Retinal Hemorrhages and Long-Term Ocular Outcomes in Neonatal Hypoxic-Ischemic Encephalopathy
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanukollu, V.M.; Ahmad, S.S. Retinal Hemorrhage. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Emerson, M.V.; Pieramici, D.J.; Stoessel, K.M.; Berreen, J.P.; Gariano, R.F. Incidence and rate of disappearance of retinal hemorrhage in newborns. Ophthalmology 2001, 108, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.A.; May, K.; Talbot, J.F.; Parsons, M.A. Incidence, distribution, and duration of birth-related retinal hemorrhages: A prospective study. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus. 2006, 10, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, Y.; Yang, Y.; Li, Z.; Lin, Y.; Liu, R.; Wei, C.; Ding, X. Birth-related retinal hemorrhages in healthy full-term newborns and their relationship to maternal, obstetric, and neonatal risk factors. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Callaway, N.F.; Ludwig, C.A.; Blumenkranz, M.S.; Jones, J.M.; Fredrick, D.R.; Moshfeghi, D.M. Retinal and optic nerve hemorrhages in the newborn infant: One-year results of the newborn eye screen test study. Ophthalmology 2016, 123, 1043–1052. [Google Scholar] [CrossRef]
- Christensen, R.D.; Baer, V.L.; Yaish, H.M. Thrombocytopenia in late preterm and term neonates after perinatal asphyxia. Transfusion 2015, 55, 187–196. [Google Scholar] [CrossRef]
