The Role and Appropriate Selection of Guidewires in Biliopancreatic Endoscopy
Abstract
1. Introduction
2. Guidewire Characteristics
2.1. Material Composition and Structure
2.2. Length
2.3. Diameter
2.4. Tip Shape
2.5. Stiffness and Maneuvrability
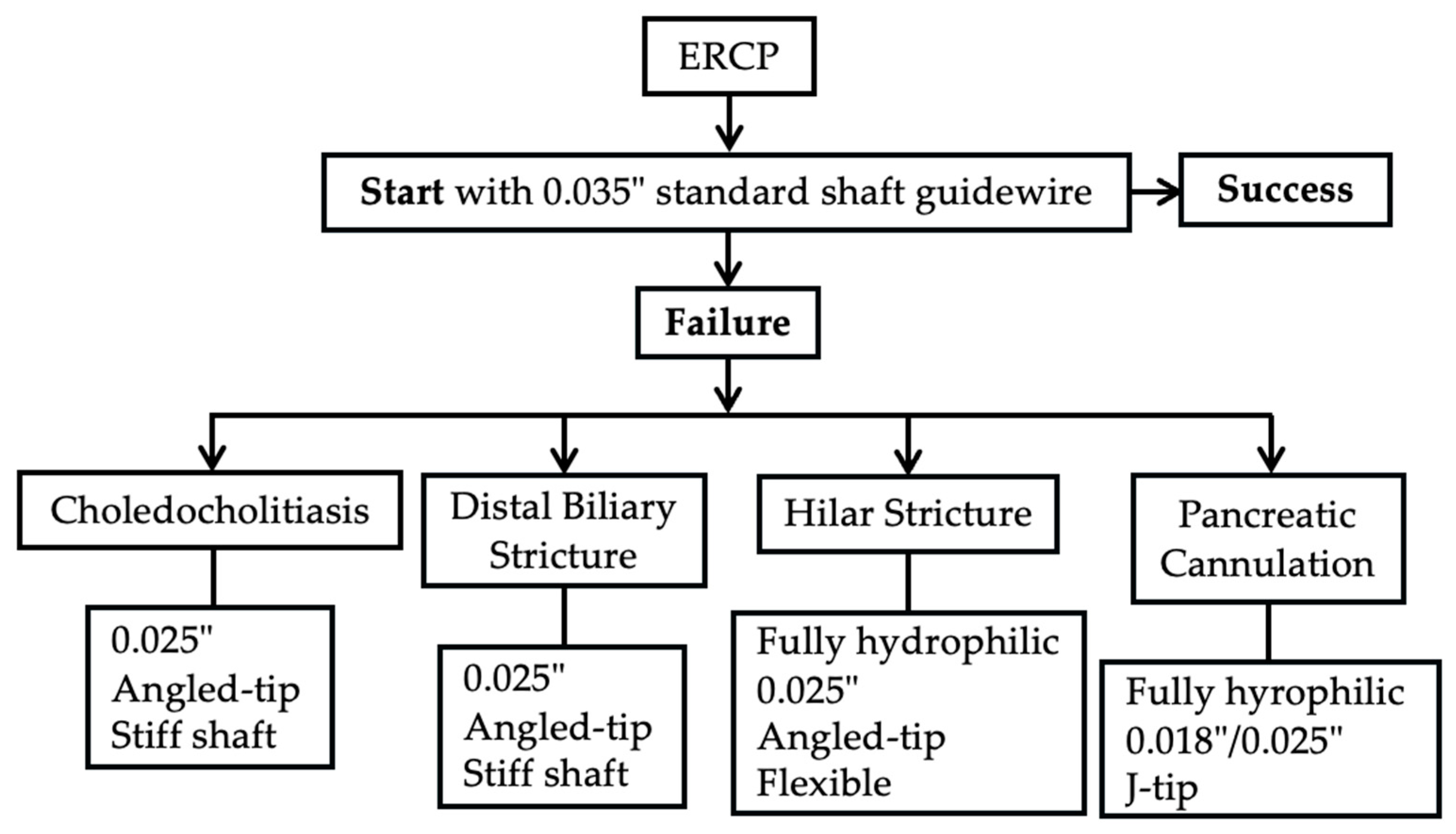
3. ERCP
3.1. Biliary Duct Cannulation
3.2. Pancreatic Duct Cannulation
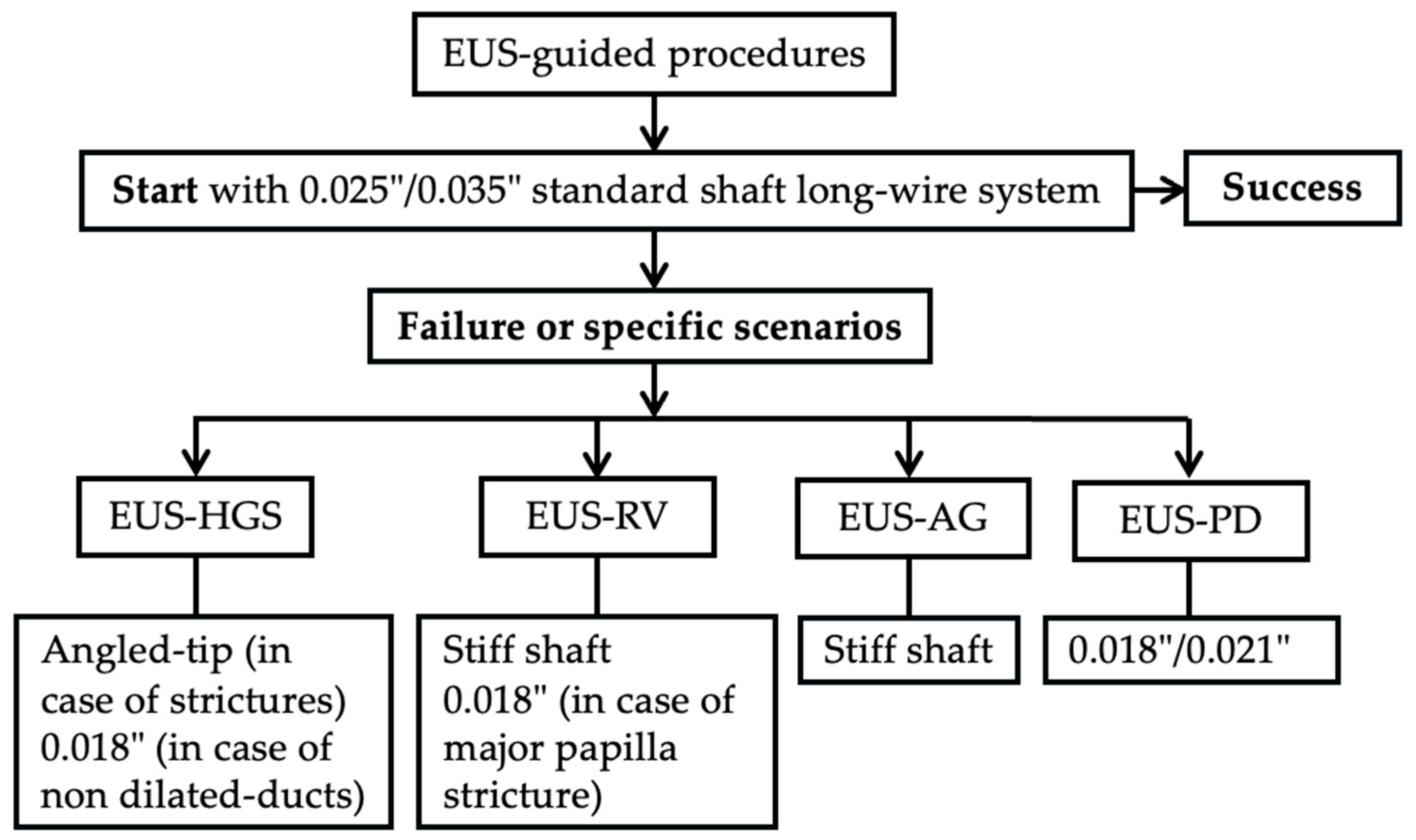
4. EUS
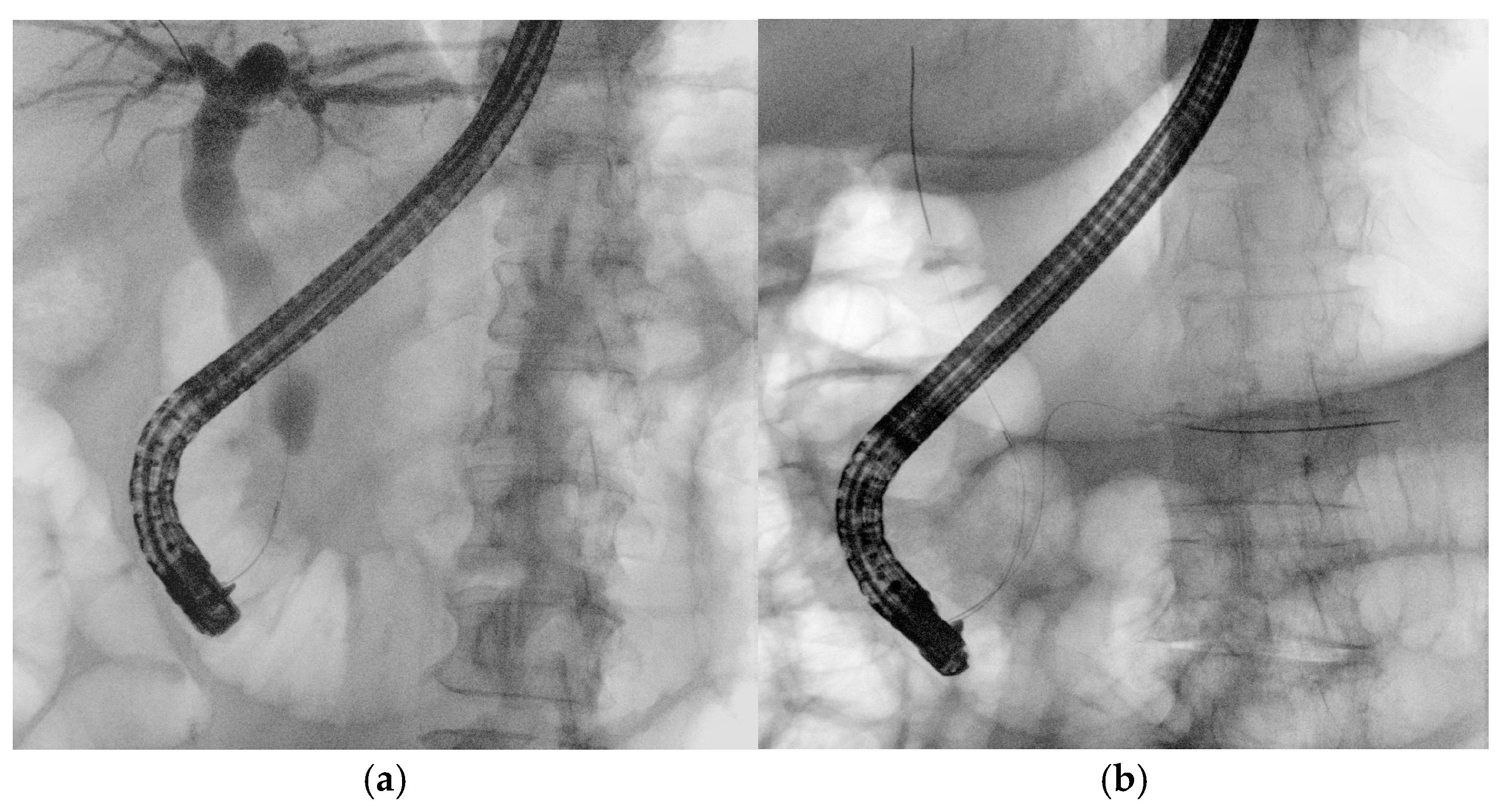
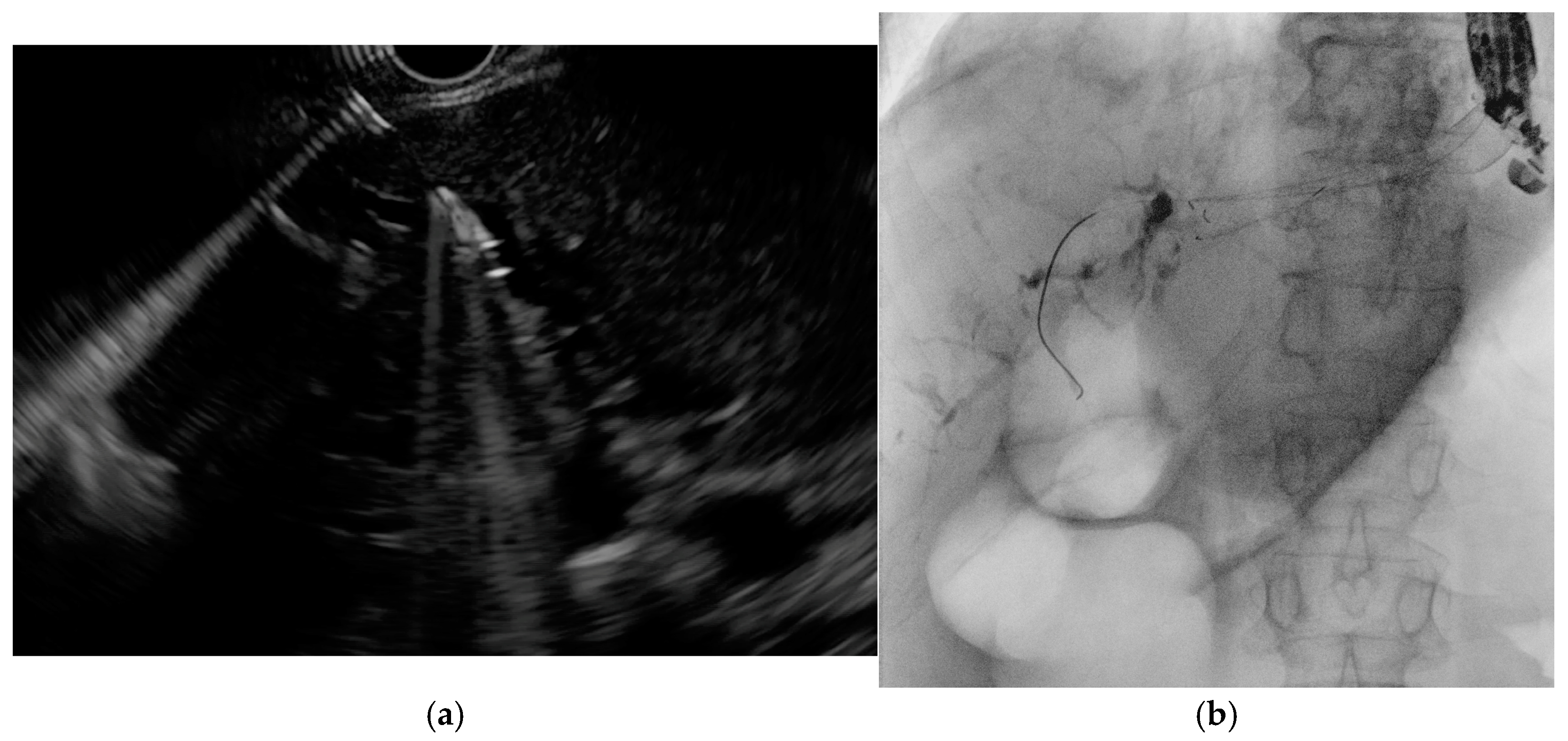
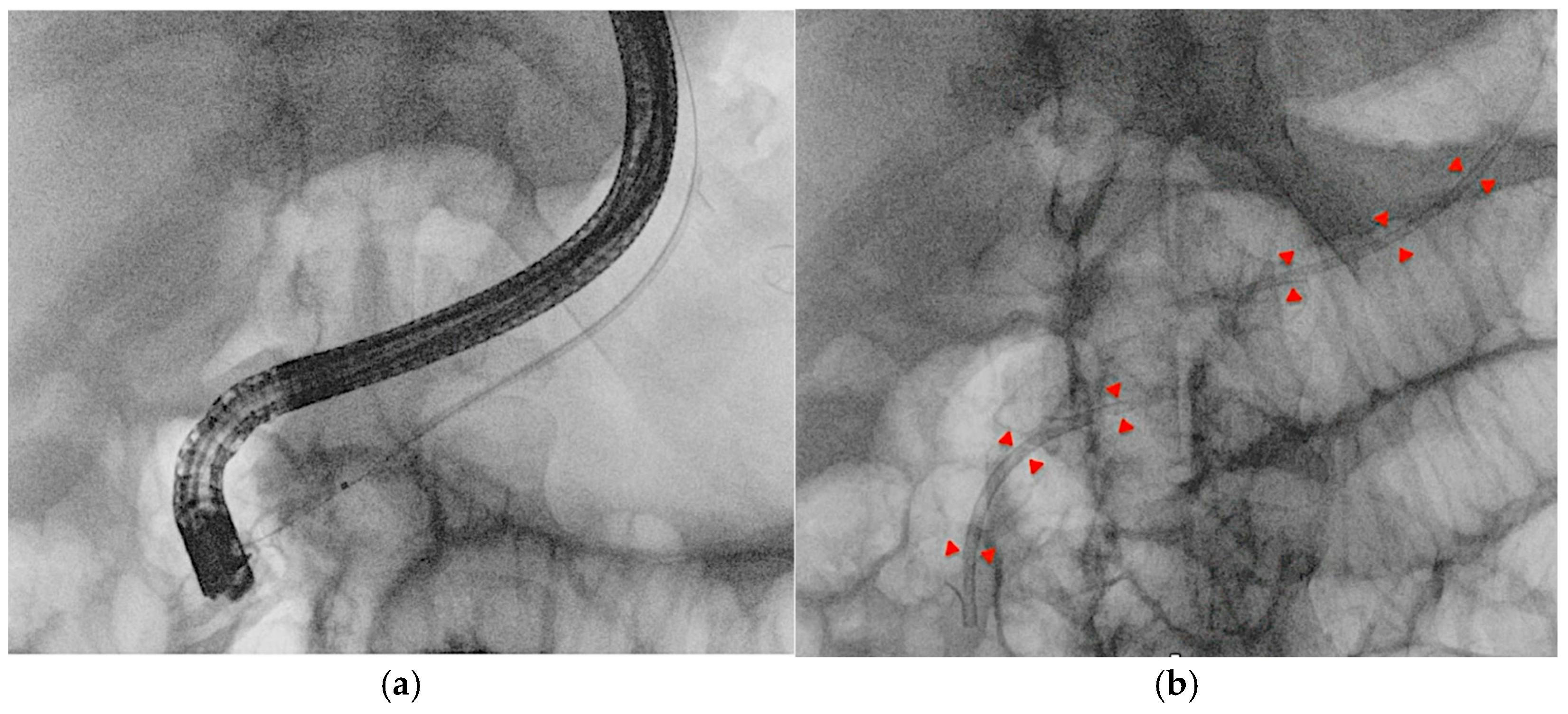
4.1. EUS-Guided Biliary Drainage (EUS-BD)
4.2. EUS–Pancreatic Drainage (EUS-PD)
4.3. Other EUS-Guided Procedures
5. Guidewire-Related Complications and Safety
5.1. ERCP-Related Complications
5.2. EUS-Related Complications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singhvi, G.; Dea, K.S. Guidewires in ERCP. Gastrointest. Endosc. 2013, 77, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Girotra, M.; Akshintala, S.V.; Chen, D.; Chen, Y.-I.; Das, K.K.; Kahn, A.; Mishra, G.; Muthusamy, R.V.; Obando, V.J.; et al. Guidewires in GI endoscopy. iGIE 2023, 2, 386–394. [Google Scholar] [CrossRef]
- Kwon, C.-I.; Koh, H.D.; Song, J.T.; Park, S.W.; Lee, H.D.; Jeong, S. Technical Reports of Endoscopic Retrograde Cholangiopancreatography Guidewires on the Basis of Physical Properties. Clin. Endosc. 2020, 53, 65–72. [Google Scholar] [CrossRef]
- Somogyi, L.; Chuttani, R.; Croffie, J.; Disario, J.; Liu, J.; Mishkin, D.; Shah, R.; Tierney, W.; Song, K.W.M.L.; Petersen, T.B. Guidewires for use in GI endoscopy. Gastrointest. Endosc. 2007, 65, 571–576. [Google Scholar] [CrossRef]
- Katsinelos, P.; Paroutoglou, G.; Kountouras, J.; Chatzimavroudis, G.; Zavos, C.; Pilpilidis, I.; Tzelas, G.; Tzovaras, G. A comparative study of standard ERCP catheter and hydrophilic guide wire in the selective cannulation of the common bile duct. Endoscopy 2008, 40, 302–307. [Google Scholar] [CrossRef]
- Reddy, C.S.; Draganov, V.P. ERCP wire systems: The long and the short of it. World J. Gastroenterol. 2009, 15, 55. [Google Scholar] [CrossRef]
- Enestvedt, K.B.; Kothari, S.; Pannala, R.; Yang, J.; Fujii-Lau, L.L.; Hwang, H.J.; Konda, V.; Manfredi, M.; Maple, T.J.; Murad, M.F.; et al. Devices and techniques for ERCP in the surgically altered GI tract. Gastrointest. Endosc. 2016, 83, 1061–1075. [Google Scholar] [CrossRef]
- Shah, J.R.; Somogyi, L.; Petersen, T.B.; Tierney, M.W.; Adler, G.D.; Chand, B.; Conway, D.J.; Croffie, M.B.J.; Disario, A.J.; Mishkin, S.D.; et al. Short-wire ERCP systems. Gastrointest. Endosc. 2007, 66, 650–657. [Google Scholar] [CrossRef]
- Draganov, V.P.; Kowalczyk, L.; Fazel, A.; Moezardalan, K.; Pan, J.-J.; Forsmark, E.C. Prospective Randomized Blinded Comparison of a Short-Wire Endoscopic Retrograde Cholangiopancreatography System with Traditional Long-Wire Devices. Dig. Dis. Sci. 2010, 55, 510–515. [Google Scholar] [CrossRef]
