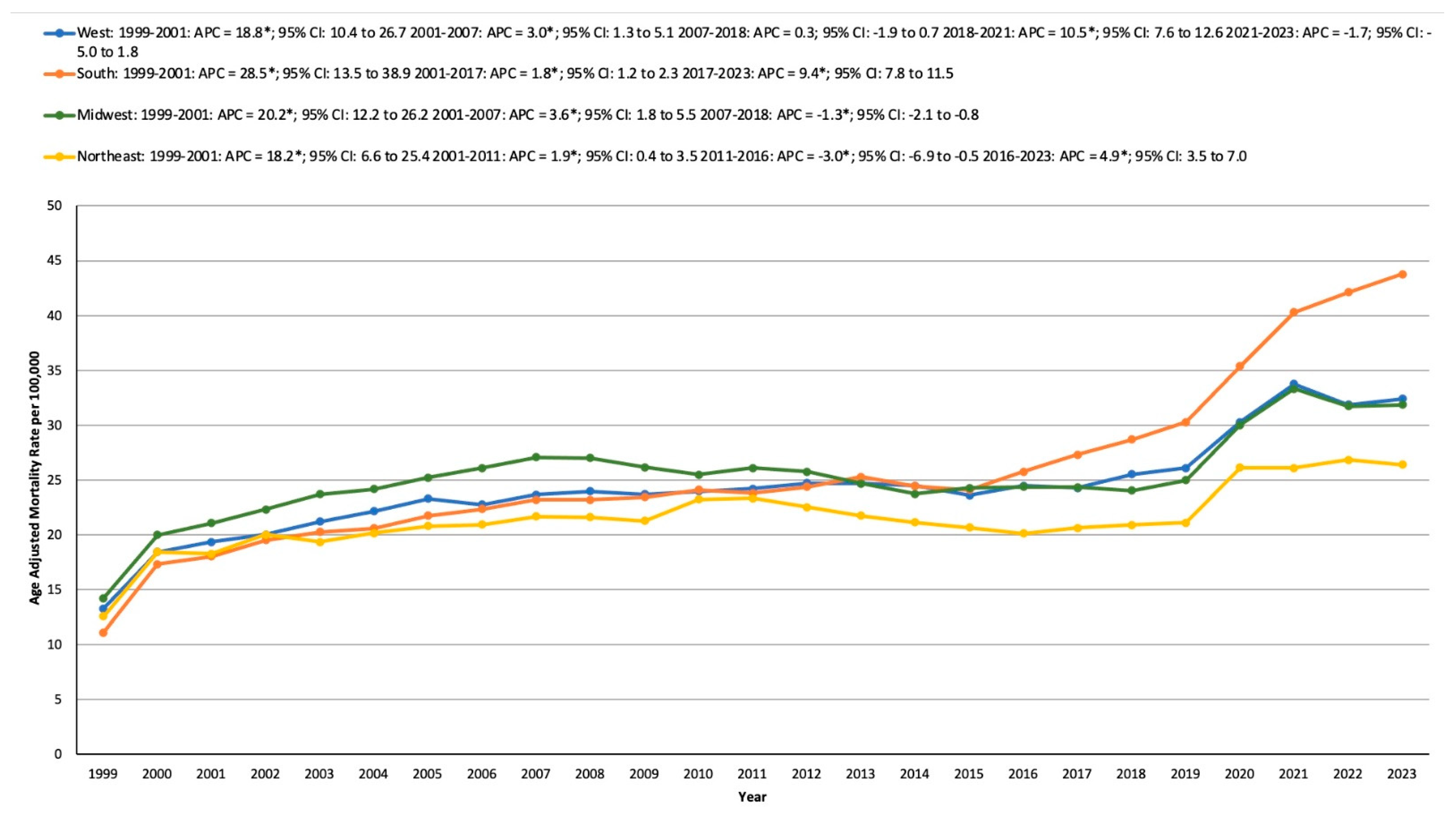
3.4. HTN- and Cancer-Related AAMR Stratified by Geographical Region
3.4.1. Statewide
3.4.2. Census Region
3.4.3. Urban and Rural Areas
3.5. HTN- and Cancer-Related AAMR Stratified by Ten-Year Age Groups
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Y.; Shen, J.; Luo, J.; Li, N.; Liao, X.; Zhang, Y. Disagreements in risk of bias assessment for randomized controlled trials in hypertension-related Cochrane reviews. Trials 2024, 25, 405. [Google Scholar] [CrossRef]
- Burlacu, A.; Kuwabara, M.; Brinza, C.; Kanbay, M. Key Updates to the 2024 ESC Hypertension Guidelines and Future Perspectives. Medicina 2025, 61, 193. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Y.; Zhang, Y.; Yang, R.; Yang, Z.; Liu, L.; Yao, Y.; Fang, X.; Xie, Q.; Deng, H.; et al. Copublication promotes dissemination hypertension guidelines: A retrospective cohort study. Hypertens. Res. 2024, 47, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Connelly, P.J.; Currie, G.; Delles, C. Sex Differences in the Prevalence, Outcomes and Management of Hypertension. Curr. Hypertens. Rep. 2022, 24, 185–192. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Han, S.; Litten, K.P.; Mehta, S.; Ng, B.P. Prevalence of primary and secondary hypertension among hospitalized patients with cancer in the United States. Chronic Illn. 2025, 21, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B.; Geara, A.S.; Hogan, J.J.; Townsend, R.R. Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC CardioOncol. 2019, 1, 238–251. [Google Scholar] [CrossRef]
- Ameri, P.; Dent, S. Cancer Prevention and Early Detection in Patients with Cardiovascular Disease: A Goal of Bidirectional Cardio-Oncology. JACC CardioOncol. 2024, 6, 962–964. [Google Scholar] [CrossRef]
- Jain, M.; Townsend, R.R. Chemotherapy agents and hypertension: A focus on angiogenesis blockade. Curr. Hypertens. Rep. 2007, 9, 320–328. [Google Scholar] [CrossRef]
- Gupta, P.; Canonico, M.E.; Faaborg-Andersen, C.; Prabhu, N.; Kondapalli, L.; Quintana, R.A. Updates in the management of cancer therapy-related hypertension. Curr. Opin. Cardiol. 2024, 39, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.J.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef]
- Ceren, İ.; Çitir Durmuşoğlu, B.N.; Şener, Y.Z.; Bozduman Habip, F.; Köroğlu, S.; Demir, N.; Ateş, Ö.; Eroğlu Büyüköner, E. Predictors and Clinical Impacts of Impaired Heart Rate Variability in Women with Breast Cancer. Medicina 2025, 61, 608. [Google Scholar] [CrossRef] [PubMed]