- Yanli, Z.; Qi, Z.; Yu, L.; Haike, G. Risk Factors Affecting the Severity of Full-Term Neonatal Retinal Hemorrhage. J. Ophthalmol. 2017, 2017, 4231489. [Google Scholar] [CrossRef]
- Ling, R.; James, B. White-centred retinal haemorrhages (Roth spots). Postgrad. Med. J. 1998, 74, 581–582. [Google Scholar] [CrossRef]
- Arora, N.; Dhibar, D.P.; Bashyal, B.; Agarwal, A. Roth’s Spots, a clinical diagnostic clue for Infective Endocarditis. Perm. J. 2020, 24, 20.038. [Google Scholar] [CrossRef]
- Chandra, A.; Chakraborty, U.; Ganai, S.; Ray, A.K. Roth spots in acute myeloid leukaemia. BMJ Case Rep. 2020, 13, e238133. [Google Scholar] [CrossRef]
- Togioka, B.M.; Arnold, M.A.; Bathurst, M.A.; Ziegfeld, S.M.; Nabaweesi, R.; Colombani, P.M.; Chang, D.C.; Abdullah, F. Retinal hemorrhages and shaken baby syndrome: An evidence-based review. J. Emerg. Med. 2009, 37, 98–106. [Google Scholar] [CrossRef]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Eris, E.; Eris, D.; Seymen, Z.; Karasu, B.; Dıracoglu, A.; Perente, I.; Cömert, S. Retinal haemorrhage rates and resolution time of retinal haemorrhage in newborns after hypothermic treatment for hypoxic–ischemic encephalopathy. Arch. Pediatr. 2020, 27, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, H.B.; Sarnat, M.S. Neonatal encephalopathy following fetal distress: A clinical and electroencephalographic study. Arch. Neurol. 