- Sung, J.M.; Jo, H.J.; Lee, S.H.; Park, Y.J.; Bang, S.; Chung, J.M. Comparison of Physician-Controlled Maneuver and Assistant-Controlled Maneuver during Endoscopic Retrograde Cholangiopancreatography. Yonsei Med. J. 2024, 65, 34. [Google Scholar] [CrossRef]
- Buxbaum, J.; Leonor, P.; Tung, J.; Lane, C.; Sahakian, A.; Laine, L. Randomized Trial of Endoscopist-Controlled vs. Assistant-Controlled Wire-Guided Cannulation of the Bile Duct. Am. J. Gastroenterol. 2016, 111, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Choe, W.J.; Kim, U.D.; Hyun, J.J.; Han, J.-H.; So, H.; Bang, J.S.; Koh, H.D.; Jeong, S. Comparison of Two Types of Guidewires for Malignant Hilar Biliary Obstruction by Endoscopic Retrograde Cholangiopancreatography: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 3590. [Google Scholar] [CrossRef]
- Aziz, M.; Iqbal, A.; Ahmed, Z.; Saleem, S.; Lee-Smith, W.; Goyal, H.; Kamal, F.; Alastal, Y.; Nawras, A.; Adler, G.D. Impact of guidewire caliber on ERCP outcomes: Systematic review and meta-analysis comparing 0.025- and 0.035-inch guidewires. Endosc. Int. Open 2022, 10, E990–E997. [Google Scholar] [CrossRef]
- Vihervaara, H.; Grönroos, M.J.; Koivisto, M.; Gullichsen, R.; Salminen, P. Angled- or straight-tipped hydrophilic guidewire in biliary cannulation: A prospective, randomized, controlled trial. Surg. Endosc. 2013, 27, 1281–1286. [Google Scholar] [CrossRef]
- Hausmann, J.; Lefa, F.; Filmann, N.; Eickhoff, A.; Zeuzem, S.; Herrmann, E.; Tantau, M.; Albert, J. Angled-tip vs. straight-tip guidewire in ERCP: A randomized, multicenter study. Scand. J. Gastroenterol. 2023, 58, 565–571. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Itoi, T.; Maetani, I.; Shigoka, H.; Ikeuchi, N.; Umeda, J.; Sofuni, A.; Itokawa, F.; Ishii, K.; Kurihara, T.; et al. Effectiveness of the J-Tip Guidewire for Selective Biliary Cannulation Compared to Conventional Guidewires (The JANGLE Study). Dig. Dis. Sci. 2015, 60, 2502–2508. [Google Scholar] [CrossRef]
- Hirose, S.; Fukasawa, M.; Takano, S.; Kadokura, M.; Shindo, H.; Takahashi, E.; Yokota, Y.; Fukasawa, Y.; Kawakami, S.; Hayakawa, H.; et al. Safety and Efficacy of a Small J-Tipped Guidewire for Pancreatic Duct Endoscopic Intervention. Can. J. Gastroenterol. Hepatol. 2019, 2019, 8947614. [Google Scholar] [CrossRef]
- Koga, T.; Tsuchiya, N.; Ishida, Y.; Kitaguchi, T.; Matsumoto, K.; Fukuyama, M.; Kojima, S.; Kojima, N.; Hirai, F. Structural factors influencing the clinical performance of 0.025-inch guidewires for pancreatobiliary endoscopy: An experimental study. Endosc. Int. Open 2024, 12, E666–E675. [Google Scholar] [CrossRef]
- Albert, J.; Lucas, K.; Filmann, N.; Herrmann, E.; Schröder, O.; Sarrazin, C.; Trojan, J.; Kronenberger, B.; Bojunga, J.; Zeuzem, S.; et al. A novel, stiff-shaft, flexible-tip guidewire for cannulation of biliary stricture during endoscopic retrograde cholangiopancreatography: A randomized trial. Endoscopy 2014, 46, 857–861. [Google Scholar] [CrossRef]
- Park, J.S.; Jeong, S.; Lee, H.D. Effectiveness of a novel highly flexible-tip guidewire on selective biliary cannulation compared to conventional guidewire: Randomized controlled study. Dig. Endosc. 2018, 30, 245–251. [Google Scholar] [CrossRef]