- Tondo, P.; Tricarico, L.; Galgano, G.; Varlese, M.P.C.; Aruanno, D.; Gallo, C.; Scioscia, G.; Brunetti, N.D.; Correale, M.; Lacedonia, D. Utility of Clustering in Mortality Risk Stratification in Pulmonary Hypertension. Bioengineering 2025, 12, 408. [Google Scholar] [CrossRef]
- Totolici, S.; Vrabie, A.-M.; Badila, E.; Weiss, E. Onco-Hypertension: A Continuously Developing Field between Cancer and Hypertension. Int. J. Mol. Sci. 2024, 25, 3442. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Kaneko, H.; Suzuki, Y.; Okada, A.; Fujiu, K.; Takeda, N.; Morita, H.; Nishiyama, A.; Yano, Y.; Node, K.; et al. Association of cancer with the risk of developing hypertension. Eur. Heart J. Qual. Care Clin. Outcomes 2024, 10, 228–234. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Ahmed, M.; Javaid, H.; Shafiq, A.; Nadeem, Z.A.; Ahsan, A.; Nofal, A.; Ahmed, R.; Alam, M.; Fudim, M.; Fonarow, G.C.; et al. Trends and Disparities in Coronary Artery Disease and Obesity-Related Mortality in the United States From 1999–2022. Endocrinol. Diabetes Metab. 2024, 7, e70010. [Google Scholar] [CrossRef]
- Ahmed, M.; Nofal, A.; Shafiq, A.; Javaid, H.; Ahsan, A.; Nadeem, Z.A.; Ahmed, R.; Alam, M.; Mamas, M.A.; Fudim, M.; et al. Rising mortality rates linked to type-2 diabetes and obesity in the United States: An observational analysis from 1999 to 2022. J. Diabetes Investig. 2024, 16, 492–500. [Google Scholar] [CrossRef]
- Ahmed, R.; Ahmed, M.; Khlidj, Y.; Rehman, O.U.; Al-Mukhtar, L.; Abou Khater, N.; Khurram Mustaq Gardezi, S.; Rashid, M.; Collins, P.; Jain, H.; et al. Nationwide Cross-Sectional Analysis of Mortality Trends in Patients with Sarcoidosis and Non-Ischemic Cardiovascular Disease-The Impact of Gender, Ethnicity, Geographical Location, and COVID-19 Pandemic. J. Clin. Med. 2024, 13, 7463. [Google Scholar] [CrossRef]
- Ansari, H.U.H.; Mahboob, E.; Samad, M.A.; Shahzad, M.; Ahmed, M.; Naqvi, S.T.H.; Qazi, S.U.; Ahmed, F.; Ross-Kenny, H.; Baniowda, M.; et al. Temporal trends and disparities in atherosclerosis-associated cerebrovascular disease mortality in the United States, 1999–2020. Curr. Probl. Cardiol. 2025, 50, 102935. [Google Scholar] [CrossRef]
- Ingram, D.D.; Franco, S.J. 2013 NCHS Urban-Rural Classification Scheme for Counties. Vital Health Stat 2 2014, 1–73. [Google Scholar] [PubMed]
- Joinpoint Regression Program. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 3 August 2024).