1976, 33, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Walas, W.; Wilińska, M.; Bekiesińska-Figatowska, M.; Halaba, Z.; Śmigiel, R. Methods for assessing the severity of perinatal asphyxia and early prognostic tools in neonates with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia. Adv. Clin. Exp. Med. 2020, 29, 1011–1016. [Google Scholar] [CrossRef]
- Egge, K.; Lyng, G.; Maltau, J. Effect of instrumental delivery on the frequency and severity of retinal hemorrhages in the newborn. Acta Obstet. Gynecol. Scand. 1981, 60, 153–155. [Google Scholar] [CrossRef]
- Laptook, A.R. Birth asphyxia and hypoxic-ischemic brain injury in the preterm infant. Clin. Perinatol. 2016, 43, 529–545. [Google Scholar] [CrossRef]
- Geddes, J.F.; Tasker, R.C.; Hackshaw, A.K.; Nickols, C.D.; Adams, G.G.W.; Whitwell, H.L.; Scheimberg, I. Dural haemorrhage in non-traumatic infant deaths: Does it explain the bleeding in ‘shaken baby syndrome’? Neuropathol. Appl. Neurobiol. 2003, 29, 14–22. [Google Scholar] [CrossRef]
- Chen, L.-N.; He, X.-P.; Huang, L.-P. A survey of high risk factors affecting retinopathy in full-term infants in China. Int. J. Ophtalmol. 2012, 5, 177. [Google Scholar]
- Pu, Q.; Li, P.; Jiang, H.; Wang, H.; Zhou, Q.; Liu, J.; Zhong, W.; Huang, H. Factors related to retinal haemorrhage in infants born at high risk. Acta Ophthalmol. 2017, 95, e477–e480. [Google Scholar] [CrossRef]
- James, M.; Connor, C.M.O.; Cullinane, A.; Murray, D.M.; Boylan, G.B. Ophthalmic outcomes following neonatal hypoxic ischaemic encephalopathy; oculomotor, biometric and refractive data in early childhood. Eye 2019, 33, 1152–1157. [Google Scholar] [CrossRef]
