Autogenous Periosteal Graft Along with Open Flap Debridement Versus Open Flap Debridement Alone for the Treatment of Grade II Furcation Defect in Chronic Periodontitis Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrolment of Protocol
2.2. Focused PICOS Question
- Probing pocket depth (PPD).
- Clinical attachment level (CAL).
- Horizontal dimension of the furcation defect.
- Vertical dimension of furcation defect.
- Radiographic measurement of bone height (BH).
- Plaque Index.
- Gingival Index.
- Sulcular bleeding index.
- Gingival recession.
- Tooth mobility.
- Transmission electron microscopy (TEM) for histological changes.
2.3. Search Strategy
2.4. Inclusion Criteria and Exclusion Criteria
- Chronic periodontitis patients.
- Patients of more than 18 years of age.
- Healthy patients without any systemic disease.
- Class II buccal/facial furcation defect in molars as per Glickman’s classification.
- Probing pocket depth (PPD) must be 5 mm or more.
- Patients with furcation involvement in the third molars were excluded.
- Smoking patients and former smokers were excluded.
- Pregnant and lactating females were excluded.
- Patients who underwent treatment prior to the study.
- Patients on oral contraceptives.
- Non-compliant patients with no oral hygiene maintenance schedule.
2.5. Screening and Data Extraction
2.6. Measurement of Outcome
- Clinical attachment level (CAL) is the distance from the cementoenamel junction (CEJ) to the base of the periodontal pocket. Pocket depth (PD) was assessed from the free gingival margin to the base of the periodontal pocket after one, two, three, four, six weeks, two months, and six months of follow-up examination. An increase in CAL indicates a loss of attachment, while a decrease in CAL indicates a gain of attachment.