- Lella, F.; Bagnolo, F.; Colombo, E.; Bonassi, U. A simple way of avoiding post-ERCP pancreatitis. Gastrointest. Endosc. 2004, 59, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Testoni, P.; Mariani, A.; Aabakken, L.; Arvanitakis, M.; Bories, E.; Costamagna, G.; Devière, J.; Dinis-Ribeiro, M.; Dumonceau, J.-M.; Giovannini, M.; et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016, 48, 657–683. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Tsoi, K.K.; Quan, W.-L.; Lau, Y.W.J.; Sung, J.Y.J. Guidewire versus conventional contrast cannulation of the common bile duct for the prevention of post-ERCP pancreatitis: A systematic review and meta-analysis. Gastrointest. Endosc. 2009, 70, 1211–1219. [Google Scholar] [CrossRef]
- Cennamo, V.; Fuccio, L.; Zagari, M.R.; Eusebi, H.L.; Ceroni, L.; Laterza, L.; Fabbri, C.; Bazzoli, F. Can a Wire-Guided Cannulation Technique Increase Bile Duct Cannulation Rate and Prevent Post-ERCP Pancreatitis?: A Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2009, 104, 2343–2350. [Google Scholar] [CrossRef]
- Maki, T.; Irisawa, A.; Yamamiya, A.; Tominaga, K.; Abe, Y.; Imbe, K.; Hoshi, K.; Yamabe, A.; Igarashi, R.; Nakajima, Y.; et al. Guide Wire Selection (Straight vs. Angled) in Endoscopic Retrograde Cholangiopancreatography Using a Normal Contrast Catheter Performed by a Trainee: A Single-Center Prospective Randomized Controlled Cross-Over Study. J. Clin. Med. 2023, 12, 2917. [Google Scholar] [CrossRef]
- Pereira-Lima, J.; Sanmartin, A.D.I.; Watte, G.; Contin, I.; Pereira-Lima, G.; Onófrio, Q.D.F.; Altmayer, S.; Santos, D.O.E.C. Biliary cannulation with contrast and guide-wire versus exclusive guide-wire: A prospective, randomized, double-blind trial. Pancreatology 2021, 21, 459–465. [Google Scholar] [CrossRef]
- Takenaka, M.; Kudo, M. Usefulness of the double-guidewire technique for endoscopic procedures in the field of biliary and pancreatic diseases. Clin. Endosc. 2022, 55, 605–614. [Google Scholar] [CrossRef]
- Shamah, S.; Okolo, P. Reverse Double-Wire Cannulation of the Pancreatic Duct. Clin. Gastroenterol. Hepatol. 2017, 15, 782–783. [Google Scholar] [CrossRef][Green Version]
- Sakai, Y. New cannulation method for pancreatic duct cannulation-bile duct guidewire-indwelling method. World J. Gastrointest. Endosc. 2011, 3, 231. [Google Scholar] [CrossRef]
- Gonda, M.; Sarita, A.M.; Sakai, A.; Kodama, Y. Successful cannulation of a difficult pancreatic duct using the uneven method. VideoGIE 2024, 9, 237–240. [Google Scholar] [CrossRef]
- Fujimori, N. Endoscopic approach through the minor papilla for the management of pancreatic diseases. World J. Gastrointest. Endosc. 2013, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Maple, T.J.; Keswani, N.R.; Edmundowicz, A.S.; Jonnalagadda, S.; Azar, R.R. Wire-assisted access sphincterotomy of the minor papilla. Gastrointest. Endosc. 2009, 69, 47–54. [Google Scholar] [CrossRef]