- Unal, S.; Kara, C.; Demirel, N.; Petriçli, S.; Kavurt, S.; Uzlu, E.; Durukan, M.; Bas, A.Y. Should Ocular Hemorrhage Screening be Conducted in Newborns with Acidosis? Am. J. Perinatol. 2024, 41, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Pehere, N.; Chougule, P.; Dutton, G.N. Cerebral visual impairment in children: Causes and associated ophthalmological problems. Indian J. Ophthalmol. 2018, 66, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Northstone, K.; Howard, M.; Harvey, I.; Harrad, R.A.; Sparrow, J.M. Prevalence and risk factors for common visual problems in children: Data from the ALSPAC study. Br. J. Ophthalmol. 2008, 92, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Robaei, D.; Rose, K.A.; Kifley, A.; Cossick, M.; Ip, J.M.; Mitchell, P. Factors associated with childhood strabismus: Findings from a population-based study. Ophthalmology 2006, 113, 1146–1153. [Google Scholar] [CrossRef]
- Pathai, S.; Cumberland, P.M.; Rahi, J.S. Prevalence of and early-life influences on childhood strabismus: Findings from the Millennium Cohort Study. Arch. Pediatr. Adolesc. Med. 2010, 164, 250–257. [Google Scholar] [CrossRef]
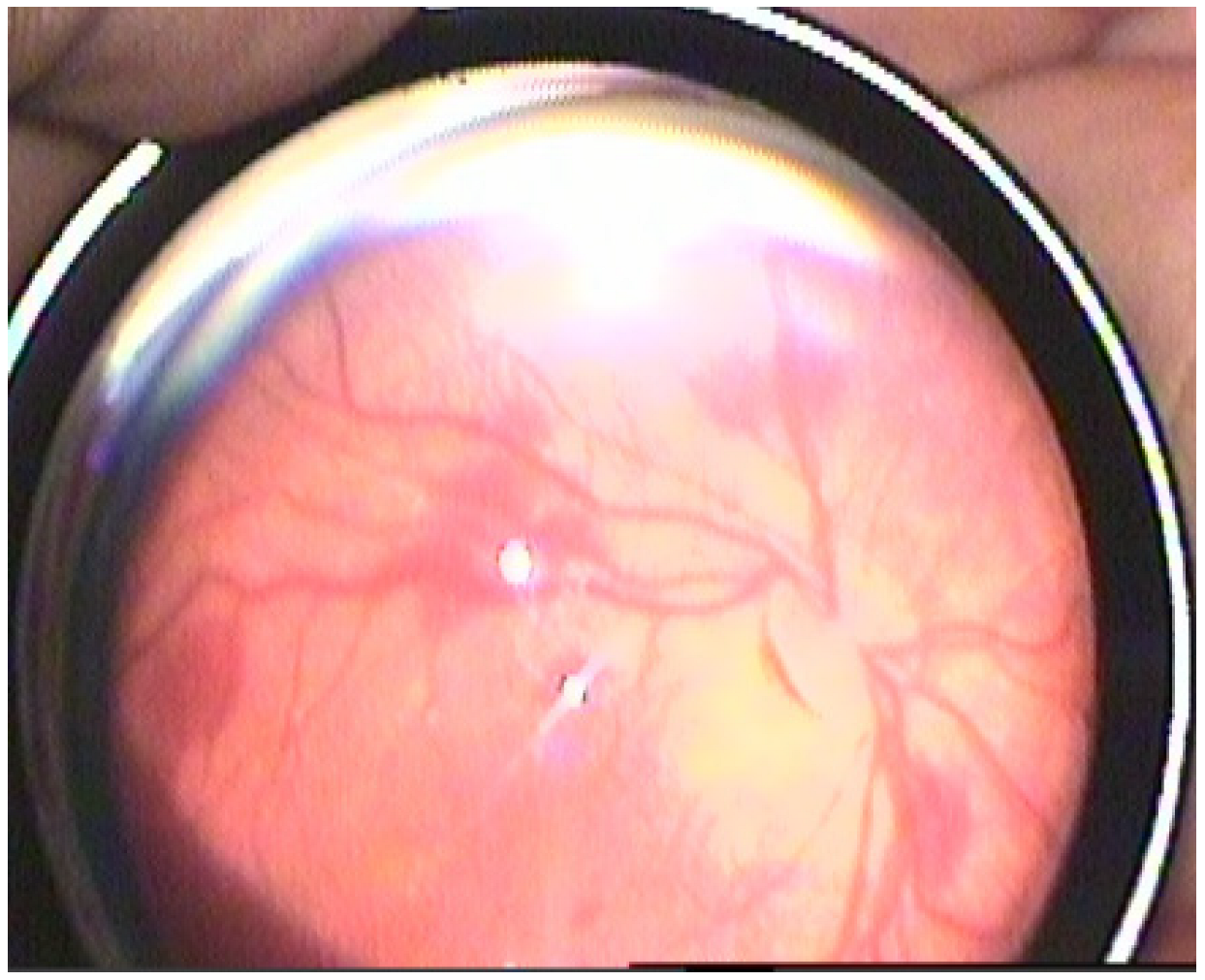
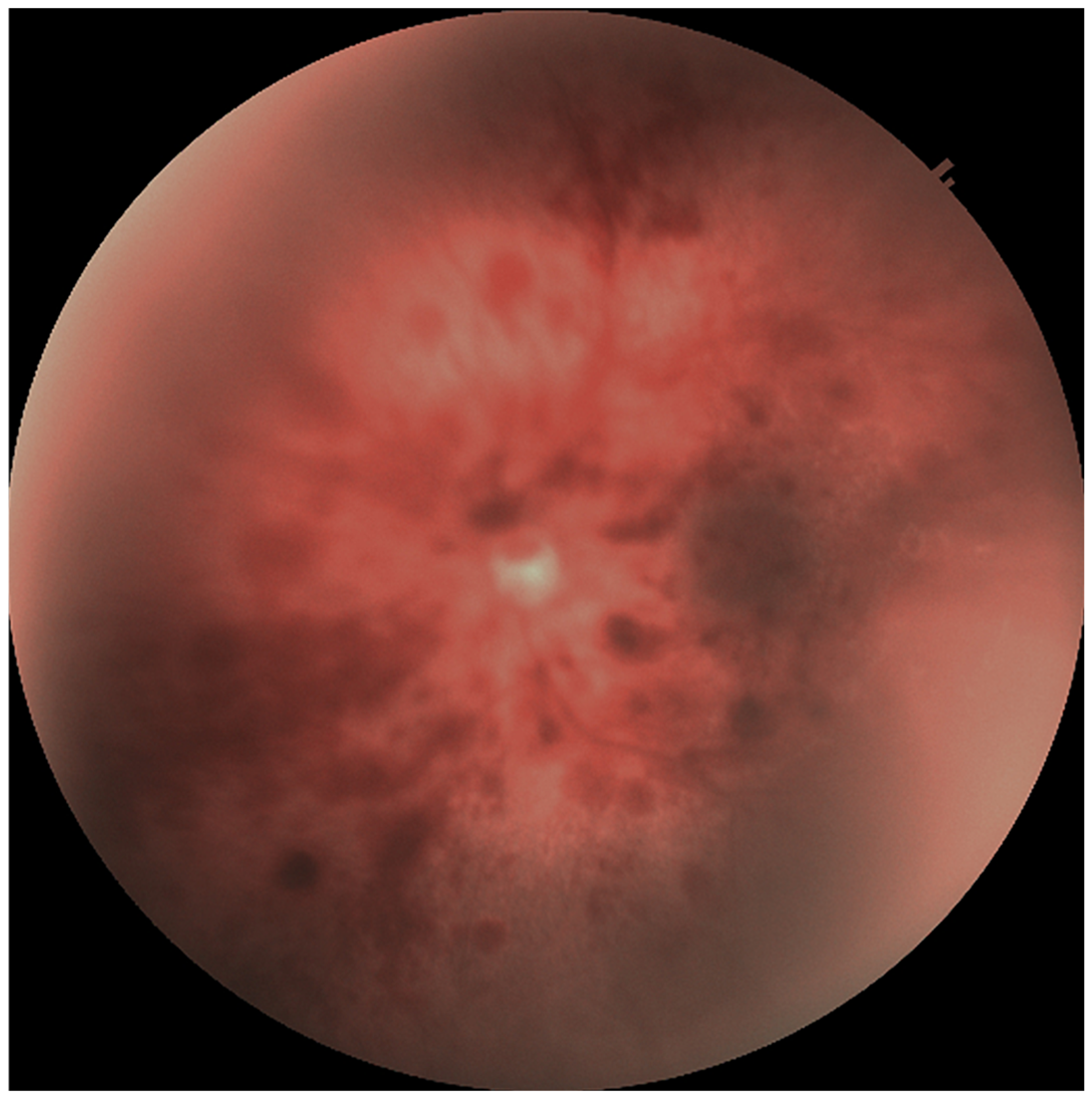
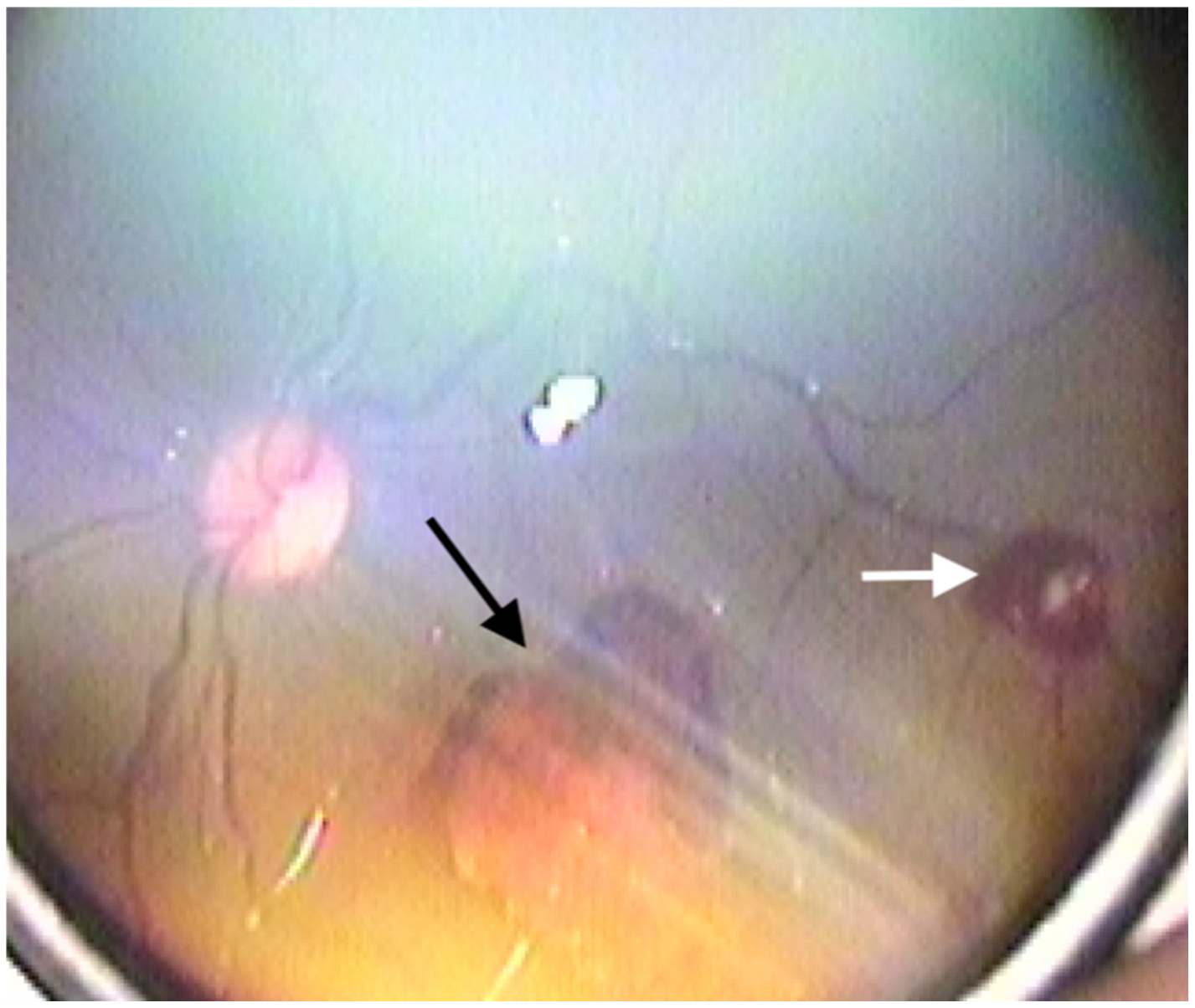
| Stage I HIE | Stage II HIE | Stage III HIE | p Value | |
|---|---|---|---|---|
| Gender n (%) | 0.354 | |||
| Female | 16 (50%) | 54 (42%) | 26 (53%) | |