- Pocket depth (PD) is a pathologic fissure between a tooth and the crevicular epithelium which is limited at its apex by the junctional epithelium. Change in the clinical attachment level (CAL) was calculated as the distance between the CEJ and the base of the periodontal pocket.
- Variation in bone defect fill (vertical and horizontal bone defect) was depicted as a greater bone density on the radiograph, which was noted at baseline and 6 months
- An increase in bone opacity from baseline to 6-month on radiographs indicated a change in bone defect fill (bone height).
- A decrease in plaque was related to a change in the Plaque Index at the 6-month recall.
- A decrease in gingival inflammation was related to a change in the Gingival Index at the 6-month recall.
- Change in gingival recession was measured from the gingival marginal to CEJ at different time intervals.
- Change in the sulcular bleeding index at different time intervals.
- Tooth mobility was measured at different time intervals.
- Transmission electron microscopy (TEM) for histological changes was evaluated post-operatively at 6 months follow-up.
2.7. Study Selection
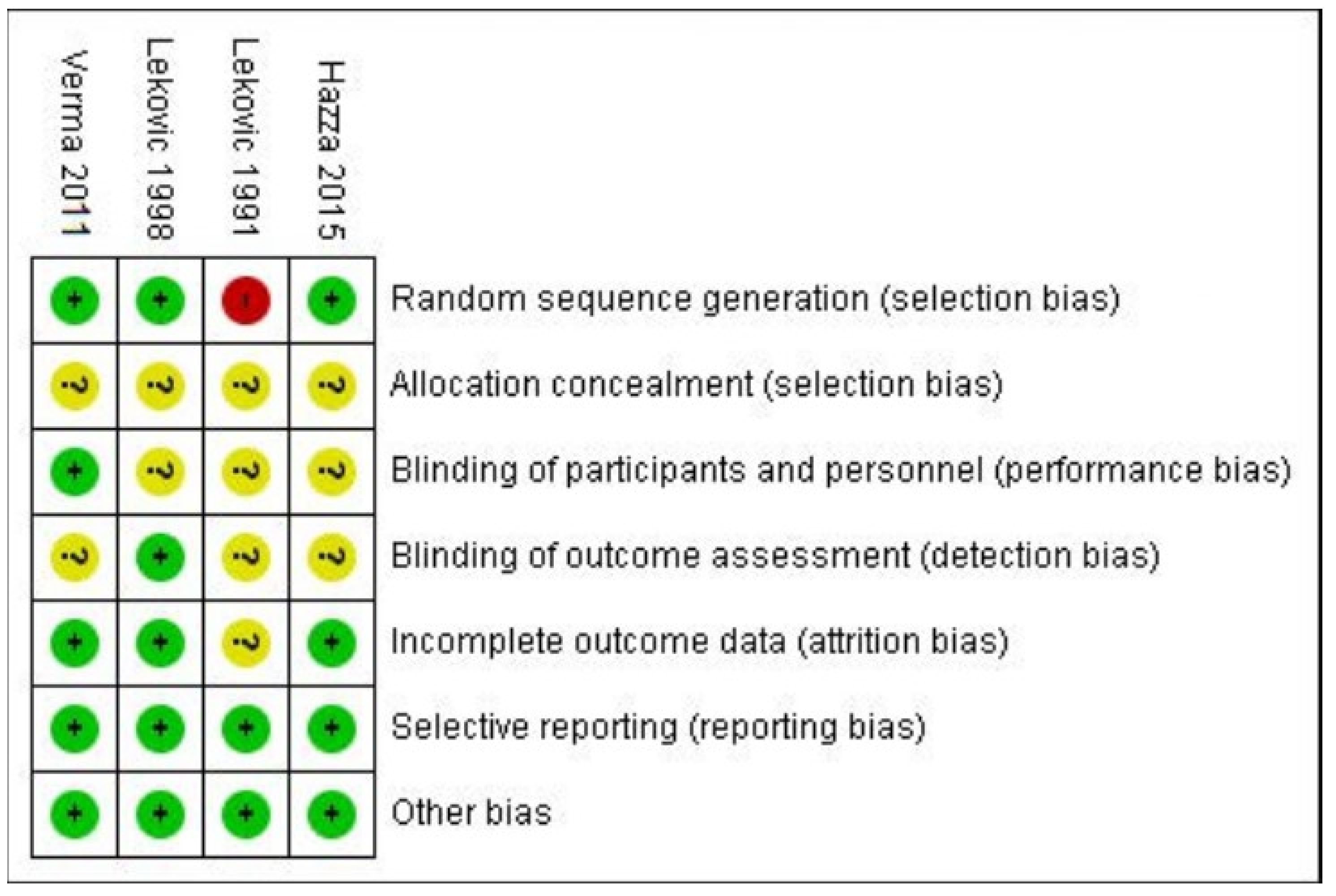
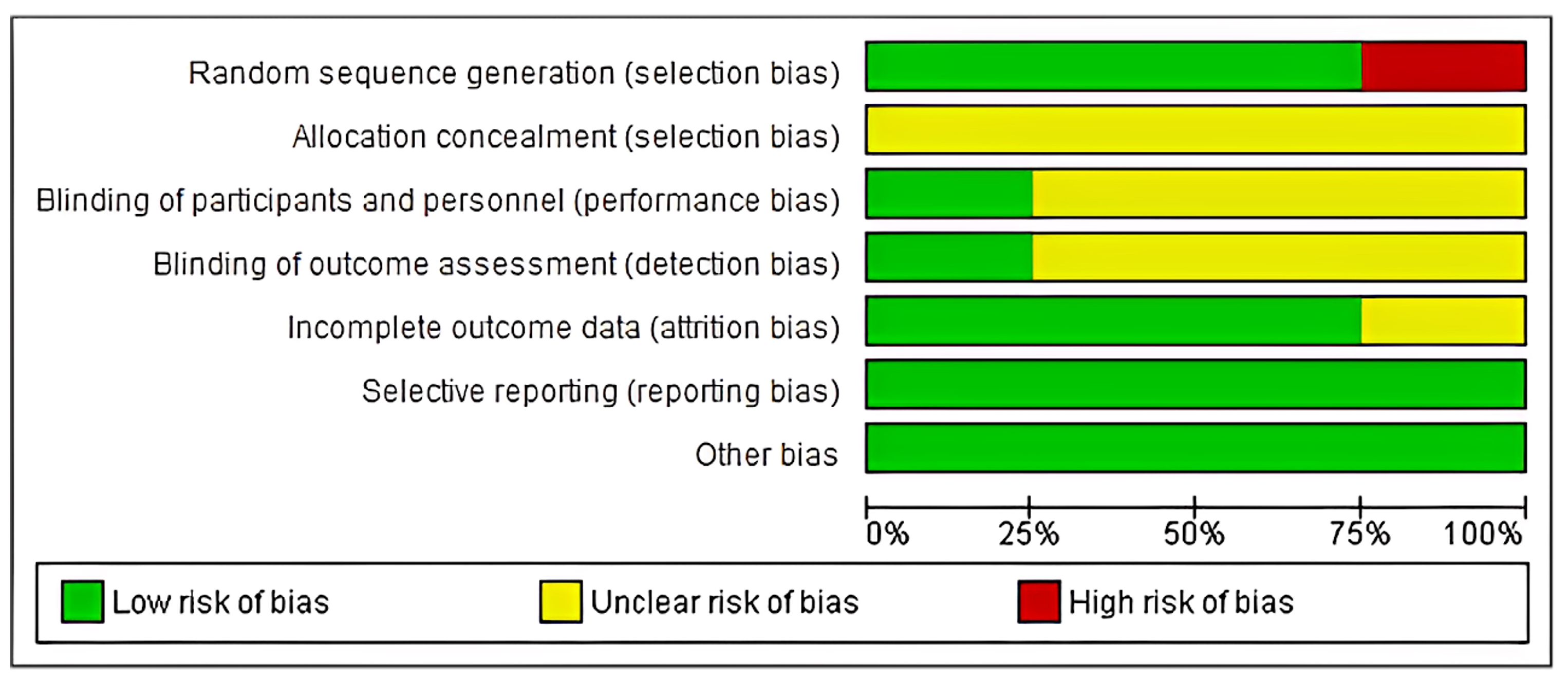
2.8. Risk of Bias
- (1)
- low risk: when all criteria were met, or one criterion was unclear/not met;
- (2)
- moderate risk: when two criteria were unclear/not met;
- (3)
- high risk: when more than two criteria were not met.