- Dhir, V.; Isayama, H.; Itoi, T.; Almadi, M.; Siripun, A.; Teoh, B.Y.A.; Ho, Y.K. Endoscopic ultrasonography-guided biliary and pancreatic duct interventions. Dig. Endosc. 2017, 29, 472–485. [Google Scholar] [CrossRef]
- Hristov, B.; Radev, D.; Uchikov, P.; Kostov, G.; Doykov, M.; Valova, S.; Tilkiyan, E. Clinical Outcomes of EUS-Guided Choledochoduodenostomy for Biliary Drainage in Unresectable Pancreatic Cancer: A Case Series. Medicina 2023, 59, 351. [Google Scholar] [CrossRef]
- Merwe, D.V.W.S.; Wanrooij, V.J.L.R.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef]
- Isayama, H.; Nakai, Y.; Itoi, T.; Yasuda, I.; Kawakami, H.; Ryozawa, S.; Kitano, M.; Irisawa, A.; Katanuma, A.; Hara, K.; et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography-guided biliary drainage: 2018. J. Hepato-Biliary-Pancreat. Sci. 2019, 26, 249–269. [Google Scholar] [CrossRef]
- Ogura, T.; Ueno, S.; Okuda, A.; Nishioka, N.; Yamada, M.; Ueshima, K.; Matsuno, J.; Yamamoto, Y.; Higuchi, K. Expanding indications for endoscopic ultrasound-guided hepaticogastrostomy for patients with insufficient dilatation of the intrahepatic bile duct using a 22G needle combined with a novel 0.018-inch guidewire (with video). Dig. Endosc. 2022, 34, 222–227. [Google Scholar] [CrossRef]
- Yane, K.; Yoshida, M.; Imagawa, T.; Morita, K.; Ihara, H.; Hanada, K.; Hirokawa, S.; Tomita, Y.; Minagawa, T.; Okagawa, Y.; et al. Usefulness of endoscopic ultrasound-guided transhepatic biliary drainage with a 22-gauge fine-needle aspiration needle and 0.018-inch guidewire in the procedure’s induction phase. DEN Open 2024, 4, e297. [Google Scholar] [CrossRef]
- Kanno, Y.; Ito, K.; Sakai, T.; Okano, H. Novel combination of a 0.018-inch guidewire, dedicated thin dilator, and 22-gauge needle for EUS-guided hepaticogastrostomy. VideoGIE 2020, 5, 355–358. [Google Scholar] [CrossRef]
- Iwashita, T.; Ogura, T.; Ishiwatari, H.; Nakai, Y.; Iwata, K.; Mukai, T.; Shimizu, M.; Isayama, H.; Yasuda, I.; Itoi, T. Utility of dedicated bougie dilator for a 0.018-inch guidewire during EUS-guided biliary drainage: A multi-center retrospective cohort study. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 810–816. [Google Scholar] [CrossRef]
- Ogura, T.; Kawai, J.; Nishiguchi, K.; Yamamoto, Y.; Higuchi, K. EUS–guided transhepatic metal stent deployment technique without tract dilation using a 0.018-inch guidewire (with video). Endosc. Ultrasound 2023, 12, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.J.; Pérez-Miranda, M.; Vazquez-Sequeiros, E.; Abadia, A.-S.M.; Pérez-Millán, A.; González-Huix, F.; Gornals, J.; Iglesias-Garcia, J.; Serna, L.D.C.; Aparicio, R.J.; et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest. Endosc. 2012, 76, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, F.C.; Braden, B.; Burmeister, S.; Aabakken, L.; Arciadacono, G.P.; Bhutani, S.M.; Götzberger, M.; Healey, J.A.; Hocke, M.; Hollerbach, S.; et al. How to perform EUS-guided biliary drainage. Endosc. Ultrasound 2022, 11, 342–354. [Google Scholar] [CrossRef]