| Male | 16 (50%) | 75 (58%) | 23 (47%) | |
| Gestational age (mean ± SD) (week) | 39.25 ± 0.84 | 38.89 ± 1.66 | 38.53 ± 1.91 | 0.144 |
| Birth weight (mean ± SD) (gram) | 3200 ± 148 | 3181 ± 663 | 3039 ± 626 | 0.340 |
| Delivery Type n (%) | <0.001 | |||
| Vaginal delivery | 24 (75%) | 76 (59%) | 16 (33%) | |
| Caesarean section | 8 (25%) | 53 (41%) | 33 (67%) |
| Grade I HIE | Grade II HIE | Grade III HIE | p Value | |
|---|---|---|---|---|
| Retinal haemorrhage (n = eye) n (%) | <0.001 | |||
| + | 4 (6.2%) | 92 (35.7%) | 82 (83.7%) | |
| − | 60 (93.8%) | 166 (64.3%) | 16 (16.3%) | |
| Roth spots (n = eye) n (%) | <0.001 | |||
| + | 4 (6.2%) | 100 (38.8%) | 76 (77.6%) | |
| − | 60 (93.8%) | 158 (61.2%) | 22 (22.4%) | |
| Resolution time of RH (day) (mean ± SD) | 10.50 ± 4.94 | 16.43 ± 3.67 | 24.92 ± 4.96 | <0.001 |
| Resolution time of RS (day) (mean ± SD) | 10.50 ± 4.94 | 12.88 ± 2.59 | 14.18 ± 1.13 | 0.004 |
| Egge Classification (n = eye) n (%) | <0.001 | |||
| Grade 1 | 4 (100%) | 14 (15.2) | - | |
| Grade 2 | - | 56 (60.9) | 32 (39%) | |
| Grade 3 | - | 22 (23.9) | 50 (61%) |
| Grade I HIE (n = 22) | Grade II HIE (n = 117) | Grade III HIE (n = 43) | p Value | |
|---|---|---|---|---|
| Spherical equivalent, Diopters, (mean ± SD) | 1.6 ± 1.8 | 1.8 ± 1.3 | 1.8 ± 1.3 | 0.798 |
| Astigmatism, Diopters, (mean ± SD) | 1.6 ± 0.9 | 1.3 ± 0.8 | 1.4 ± 0.9 | 0.384 |
| Emmetropia n (%) | 10 (45.5%) | 76 (65%) | 28 (65.1%) | 0.424 |