3. Results
| Sr. No. | Study ID | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Lekovic 1991 [28] | High | Unclear | Unclear | Unclear | Unclear | Low | Low | High risk |
| 2. | Lekovic 1998 [34] | Low | Unclear | Unclear | Low | Low | Low | Low | Low risk |
| 3. | Verma 2011 [33] | Low | Unclear | Low | Unclear | Low | Low | Low | Low risk |
| Study ID | Study Design | Sample Size | Age Group | Gender | Diagnosis | Inclusion Criteria |
|---|---|---|---|---|---|---|
| Lekovic 1991 [28] | clinical—split mouth study | 15 | - | 8 males, 7 females | Periodontitis with class 2 furcation defect in lower molars | Two Class II furcation involvements on the lower molar teeth’s facial side in adults furcation with >5 mm pocket depth after phase-I therapy. |
| Lekovic 1998 [34] | clinical split mouth study | 28 | - | - | Each side of the jaw has class II mandibular buccal furcation deformities. | (1) The presence of two Class II mandibular buccal furcation defects on each side of the jaw; radiography and clinical evaluation were used to diagnose the Class II furcation defect. (2) Both roots’ viability tests (cold and electrical pulp tests) reported positive. (3) The buccal aspect of the tooth has keratinized gingiva that is at least 2 mm thick. |
| Verma 2011 [33] | clinical split mouth study | 11 (initially 12 patients were considered) 12/12 | 28–49 years | 7 males, 5 females | Periodontitis with grade 2 furcation involvement in lower molars bilaterally. | Patients having at least one pair of bilateral buccal grade II furcation defects of lower molars Selected subjects were not under any medication during 1st month before surgery. Periodontal probing depth (PPD) at mid furcation area was 5 mm or more. Patients had good oral hygiene. Patients were free of any systemic disease. |
| Hala Hazzaa 2015 [35] | Clinical parallel design | 26 | 37–52 years | 15 females, 11 men | Chronic periodontitis along with class II buccal furcation defect in a mandibular molar. | (1) The mandibular molar has a class II buccal furcation defect, as defined by Glickman’s classification (1953). (2) There are no systemic disorders that could affect the therapy’s success, as determined by the modified Cornell medical index. (3) Adherence to plaque control guidelines following initial therapy, using the plaque index values (0 or 1) according to Silness and Löe (1964). (4) Vertical probing pocket depths (VPD) of ≥5 mm and clinical attachment levels (CAL) ≥4 mm four weeks following initial therapy. (5) Gingival margin positioned coronally to the furcation fornix. |
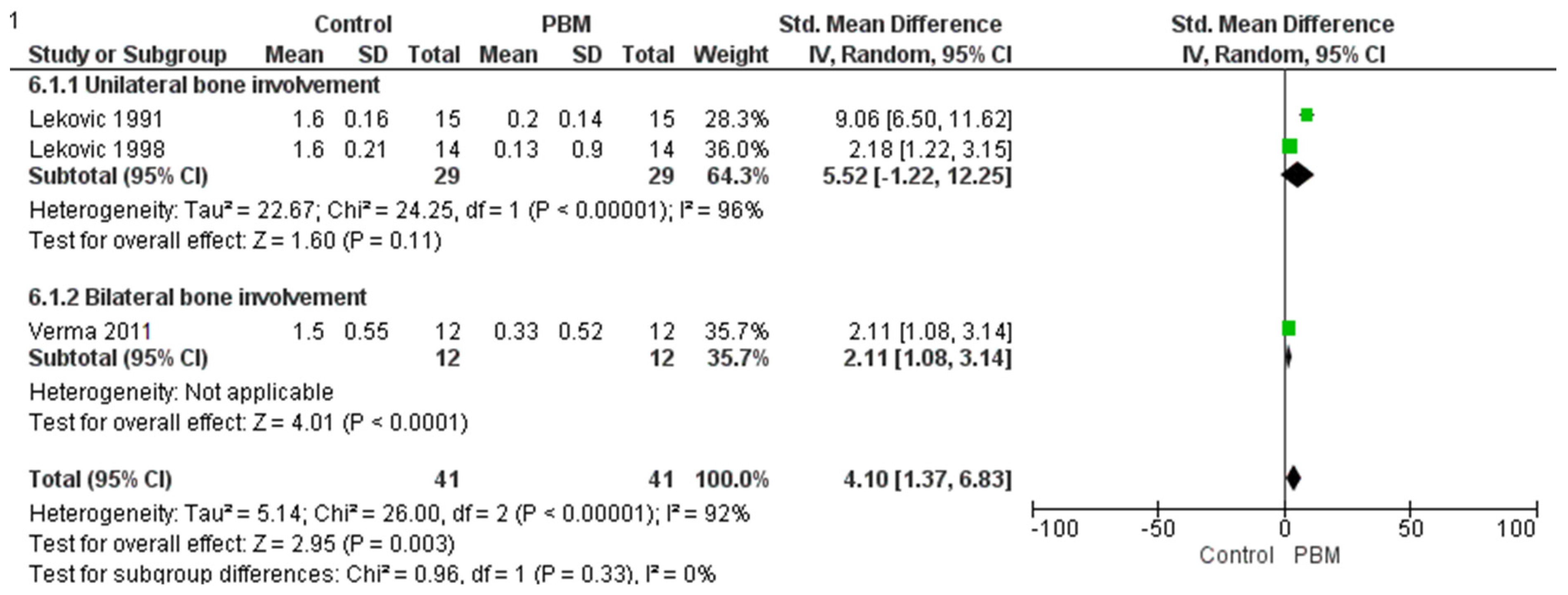
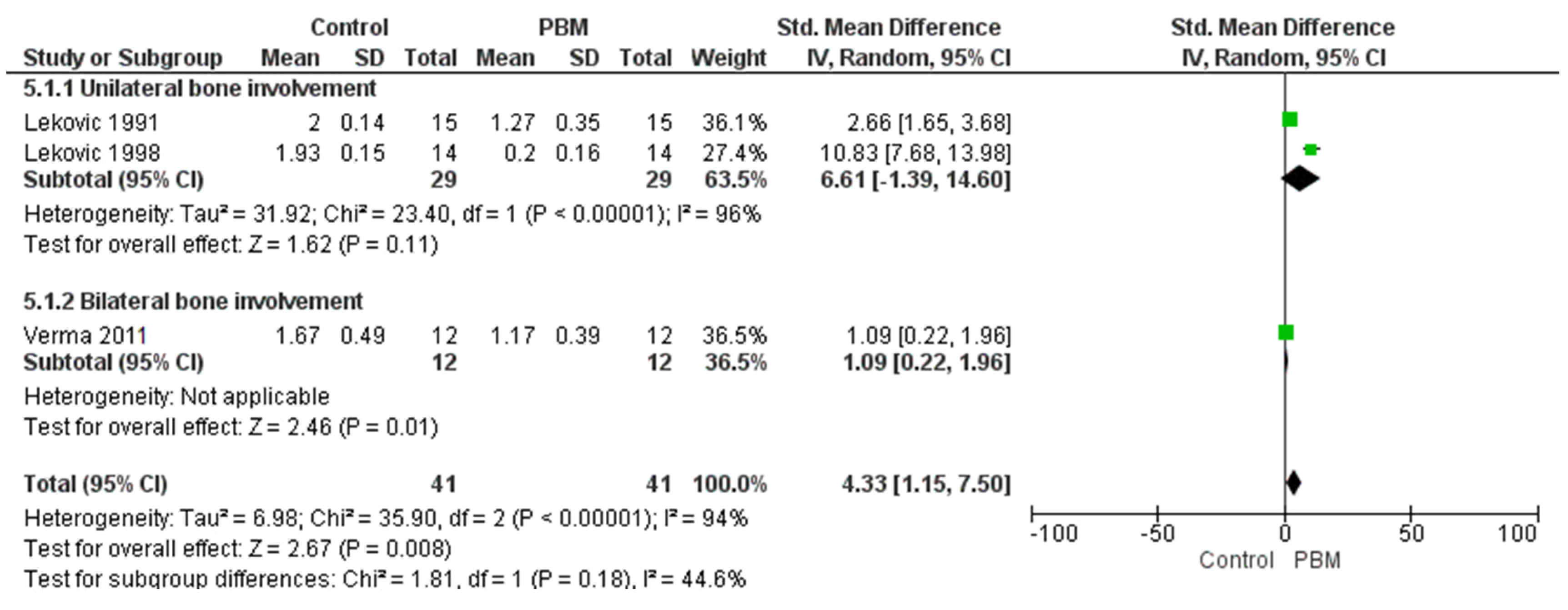
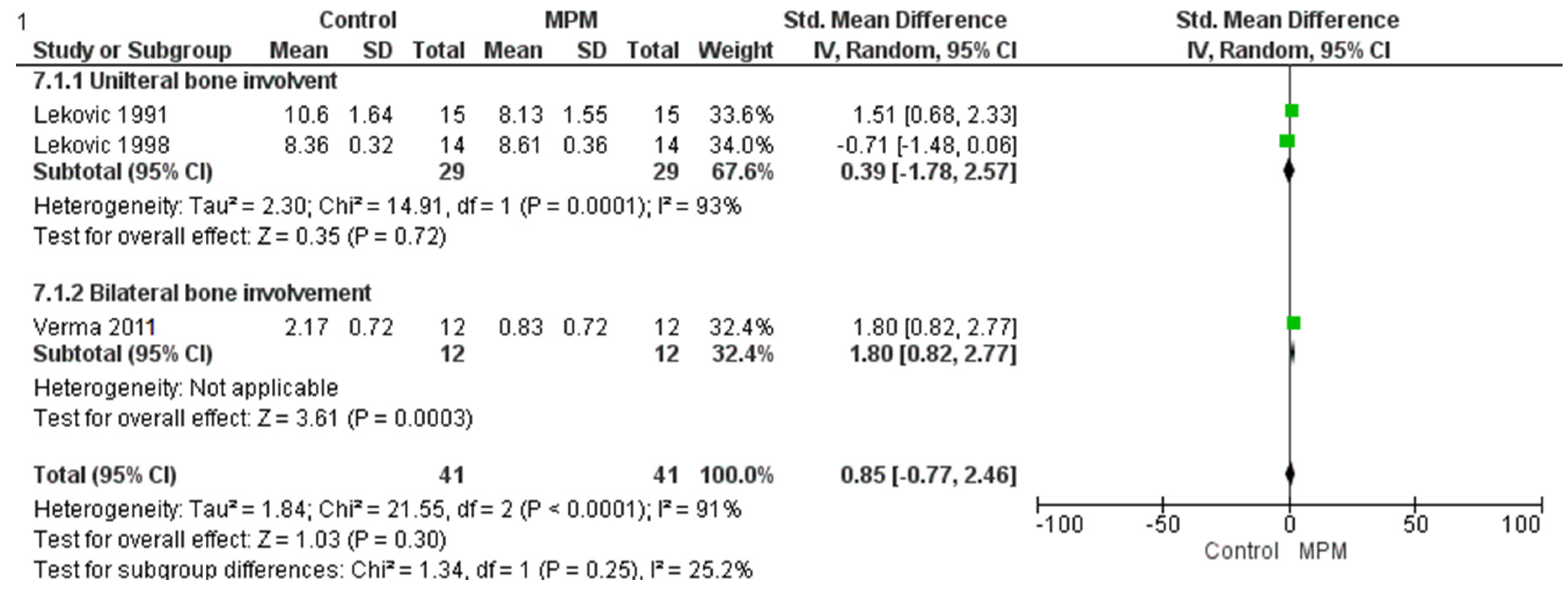
| Reference | MD in Horizontal Bone Loss | MD in Vertical Bone Loss | MD in Clinical Attachment Level | MD in PPD | MD in Gingival Recession | MD in Plaque Index | MD in Sulcular Bleeding Index | MD in Gingival Index |
|---|---|---|---|---|---|---|---|---|
| Lekovic et al. 1991 [28] | 1.60 ± 0.63 | 2.00 ± 0.54 | 2.40 ± 1.35 | 4.13 ± 0.92 | 0.73 ± 0.88 | 0.11 ± 0.24 | 0.27 ± 0.46 | - |