- Ogura, T.; Nishioka, N.; Ueno, S.; Yamada, T.; Yamada, M.; Imoto, A.; Hakoda, A.; Higuchi, K. Effect of echoendoscope angle on success of guidewire manipulation during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2021, 53, 369–375. [Google Scholar] [CrossRef]
- Ohno, A.; Fujimori, N.; Kaku, T.; Matsumoto, K.; Murakami, M.; Teramatsu, K.; Ueda, K.; Hijioka, M.; Aso, A.; Ogawa, Y. Puncture angle on an endoscopic ultrasound image is independently associated with unsuccessful guidewire manipulation of endoscopic ultrasound-guided hepaticogastrostomy: A retrospective study in Japan. Clin. Endosc. 2024, 57, 656–665. [Google Scholar] [CrossRef]
- Ueno, S.; Ogura, T.; Higuchi, K. Moving scope technique for guidewire insertion during endoscopic ultrasound-guided hepaticogastrostomy. Dig. Endosc. 2021, 33, 109. [Google Scholar] [CrossRef]
- Ogura, T.; Ueno, S.; Okuda, A.; Nishioka, N.; Higuchi, K. Jumping technique for guidewire manipulation within an intrahepatic bile duct during EUS-guided biliary drainage (with video). Endosc. Ultrasound 2021, 10, 305. [Google Scholar] [CrossRef]
- Martínez, B.; Martínez, J.; Casellas, A.J.; Aparicio, R.J. Endoscopic ultrasound-guided rendezvous in benign biliary or pancreatic disorders with a 22-gauge needle and a 0.018-inch guidewire. Endosc. Int. Open 2019, 7, E1038–E1043. [Google Scholar] [CrossRef]
- Nakai, Y.; Oyama, H.; Kanai, S.; Noguchi, K.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, K.; Saito, T.; Hamada, T.; et al. Double Guidewire Technique Using an Uneven Double Lumen Catheter for Endoscopic Ultrasound-Guided Interventions. Dig. Dis. Sci. 2021, 66, 1540–1547. [Google Scholar] [CrossRef]
- Tomishima, K.; Isayama, H.; Suzuki, A.; Ishii, S.; Takahashi, S.; Fujisawa, T. Technical review of endoscopic ultrasound-guided drainage/anastomosis and trans-endosonographically created route procedures for the treatment of pancreatic diseases. DEN Open 2025, 5, e393. [Google Scholar] [CrossRef]
- Itoi, T.; Kasuya, K.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Yasuda, I.; Nakai, Y.; Isayama, H.; Moriyasu, F. Endoscopic ultrasonography-guided pancreatic duct access: T echniques and literature review of pancreatography, transmural drainage and rendezvous techniques. Dig. Endosc. 2013, 25, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Kogure, H.; Isayama, H.; Koike, K. Endoscopic ultrasound-guided pancreatic duct drainage. Saudi J. Gastroenterol. 2019, 25, 210. [Google Scholar] [CrossRef]
- Matsunami, Y.; Itoi, T.; Sofuni, A.; Tsuchiya, T.; Kamada, K.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Fujita, M.; et al. Evaluation of a new stent for EUS-guided pancreatic duct drainage: Long-term follow-up outcome. Endosc. Int. Open 2018, 06, E505–E512. [Google Scholar] [CrossRef]
- Imoto, A.; Ogura, T.; Higuchi, K. Endoscopic Ultrasound-Guided Pancreatic Duct Drainage: Techniques and Literature Review of Transmural Stenting. Clin. Endosc. 2020, 53, 525–534. [Google Scholar] [CrossRef]