- Han, H.; Guo, W.; Shi, W.; Yu, Y.; Zhang, Y.; Ye, X.; He, J. Hypertension and breast cancer risk: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 44877. [Google Scholar] [CrossRef]
- Sionakidis, A.; McCallum, L.; Padmanabhan, S. Unravelling the tangled web of hypertension and cancer. Clin. Sci. 2021, 135, 1609–1625. [Google Scholar] [CrossRef]
- Muhandiramge, J.; Zalcberg, J.R.; Warner, E.T.; Polekhina, G.; Gibbs, P.; van Londen, G.J.; Bernstein, W.B.; Macrae, F.; Haydon, A.; Tie, J.; et al. Cardiovascular disease and stroke following cancer and cancer treatment in older adults. Cancer 2024, 130, 4138–4148. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.-J.; Abraham, R.; Surapaneni, A.L.; Schlosser, P.; Ballew, S.H.; Ozkan, B.; Flaherty, C.M.; Yu, B.; Bonventre, J.V.; Parikh, C.R.; et al. Sex Differences in Hypertension and Its Management Throughout Life. Hypertension 2024, 81, 2263–2274. [Google Scholar] [CrossRef]
- Hashmi, S.; Safdar, I.; Kazmi, M.H.; Zulfiqar, E.; Shahzad, M.; Hurjkaliani, S.; Ayyad, M.; Zuberi, N.; Ahmed, T.; Sujay, G.B.; et al. Rising Mortality Related to Diabetes Mellitus and Hypertension: Trends and Disparities in the United States (1999−2023). Clin. Cardiol. 2025, 48, e70132. [Google Scholar] [CrossRef]
- Fryar, C.D.; Kit, B.; Carroll, M.D.; Afful, J. Hypertension Prevalence, Awareness, Treatment, and Control Among Adults Age 18 and Older: United States, August 2021–August 2023. Available online: https://stacks.cdc.gov/view/cdc/164016 (accessed on 6 January 2025).
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief. 2020, 1–8. [Google Scholar] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Cook, M.B.; McGlynn, K.A.; Devesa, S.S.; Freedman, N.D.; Anderson, W.F. Sex Disparities in Cancer Mortality and Survival. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1629–1637. [Google Scholar] [CrossRef]
- Dwyer-Lindgren, L.; Kendrick, P.; Kelly, Y.O.; Sylte, D.O.; Schmidt, C.; Blacker, B.F.; Daoud, F.; Abdi, A.A.; Baumann, M.; Mouhanna, F.; et al. Life expectancy by county, race, and ethnicity in the USA, 2000–2019: A systematic analysis of health disparities. Lancet 2022, 400, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Ostchega, Y.; Hughes, J.P.; Zhang, G.; Nwankwo, T.; Graber, J.; Nguyen, D.T. Differences in Hypertension Prevalence and Hypertension Control by Urbanization Among Adults in the United States, 2013–2018. Am. J. Hypertens. 2022, 35, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ledford, S.G.; Kessler, F.; Moss, J.L.; Wang, M.; Lengerich, E.J. The Impact of the COVID-19 Pandemic on Cancer Mortality in Pennsylvania: A Retrospective Study with Geospatial Analysis. Cancers 2023, 15, 4788. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Sommers, B.D.; Aboulafia, G.; Phelan, J.; Orav, E.J.; Epstein, A.M.; Figueroa, J.F. Coverage and Access Changes During Medicaid Unwinding. JAMA Health Forum 2024, 5, e242193. [Google Scholar] [CrossRef]
- Presant, C.A.; Till, C.; Vaidya, R.; Ashing, K.T.; Warren, G.W.; Sun, V.; Salgia, R.; Massarelli, E.; Mortimer, J.E.; Pal, S.; et al. Smoking prevalence and association with sociodemographic variables in cancer clinical trial participants. Cancer 2025, 131, e35560. [Google Scholar] [CrossRef]
- Jareebi, M.A. The Association Between Smoking Behavior and the Risk of Hypertension: Review of the Observational and Genetic Evidence. J. Multidiscip. Heal. 2024, 17, 3265–3281. [Google Scholar] [CrossRef]
- Leventhal, A.M.; Dai, H.; Higgins, S.T. Smoking Cessation Prevalence and Inequalities in the United States: 2014–2019. JNCI J. Natl. Cancer Inst. 2022, 114, 381–390. [Google Scholar] [CrossRef]