| Lekovic et al. 1998 [34] | 1.60 ± 0.21 | 1.93 ± 0.15 | 2.71 ± 0.40 | 3.66 ± 0.24 | 1.07 ± 0.37 | 0.14 ± 0.05 | 0.02 ± 0.04 | - |
| Verma et al. 2011 [33] | 1.50 ± 0.55 | 1.67 ± 0.49 | 2.17 ± 0.72 | - | - | - | - | 0.83 + 0.19 |
| Hazzaa et al. 2015 [35] | 59.7 ± 13.4 | 60 ± 8.6 | 63.7 + 8.8 | - | - | - | - |
3.1. Study Characteristics
3.2. Statistical Analysis
3.3. Horizontal Bone Change
3.4. Vertical Bone Change Group
3.5. Clinical Attachment Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBR | Guided Bone Regeneration |
| GTR | Guided Tissue Regeneration |
| ePTFE | Expanded Polytetrafluoroethylene |
| CAL | Clinical Attachment Level |
| OFD | Open Flap Debridement |
| CPRT | Combined Periodontal Regenerative Therapy |
| PROSPERO | Prospective Register of Systematic Reviews |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PICOS | Population, Intervention, Comparison, Outcome, Study design |
| PPD | Probing Pocket Depth |
| BH | Bone Height |
| TEM | Transmission Electron Microscopy |
| RCT | Randomized Controlled Trial |
| MeSH | Medical Subject Headings |
| TEM | Transmission electron microscopy |
| CEJ | Cemento-enamel Junction |
| PBM | Periosteal Barrier Membrane |
| MPM | Modified Periodontal Management |
References
- Cho, Y.D.; Kim, K.H.; Lee, Y.M.; Ku, Y.; Seol, Y.J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.M.; Romano, F.; Giraudi, M.; La Bruna, N.; Citterio, F.; Mariani, G.M.; Baima, G.; Aimetti, M. Effect of Post-Surgical Flap Position on Soft Tissue Regrowth and Keratinized Tissue Increase Following Fibre Retention Osseous Resective Surgery: A 6-Month Randomized Study with Multilevel Analysis. BMC Oral Health 2023, 23, 472. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Giraudi, M.; Citterio, F.; Fratini, A.; Gualini, G.; Piccoli, G.M.; Mariani, G.M.; Romano, F.; Aimetti, M. Pocket Elimination after Osseous Resective Surgery: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2020, 47, 756–767. [Google Scholar] [CrossRef]
- Donos, N.; Mardas, N.; Chadha, V. Clinical Outcomes of Implants Following Lateral Bone Augmentation: Systematic Assessment of Available Options (Barrier Membranes, Bone Grafts, Split Osteotomy). J. Clin. Periodontol. 2008, 35 (Suppl. S8), 173–202. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Buser, D. Guided Bone Regeneration for Dental Implants. Curr. Opin. Periodontol. 1996, 3, 168–177. [Google Scholar] [PubMed]
- Alpiste Illueca, F.M.; Buitrago Vera, P.; de Grado Cabanilles, P.; Fuenmayor Fernandez, V.; Gil Loscos, F.J. Periodontal Regeneration in Clinical Practice. Med. Oral Patol. Oral Cir. Bucal 2006, 11, E382–E392. [Google Scholar]
- Melcher, A.H. On the Repair Potential of Periodontal Tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel Matrix Derivative (Emdogain®) for Periodontal Tissue Regeneration in Intrabony Defects. Cochrane Database Syst. Rev. 2009, 2009, CD003875. [Google Scholar] [CrossRef]