- Inoue, T.; Kitano, R.; Ibusuki, M.; Kitada, T.; Sakamoto, K.; Arai, J.; Ito, K. One-step primary endoscopic ultrasound-guided choledochoduodenostomy without lumen-apposing metal stent using a Franseen needle and an ultra-stiff high-sliding guidewire. Endoscopy 2024, 56, E726–E727. [Google Scholar] [CrossRef]
- Sasaki, T.; Sato, Y.; Maegawa, Y.; Okamoto, T.; Sasahira, N. One-step endoscopic ultrasound-guided pancreatic duct drainage using a 7-Fr ultra-tapered plastic stent and a novel 0.035-inch guidewire. Endoscopy 2024, 56, E1098–E1099. [Google Scholar] [CrossRef]
- Shimizu, H.; Suzuki, R.; Sato, Y.; Takagi, T.; Abe, N.; Irie, H.; Sugimoto, M.; Yanagita, T.; Kobashi, R.; Hashimoto, M.; et al. Transjejunal endoscopic ultrasound-guided pancreatic drainage for pancreatic jejunostomy stricture using a forward-viewing echoendoscope in a patient with altered anatomy. DEN Open 2022, 2, e114. [Google Scholar] [CrossRef]
- Luengo, C.M.; Consiglieri, F.C.; Puga, M.; Gornals, B.J. A novel rescue maneuver for a distal dislodged lumen-apposing metal stent (LAMS): “LAMS-in-LAMS” technique. Rev. Esp. Enfermedades Dig. 2019, 111, 243–245. [Google Scholar] [CrossRef]
- Wannhoff, A.; Ruh, N.; Meier, B.; Riecken, B.; Caca, K. Endoscopic gastrointestinal anastomoses with lumen-apposing metal stents: Predictors of technical success. Surg. Endosc. 2021, 35, 1997–2004. [Google Scholar] [CrossRef]
- Itoi, T.; Ishii, K.; Ikeuchi, N.; Sofuni, A.; Gotoda, T.; Moriyasu, F.; Dhir, V.; Teoh, B.Y.A.; Binmoeller, F.K. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut 2016, 65, 193–195. [Google Scholar] [CrossRef]
- Chavan, R.; Singla, V.; Sundaram, S.; Zanwar, S.; Shah, C.; Sud, S.; Singh, P.; Gandhi, C.; Bhatt, P.; Goel, A.; et al. Outcomes of endoscopic ultrasound-guided gallbladder drainage: A multicenter study from India (with video). Indian J. Gastroenterol. 2024, 43, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Omoto, S.; Kudo, M. EUS-guided drainage of the gallbladder using a novel 0.018-inch guidewire for preventing bile leakage (with video). Endosc. Ultrasound 2022, 11, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Stapfer, M.; Selby, R.R.; Stain, C.S.; Katkhouda, N.; Parekh, D.; Jabbour, N.; Garry, D. Management of Duodenal Perforation After Endoscopic Retrograde Cholangiopancreatography and Sphincterotomy. Ann. Surg. 2000, 232, 191–198. [Google Scholar] [CrossRef]
- Lee, H.T.; Jung, K.Y.; Park, S.-H. Preparation of High-Risk Patients and the Choice of Guidewire for a Successful Endoscopic Retrograde Cholangiopancreatography Procedure. Clin. Endosc. 2014, 47, 334. [Google Scholar] [CrossRef]
- Vezakis, A.; Fragulidis, G.; Polydorou, A. Endoscopic retrograde cholangiopancreatography-related perforations: Diagnosis and management. World J. Gastrointest. Endosc. 2015, 7, 1135–1141. [Google Scholar] [CrossRef]