| Hyperopia n (%) | 5 (22.7%) | 20 (17.1%) | 5 (11.6%) | |
| Myopia n (%) | 3 (13.6%) | 5 (4.3%) | 2 (4.7%) | |
| Astigmatism n (%) | 4 (18.2%) | 16 (13.7%) | 8 (18.6%) | |
| Strabismus n (%) | 1 (4.5%) | 18 (15.4%) | 15 (34.9%) | 0.005 |
| Esotropia | - | 3 (2.6%) | - | |
| Exotropia | 1(4.5%) | 15 (12.8%) | 15 (34.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabataş, E.U.; Aydoğan, S.; Bilgiç, A.A.; Dinlen Fettah, N.; Kabataş, N.; Dilli, D.; Zenciroğlu, A. Retinal Hemorrhages and Long-Term Ocular Outcomes in Neonatal Hypoxic-Ischemic Encephalopathy. Medicina 2025, 61, 906. https://doi.org/10.3390/medicina61050906
Kabataş EU, Aydoğan S, Bilgiç AA, Dinlen Fettah N, Kabataş N, Dilli D, Zenciroğlu A. Retinal Hemorrhages and Long-Term Ocular Outcomes in Neonatal Hypoxic-Ischemic Encephalopathy. Medicina. 2025; 61(5):906. https://doi.org/10.3390/medicina61050906
Chicago/Turabian StyleKabataş, Emrah Utku, Seda Aydoğan, Ahmet Alp Bilgiç, Nurdan Dinlen Fettah, Naciye Kabataş, Dilek Dilli, and Ayşegül Zenciroğlu. 2025. "Retinal Hemorrhages and Long-Term Ocular Outcomes in Neonatal Hypoxic-Ischemic Encephalopathy" Medicina 61, no. 5: 906. https://doi.org/10.3390/medicina61050906
APA StyleKabataş, E. U., Aydoğan, S., Bilgiç, A. A., Dinlen Fettah, N., Kabataş, N., Dilli, D., & Zenciroğlu, A. (2025). Retinal Hemorrhages and Long-Term Ocular Outcomes in Neonatal Hypoxic-Ischemic Encephalopathy. Medicina, 61(5), 906. https://doi.org/10.3390/medicina61050906