- Sasaki, J.I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier Membranes for Tissue Regeneration in Dentistry. Biomater. Investig. Dent. 2020, 8, 54–63. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E. Treatment of Mandibular 3-Wall Intrabony Defects by Flap Debridement and Expanded Polytetrafluoroethylene Barrier Membranes. J. Periodontol. 1993, 64, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Gottlow, J.; Nyman, S.; Lindhe, J.; Karring, T.; Wennström, J. New Attachment Formation in the Human Periodontium by Guided Tissue Generation. J. Clin. Periodontol. 1986, 13, 604–616. [Google Scholar] [CrossRef]
- Blumenthal, N.M.; Steinberg, J. The Use of Collagen Membrane Barriers in Conjunction with Combined Demineralized Bone-Collagen Gel Implant in Human Infrabony Defects. J. Periodontol. 1990, 61, 319–327. [Google Scholar] [CrossRef]
- Cortellini, P.; Pini Prato, G.; Tonetti, M.S. Periodontal Regeneration of Human Intrabony Defects with Bioresorbable Membranes: A Controlled Clinical Trial. J. Periodontol. 1996, 67, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Christgau, M.; Schmalz, G.; Wenzel, A.; Hiller, K.A. Periodontal Regeneration of Intrabony Defects with Resorbable and Nonresorbable Membranes: 30-Month Result. J. Clin. Periodontol. 1997, 24, 17–21. [Google Scholar] [CrossRef]
- Jepsen, S.; Eberhard, J.; Herrera, D.; Needleman, I. A Systematic Review of Guided Tissue Regeneration for Periodontal Furcation Defects. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 103–116. [Google Scholar] [CrossRef]
- Kwan, S.K.; Lekovic, V.; Camargo, P.M.; Klokkevold, P.R.; Kenney, E.B.; Nedic, M.; Dimitrijevic, B. The Use of Autogenous Periosteal Grafts as Barriers for the Treatment of Intrabony Defects in Humans. J. Periodontol. 1998, 69, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Gamal, A.Y.; Mailhot, J.M. A Novel Marginal Periosteal Pedicle Graft as an Autogenous Guided Tissue Membrane for the Treatment of Intrabony Periodontal Defects. J. Int. Acad. Periodontol. 2008, 10, 106–117. [Google Scholar]
- Paolantonio, M.; Femminella, B.; Coppolino, E.; Sammartino, G.; D’Arcangelo, C.; Perfetti, G.; Perinetti, G. Autogenous Periosteal Barrier Membranes and Bone Grafts in the Treatment of Periodontal Intrabony Defects of Single-Rooted Teeth: A 12-Month Reentry Randomized Controlled Clinical Trial. J. Periodontol. 2010, 81, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- McClain, P.K.; Schallhorn, R.G. Long-Term Assessment of Combined Osseous Composite Grafting, Root Conditioning, and Guided Tissue Regeneration. Int. J. Periodontics Restor. Dent. 1993, 13, 9–27. [Google Scholar]
- Stahl, S.S.; Froum, S. Histologic healing responses in human vertical lesions following the use of osseous allografts and barrier membranes. J. Clin. Periodontol. 1991, 18, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, C.; Shi, H.; Luo, X.; Sun, H.; Wang, Q.; Zhang, D. Advances in Barrier Membranes for Guided Bone Regeneration Techniques. Front. Bioeng. Biotechnol. 2022, 10, 921576. [Google Scholar] [CrossRef]