- Wu, M.H.; Dixon, E.; May, R.G.; Sutherland, R.F. Management of perforation after endoscopic retrograde cholangiopancreatography (ERCP): A population-based review. HPB 2006, 8, 393–399. [Google Scholar] [CrossRef]
- Canena, J.; Liberato, M.; Horta, D.; Romão, C.; Coutinho, A. Short-term stenting using fully covered self-expandable metal stents for treatment of refractory biliary leaks, postsphincterotomy bleeding, and perforations. Surg. Endosc. 2013, 27, 313–324. [Google Scholar] [CrossRef]
- Zejnullahu, A.V.; Zejnullahu, A.V. Fractured guide wire in the main pancreatic duct during ERCP: A case report. Int. J. Surg. Case Rep. 2023, 102, 107843. [Google Scholar] [CrossRef]
- Fry, L.C.; Linder, J.D.; Mönkemüller, K.E. Cholangitis as a result of hydrophilic guidewire fracture. Gastrointest. Endosc. 2002, 56, 943–944. [Google Scholar] [CrossRef]
- Vats, P.; Jain, K.P.; Khanna, S.; Srivastava, A.; Patel, K.R. Bleeding From Unintentional Portal Vein Stenting During Endoscopic Retrograde Cholangiopancreatography Managed With Portal Vein Stent Graft Placement—A Case Report With a Detailed Review of the Literature. J. Clin. Exp. Hepatol. 2025, 15, 102468. [Google Scholar] [CrossRef]
- Ogura, T.; Higuchi, K. Technical Review of Developments in Endoscopic Ultrasound-Guided Hepaticogastrostomy. Clin. Endosc. 2021, 54, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Uba, Y.; Hattori, N.; Matsuno, J.; Nishikawa, H. Endoscopic ultrasound-guided hepaticogastrostomy for patients with frequent respiratory fluctuations using a novel hybrid guidewire to prevent guidewire shearing. Endoscopy 2024, 56, E743–E744. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhara, V.; Khashab, A.M.; Muthusamy, R.V.; Acosta, D.R.; Agrawal, D.; Bruining, H.D.; Eloubeidi, A.M.; Fanelli, D.R.; Faulx, L.A.; Gurudu, R.S.; et al. Adverse events associated with ERCP. Gastrointest. Endosc. 2017, 85, 32–47. [Google Scholar] [CrossRef]
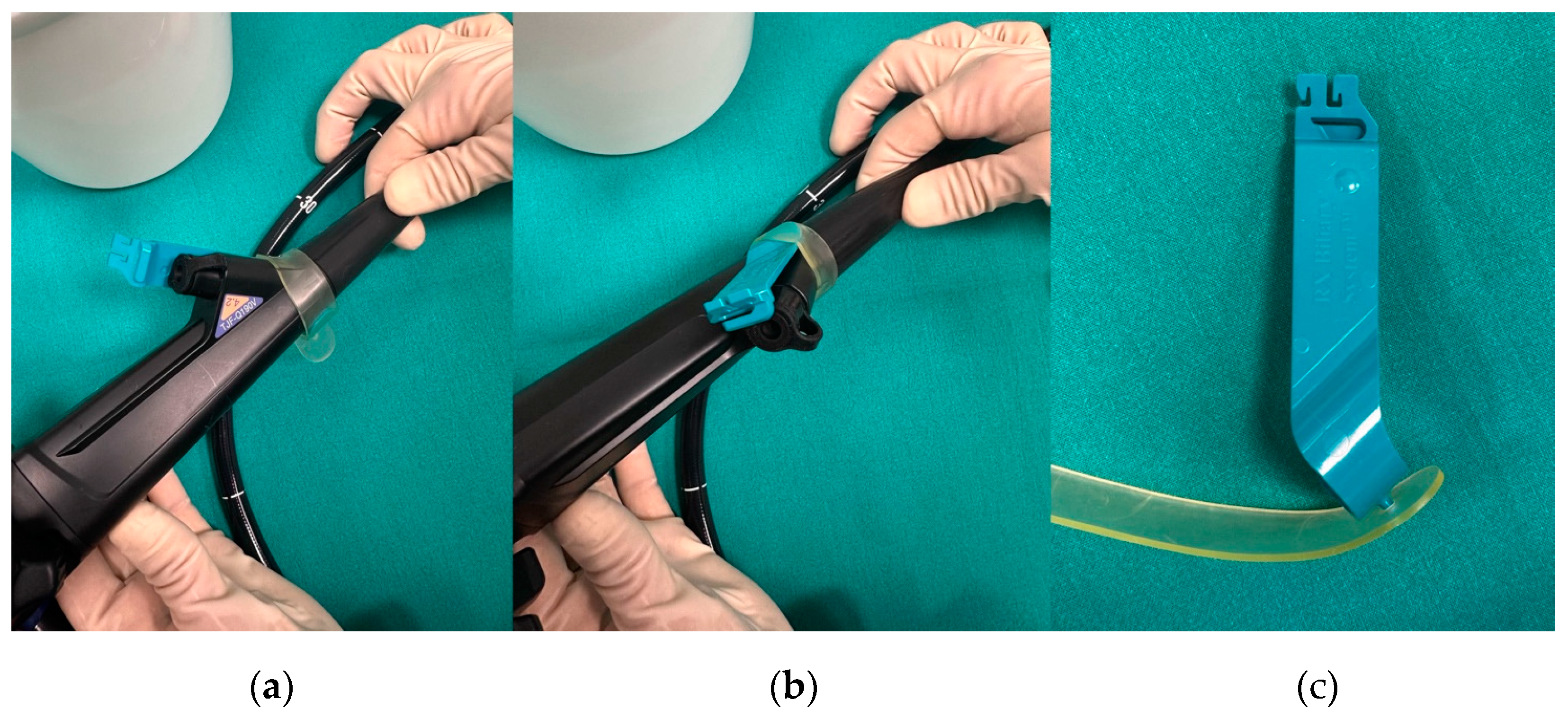
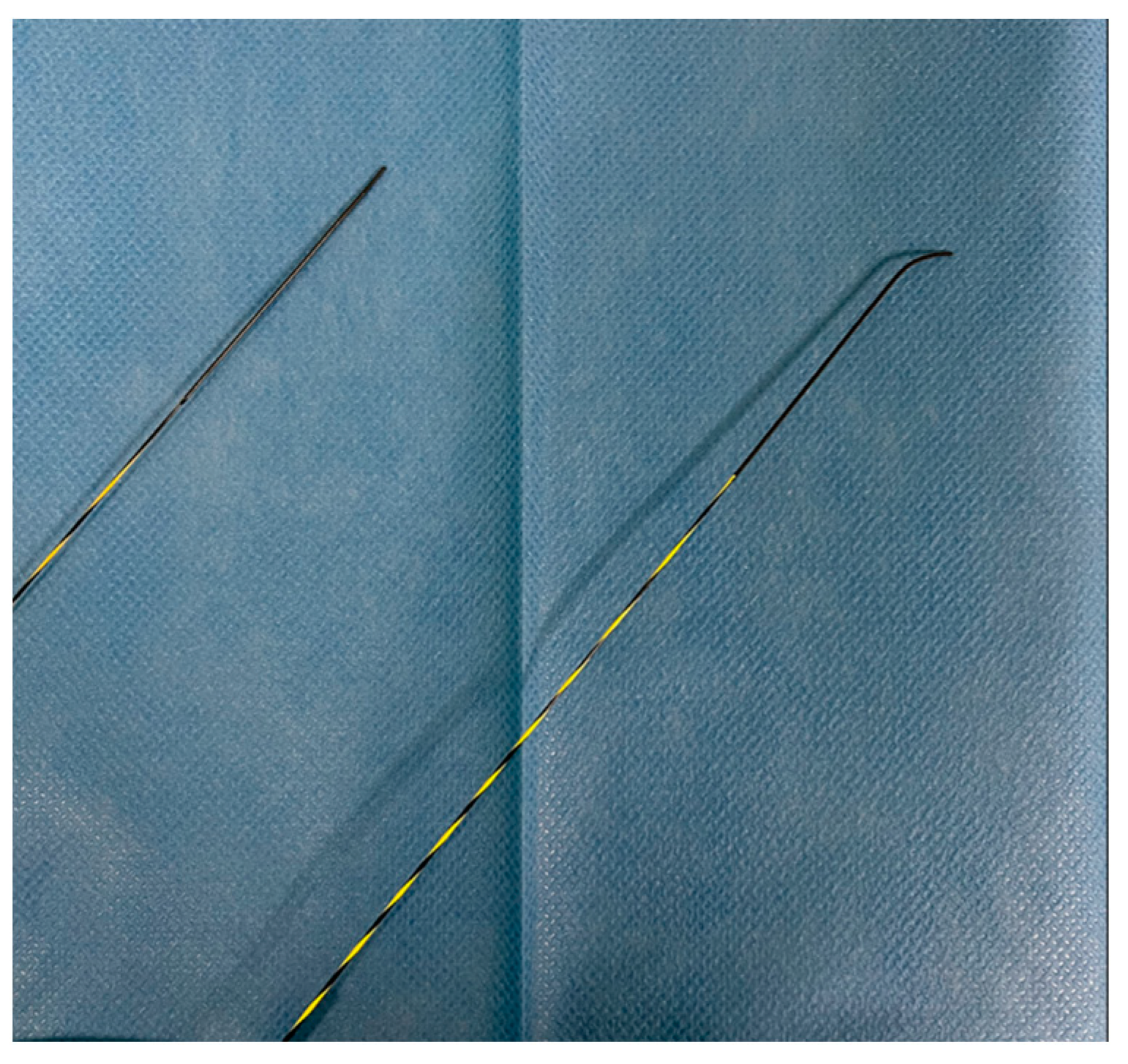
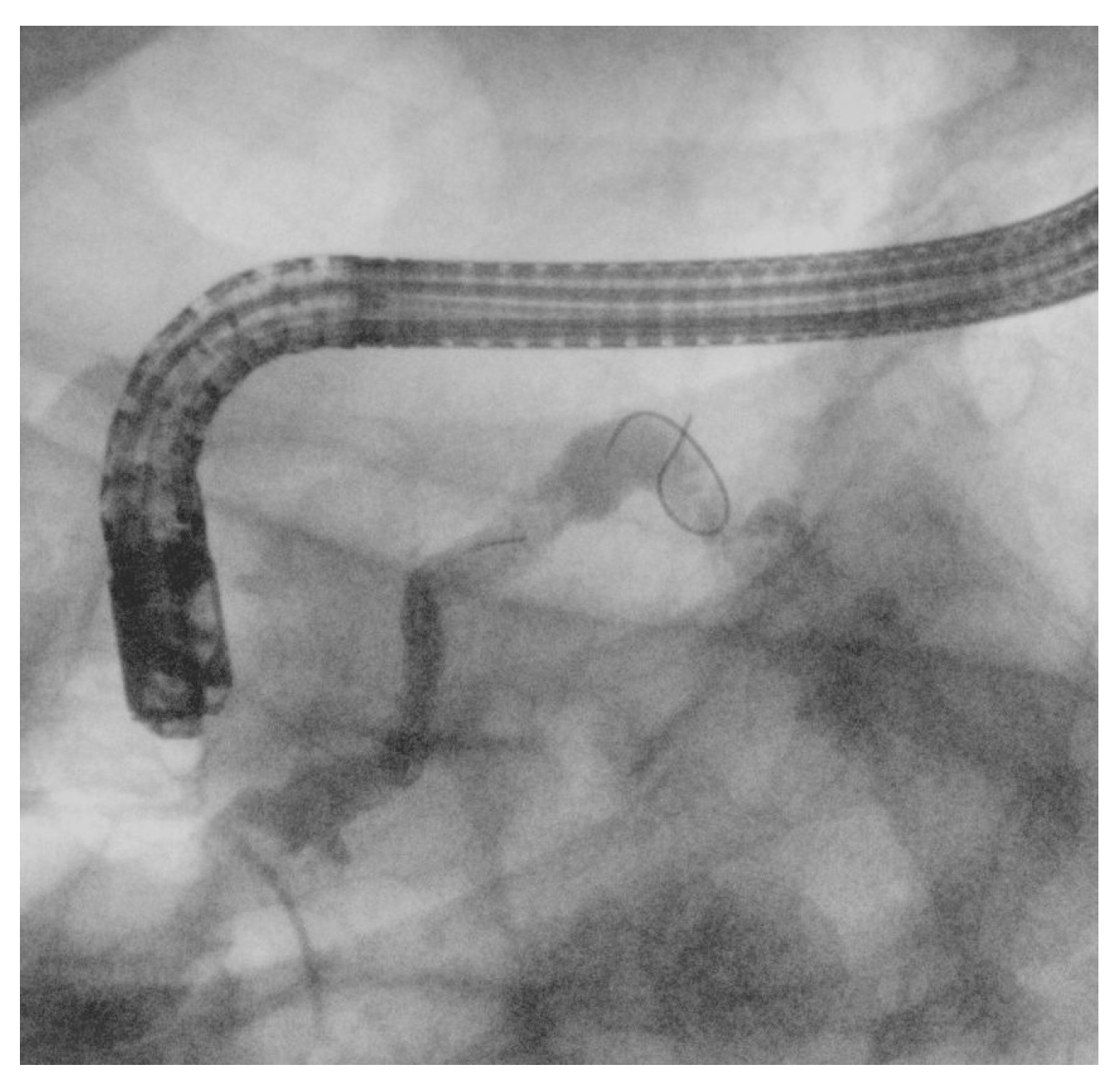
| Structure | Length | Diameter | Tip Shape | Stiffness |
|---|---|---|---|---|
| Hydrophobic shaft with hydrophilic tip | Long wire system (450, 480 cm) | Standard (0.035-inch) | Straight | Standard |
| Fully hydrophilic | Short wire system (260 cm) | Thin (0.018- and 0.025-inch) | Angled, “J”-shaped | Stiff, ultra stiff |
| Guidewire | Manufacturer, Country | Diameter (inch) | Length (cm) | Shaft Style | Tip Shape | Tip (cm) |
|---|---|---|---|---|---|---|
| Fielder25 | Asahi, Intecc, Tokyo, Japan | 0.025 | 450 | Standard (flexible) | Straight, angled | 7.5 |
| M-Through | Asahi, Intecc, Tokyo, Japan | 0.025 | 450 | Standard (stiff), Flex | Straight, angled | 7.5 |
| Dreamwire | BS, Marlborough, MA, USA | 0.035 | 260, 450 | Standard, stiff | Straight, angled | 10 |
| EndoSelector | BS, Marlborough, MA, USA | 0.025 | 450 | Standard | Staight, angled | 7 |
| Hydra Jagwire | BS, Marlborough, MA, USA | 0.035 | 260, 450 | Standard, stiff | Straight, angled | 5, 10 |