- Zavala, V.A.; Bracci, P.M.; Carethers, J.M.; Carvajal-Carmona, L.; Coggins, N.B.; Cruz-Correa, M.R.; Davis, M.; de Smith, A.J.; Dutil, J.; Figueiredo, J.C.; et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 2021, 124, 315–332. [Google Scholar] [CrossRef]
- Al-Quteimat, O.M.; Amer, A.M. The Impact of the COVID-19 Pandemic on Cancer Patients. Am. J. Clin. Oncol. 2020, 43, 452. [Google Scholar] [CrossRef]
- Bakouny, Z.; Hawley, J.E.; Choueiri, T.K.; Peters, S.; Rini, B.I.; Warner, J.L.; Painter, C.A. COVID-19 and Cancer: Current Challenges and Perspectives. Cancer Cell 2020, 38, 629–646. [Google Scholar] [CrossRef]
- Golestaneh, L.; Neugarten, J.; Fisher, M.; Billett, H.H.; Gil, M.R.; Johns, T.; Yunes, M.; Mokrzycki, M.H.; Coco, M.; Norris, K.C.; et al. The association of race and COVID-19 mortality. eClinicalMedicine 2020, 25, 100455. [Google Scholar] [CrossRef] [PubMed]
- Yusni, Y.; Rahman, S.; Naufal, I. Positive correlation between body weight and body mass index with blood pressure in young adults. Narra J. 2024, 4, e533. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.D.; Haider, M.R.; Roy, S.; Hashan, M.R.; Baral, A.; Tamanna, N.; Mazumder, A.; Haider, S.S.; Datta, B. Association Between Abdominal Obesity, Body Mass Index, and Hypertension in India: Evidence from a Large Nationally Representative Data. J. Clin. Hypertens. 2025, 27, e70034. [Google Scholar] [CrossRef] [PubMed]
- Kosami, K.; Kuwabara, M.; Okayama, A.; Ae, R. Body Mass Index and Weight Change as Predictors of Hypertension Development: A Sex-Specific Analysis. Nutrients 2024, 17, 119. [Google Scholar] [CrossRef]
- Mahler, B.; Băiceanu, D.; Panciu, T.C.; Florea, R.M.; Iorga, A.L.; Gnat, M.; German, C.F.; Pârvu, S.; Paraschiv, D.; Manea, D.; et al. Air Pollutants and Their Impact on Chronic Diseases—A Retrospective Study in Bucharest, Romania. Atmosphere 2023, 14, 867. [Google Scholar] [CrossRef]
- Nayebare, S.R.; Aburizaiza, O.S.; Siddique, A.; Carpenter, D.O.; Mirza, H.M.; Zeb, J.; Aburiziza, A.J.; Khwaja, H.A. Exposure to Black Carbon (BC) and the Secondary Aerosol (p-SO42− and p-NO3−) Components of Fine Particulate Matter (PM2.5), and Cardiopulmonary Morbidity in Jeddah, Saudi Arabia. Atmosphere 2025, 16, 168. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, I.A.; Asghar, S.; Masud, L.; Hafeez, M.A.; Hurjkaliani, S.; Zulfiqar, E.; Shahzad, M.; Ahmed, H.; Khan, S.; Ahmed, S.; et al. The Interplay of Cancer and Hypertension: Rising Mortality and Widening Disparities Across the United States (1999–2023). Medicina 2025, 61, 917. https://doi.org/10.3390/medicina61050917
Nasser IA, Asghar S, Masud L, Hafeez MA, Hurjkaliani S, Zulfiqar E, Shahzad M, Ahmed H, Khan S, Ahmed S, et al. The Interplay of Cancer and Hypertension: Rising Mortality and Widening Disparities Across the United States (1999–2023). Medicina. 2025; 61(5):917. https://doi.org/10.3390/medicina61050917
Chicago/Turabian StyleNasser, Ibrahim Ali, Shereen Asghar, Laraib Masud, Muhammad Ali Hafeez, Sonia Hurjkaliani, Eeshal Zulfiqar, Maryam Shahzad, Husain Ahmed, Shahrukh Khan, Sajeel Ahmed, and et al. 2025. "The Interplay of Cancer and Hypertension: Rising Mortality and Widening Disparities Across the United States (1999–2023)" Medicina 61, no. 5: 917. https://doi.org/10.3390/medicina61050917
APA StyleNasser, I. A., Asghar, S., Masud, L., Hafeez, M. A., Hurjkaliani, S., Zulfiqar, E., Shahzad, M., Ahmed, H., Khan, S., Ahmed, S., Abdul, Q., Noushad, M. A., Nusrat, R., Azhar, S., Ward, C. D., Ahmed, M., & Ahmed, R. (2025). The Interplay of Cancer and Hypertension: Rising Mortality and Widening Disparities Across the United States (1999–2023). Medicina, 61(5), 917. https://doi.org/10.3390/medicina61050917