- Femminella, B.; Traini, T.; Romano, L.; Tullio, M.; D’Arcangelo, C.; Paolantonio, M. Periodontal Healing of a Human Intrabony Defect Treated by Autogenous Periosteal Barrier Membrane and Bone Graft: A Clinical and Histologic Case Report. Clin. Adv. Periodontics 2012, 2, 162–170. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, N.; Yang, M.; Sun, T.; Zhang, J.; Zhao, Y.; Huo, N.; Li, Z. Periosteum and Development of the Tissue-Engineered Periosteum for Guided Bone Regeneration. J. Orthop. Transl. 2022, 33, 41–54. [Google Scholar] [CrossRef]
- Kumar, A.; Lal, N.; Singhal, R.; Rastogi, P. Comparative Evaluation of Periosteum as a Barrier Membrane with and without an Alloplastic Bone Graft in Periodontal Osseous Defects: A 9-Month Follow-Up Study. J. Indian Soc. Periodontol. 2014, 18, 493–496. [Google Scholar]
- Mizuno, H.; Hata, K.; Kojima, K.; Bonassar, L.J.; Vacanti, C.A.; Ueda, M. A Novel Approach to Regenerating Periodontal Tissue by Grafting Autologous Cultured Periosteum. Tissue Eng. 2006, 12, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Ritsila, V.; Alhopuro, S.; Rintala, A. Bone Formation with Free Periosteum from Tibia. Scand. Plast. Reconstr. Surg. 1972, 6, 51–56. [Google Scholar]
- Lekovic, V.; Kenney, E.B.; Carranza, F.A.; Martignoni, M. The Use of Autogenous Periosteal Grafts as Barriers for the Treatment of Class II Furcation Involvements in Lower Molars. J. Periodontol. 1991, 62, 775–780. [Google Scholar] [CrossRef]
- Mahajan, A. Treatment of Multiple Gingival Recession Defects Using Periosteal Pedicle Graft: A Case Series. J. Periodontol. 2010, 81, 1426–1431. [Google Scholar] [CrossRef]
- Tobon-Arroyave, S.I.; Dominguez-Mejia, J.S.; Florez-Moreno, G.A. Periosteal Grafts as Barriers in Periradicular Surgery: Report of Two Cases. Int. Endod. J. 2004, 37, 632–642. [Google Scholar] [CrossRef]
- Saimbi, C.S.; Gautam, A.; Khan, M.A.; Nandlal. Periosteum as a Barrier Membrane in the Treatment of Intrabony Defect: A New Technique. J. Indian Soc. Periodontol. 2014, 18, 331–335. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; John Wiley & Sons: Chichester, UK, 2022. [Google Scholar]
- Verma, V.; Saimbi, C.S.; Khan, M.A.; Goel, A. Use of Periosteal Membrane as a Barrier Membrane for the Treatment of Buccal Grade II Furcation Defects in Lower Molars: A Novel Technique. Indian J. Dent. Res. 2011, 22, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Lekovic, V.; Klokkevold, P.R.; Camargo, P.M.; Kenney, E.B.; Nedic, M.; Weinlaender, M. Evaluation of Periosteal Membranes and Coronally Positioned Flaps in the Treatment of Class II Furcation Defects: A Comparative Clinical Study in Humans. J. Periodontol. 1998, 69, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Hazzaa, H.; Adawy, H.; Magdi, H. A Novel Surgical Approach for Treatment of Class II Furcation Defects Using Marginal Periosteal Membrane. J. Int. Acad. Periodontol. 2015, 17, 20–31. [Google Scholar] [PubMed]
- Dannewitz, B.; Zeidler, A.; Hüsing, J.; Saure, D.; Pfefferle, T.; Eickholz, P.; Pretzl, B. Loss of Molars in Periodontally Treated Patients: Results 10 Years and More After Active Periodontal Therapy. J. Clin. Periodontol. 2016, 43, 53–62. [Google Scholar] [CrossRef]