| Jagwire | BS, Marlborough, MA, USA | 0.025, 0.035 | 260, 450 | Standard, stiff | Straight, angled | 5 |
| Jagwire Revolution | BS, Marlborough, MA, USA | 0.025 | 260, 450 | Standard | Straight, angled | 5 |
| NaviPro | BS, Marlborough, MA, USA | 0.018, 0.025, 0.035 | 260 | FH Standard, stiff | Straight, angled | 3 |
| NovaGold | BS, Marlborough, MA, USA | 0.018 | 260, 480 | Standard | Straight | 6 |
| XWire | ConMed, Westborough, MA, USA | 0.025, 0.035 | 260, 450 | Standard, stiff | Straight, angled | 5 |
| FXWire | ConMed, Westborough, MA, USA | 0.035 | 260, 450 | Standard | Straight, angled | 5 |
| Acrobat 2 | Cook Medical, Bloomington, IN, USA | 0.025, 0.035 | 260, 450 | Standard | Straight, angled | 4 |
| Tracer Metro | Cook Medical, Bloomington, IN, USA | 0.021, 0.025, 0.035 | 260, 480 | Standard | Straight, angled | 5 |
| Roadrunner | Cook Medical, Bloomington, IN, USA | 0.018 | 260, 480 | Standard | Straight, angled | 3 |
| Delta Wire | Cook Medical, Bloomington, IN, USA | 0.025, 0.035 | 260 | FH | Straight, angled | N/A |
| GPS Wire | Fujifilm, Tokyo, Japan | 0.035 | 260, 450 | Standard | Straight, J-tip | N/A |
| J-Wire Series | J-MIT, Tokyo, Japan | 0.018, 0.025 | 450 | N/A | Angled | 5 |
| RewoWave | Olympus, Tokyo, Japan | 0.025, 0.035 | 260, 450 | Standard, stiff, ultra stiff | Straight, angled | 5, 7 |
| VisiGlide, VisiGlide 2 | Olympus, Tokyo, Japan | 0.025, 0.035 | 270, 450 | Standard | Straight, angled | 7 |
| Glidewire | Olympus, Tokyo, Japan | 0.018, 0.025, 0.035 | 260, 450 | FH Standard, stiff | Straight, angled | 3, 5, 8 |
| Optimos | Taewong, China | 0.025, 0.035 | 260, 450 | Standard | Straight, angled | 5.5, 6, 7, 7.5 |
| Radifocus | Terumo, Tokyo, Japan | 0.018, 0.025, 0.035 | 260, 450 | FH Standard, stiff | Straight, angled, J-tip | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, D.; Delogu, C.; Mazza, S.; Mauro, A.; Bartolotta, E.; Cappellini, A.; Scalvini, D.; Torello Viera, F.; Bardone, M.; Anderloni, A. The Role and Appropriate Selection of Guidewires in Biliopancreatic Endoscopy. Medicina 2025, 61, 913. https://doi.org/10.3390/medicina61050913
Alfieri D, Delogu C, Mazza S, Mauro A, Bartolotta E, Cappellini A, Scalvini D, Torello Viera F, Bardone M, Anderloni A. The Role and Appropriate Selection of Guidewires in Biliopancreatic Endoscopy. Medicina. 2025; 61(5):913. https://doi.org/10.3390/medicina61050913
Chicago/Turabian StyleAlfieri, Daniele, Claudia Delogu, Stefano Mazza, Aurelio Mauro, Erica Bartolotta, Alessandro Cappellini, Davide Scalvini, Francesca Torello Viera, Marco Bardone, and Andrea Anderloni. 2025. "The Role and Appropriate Selection of Guidewires in Biliopancreatic Endoscopy" Medicina 61, no. 5: 913. https://doi.org/10.3390/medicina61050913
APA StyleAlfieri, D., Delogu, C., Mazza, S., Mauro, A., Bartolotta, E., Cappellini, A., Scalvini, D., Torello Viera, F., Bardone, M., & Anderloni, A. (2025). The Role and Appropriate Selection of Guidewires in Biliopancreatic Endoscopy. Medicina, 61(5), 913. https://doi.org/10.3390/medicina61050913