- Salvi, G.E.; Mischler, D.C.; Schmidlin, K.; Matuliene, G.; Pjetursson, B.E.; Brägger, U.; Lang, N.P. Risk Factors Associated with the Longevity of Multi-Rooted Teeth: Long-Term Outcomes After Active and Supportive Periodontal Therapy. J. Clin. Periodontol. 2014, 41, 701–707. [Google Scholar] [CrossRef]
- Siaili, M.; Chatzopoulou, D.; Gillam, D.G. An Overview of Periodontal Regenerative Procedures for the General Dental Practitioner. Saudi Dent. J. 2018, 30, 26–37. [Google Scholar] [CrossRef]
- Ritsila, V.; Alhopuro, S.; Gulling, U.; Rintala, A. The Use of Free Periosteum for Bone Formation in Congenital Clefts of the Maxilla. Scand. J. Plast. Reconstr. Surg. 1972, 6, 57–60. [Google Scholar]
- Cohen, J.; Lacroix, P. Bone and Cartilage Formation by Periosteum: Assay of Experimental Autogenous Grafts. J. Bone Jt. Surg. 1955, 37A, 717–730. [Google Scholar] [CrossRef]
- Goldman, H.M.; Smukler, H. Controlled Surgical Stimulation of Periosteum. J. Periodontol. 1978, 49, 518–522. [Google Scholar] [CrossRef]
- Alauddin, M.S.; Abdul Hayei, N.A.; Sabarudin, M.A.; Mat Baharin, N.H. Barrier Membrane in Regenerative Therapy: A Narrative Review. Membranes 2022, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Sam, G.; Pillai, B.R.M. Evolution of Barrier Membranes in Periodontal Regeneration—“Are the Third Generation Membranes Really Here?”. J. Clin. Diagn. Res. JCDR 2014, 8, ZE14–ZE17. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.H.; Park, J.C.; Cha, J.K.; Kim, Y.T.; Jung, U.W.; Kim, C.S.; Choi, S.H.; Kim, C.K. Dimensional Change of the Healed Periosteum on Surgically Created Defects. J. Periodontal Implant Sci. 2011, 41, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Esfahanian, V.; Golestaneh, H.; Moghaddas, O.; Ghafari, M.R. Efficacy of Connective Tissue with and without Periosteum in Regeneration of Intrabony Defects. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 189–196. [Google Scholar]
- Bunæs, D.F.; Lie, S.A.; Åstrøm, A.N.; Mustafa, K.; Leknes, K.N. Site-Specific Treatment Outcome in Smokers Following 12 Months of Supportive Periodontal Therapy. J. Clin. Periodontol. 2016, 43, 1086–1093. [Google Scholar] [CrossRef]
- Bhandare, J.; Mahale, S.A.; Abullais, S.S.; Katkurwar, A.; AlQahtani, S.M.; Algarni, Y.A.; Asif, S.M. Appraising and Comparing the Role of Autogenous Periosteal Graft as a Barrier Membrane in the Treatment of Intrabony Defects in Chronic Periodontitis Cases: A Systematic Review and Meta-Analysis. Int. J. Med. Sci. 2024, 21, 253–264. [Google Scholar] [CrossRef]
- Abu-Shahba, A.G.; Wilkman, T.; Kornilov, R.; Adam, M.; Salla, K.M.; Lindén, J.; Lappalainen, A.K.; Björkstrand, R. Periosteal Flaps Enhance Prefabricated Engineered Bone Reparative Potential. J. Dent. Res. 2022, 101, 166–176. [Google Scholar] [CrossRef]
- Hirata, A.; Maruyama, Y.; Onishi, K.; Hayashi, A.; Saze, M.; Okada, E. A Vascularized Artificial Bone Graft Using the Periosteal Flap and Porous Hydroxyapatite: Basic Research and Preliminary Clinical Application. Wound Repair Regen. 2004, 12, 1067–1927. [Google Scholar]
- Barckman, J.; Baas, J.; Sørensen, M.; Bechtold, J.E.; Søballe, K. Periosteal Augmentation of Allograft Bone and Its Effect on Implant Fixation—An Experimental Study on 12 Dogs. Open Orthop. J. 2013, 7, 18–24. [Google Scholar] [CrossRef]
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum: Biology and Applications in Craniofacial Bone Regeneration. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef]

| Sr No | Database | Search Term |
|---|---|---|
| 1 | PUBMED | ((Open flap debridement) OR (root debridement)) AND ((periosteum as barrier membrane) OR (periosteal pedicle graft)) AND (class II furcation deficiency, class II furcation involvement, OR grade II furcation involvement) AND ((Chronic Periodontitis(Other term)) AND ((RCT OR (Randomized Controlled Trial)) |
| 2 | EBSCO | ((Open flap debridement) AND (periosteum as barrier membrane) OR (periosteal pedicle graft)) AND ((Chronic Periodontitis)(Periodontitis)) AND (RCT) |
| 3 | Google Scholar | ((Open flap debridement) OR (Periodontal Surgery) AND (periosteum as barrier membrane) OR (periosteal pedicle graft)) AND (grade II furcation) AND ((Chronic Periodontitis)(Periodontitis)) AND (RCT) |
| 4 | SCOPUS | ((Open flap debridement) OR (Periodontal Flap Surgery) AND (periosteal pedicle graft)) AND ((grade II furcation) OR (Class II Furcation defect)) AND ((Chronic Periodontitis)(Periodontitis)) AND (RCT) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahale, S.A.; Dhadse, P.; Thosar, S.; Bhandari, V.; Patil, A.; Syed, S.; Bapat, R.A.; Chaubal, T.; Eusufzai, S.Z.; Abullais, S.S. Autogenous Periosteal Graft Along with Open Flap Debridement Versus Open Flap Debridement Alone for the Treatment of Grade II Furcation Defect in Chronic Periodontitis Patients: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 905. https://doi.org/10.3390/medicina61050905
Mahale SA, Dhadse P, Thosar S, Bhandari V, Patil A, Syed S, Bapat RA, Chaubal T, Eusufzai SZ, Abullais SS. Autogenous Periosteal Graft Along with Open Flap Debridement Versus Open Flap Debridement Alone for the Treatment of Grade II Furcation Defect in Chronic Periodontitis Patients: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(5):905. https://doi.org/10.3390/medicina61050905
Chicago/Turabian StyleMahale, Swapna A., Prasad Dhadse, Sumedha Thosar, Vedant Bhandari, Akhil Patil, Sadatullah Syed, Ranjeet Ajit Bapat, Tanay Chaubal, Sumaiya Zabin Eusufzai, and Shahabe Saquib Abullais. 2025. "Autogenous Periosteal Graft Along with Open Flap Debridement Versus Open Flap Debridement Alone for the Treatment of Grade II Furcation Defect in Chronic Periodontitis Patients: A Systematic Review and Meta-Analysis" Medicina 61, no. 5: 905. https://doi.org/10.3390/medicina61050905
APA StyleMahale, S. A., Dhadse, P., Thosar, S., Bhandari, V., Patil, A., Syed, S., Bapat, R. A., Chaubal, T., Eusufzai, S. Z., & Abullais, S. S. (2025). Autogenous Periosteal Graft Along with Open Flap Debridement Versus Open Flap Debridement Alone for the Treatment of Grade II Furcation Defect in Chronic Periodontitis Patients: A Systematic Review and Meta-Analysis. Medicina, 61(5), 905. https://doi.org/10.3390/medicina61050905