Meta-Analysis of 16S rRNA Sequencing Reveals Altered Fecal but Not Vaginal Microbial Composition and Function in Women with Endometriosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Datasets
2.2. 16S rRNA Preprocessing
2.3. Microbiome Community Analysis
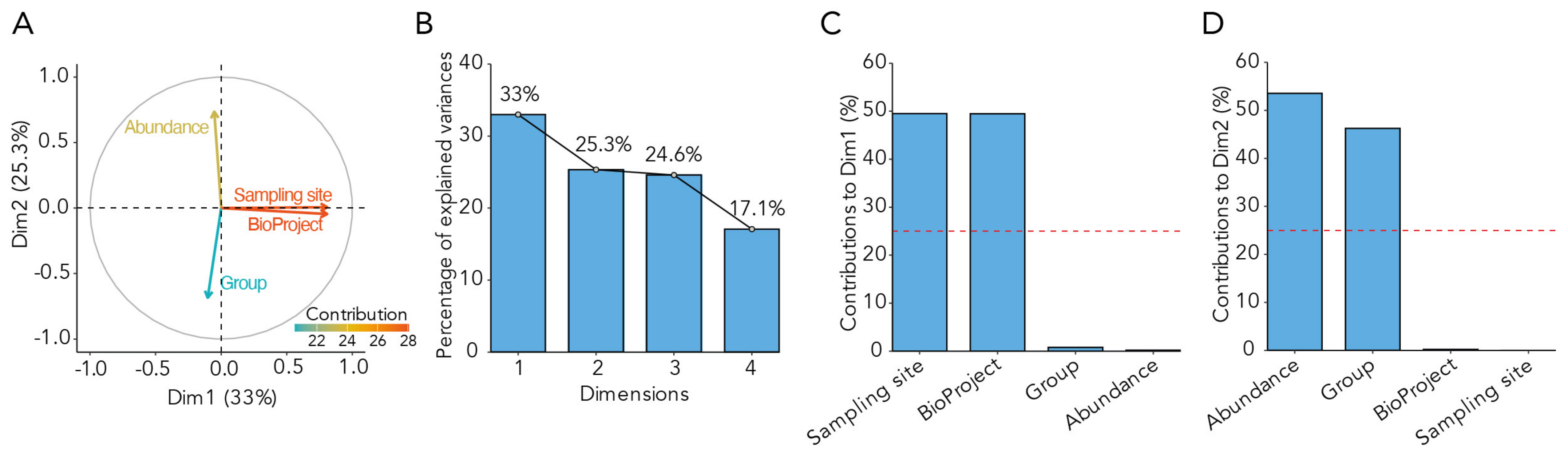
2.4. Analysis of Confounding Factors
2.5. The Microbial Endometriosis Index (MEI)
2.6. Statistics
2.6.1. Taxonomic Analysis
2.6.2. Functional Microbiome Analysis
2.7. Study Approval
2.8. Data Availability
3. Results
3.1. Data Features Included in the Meta-Analysis
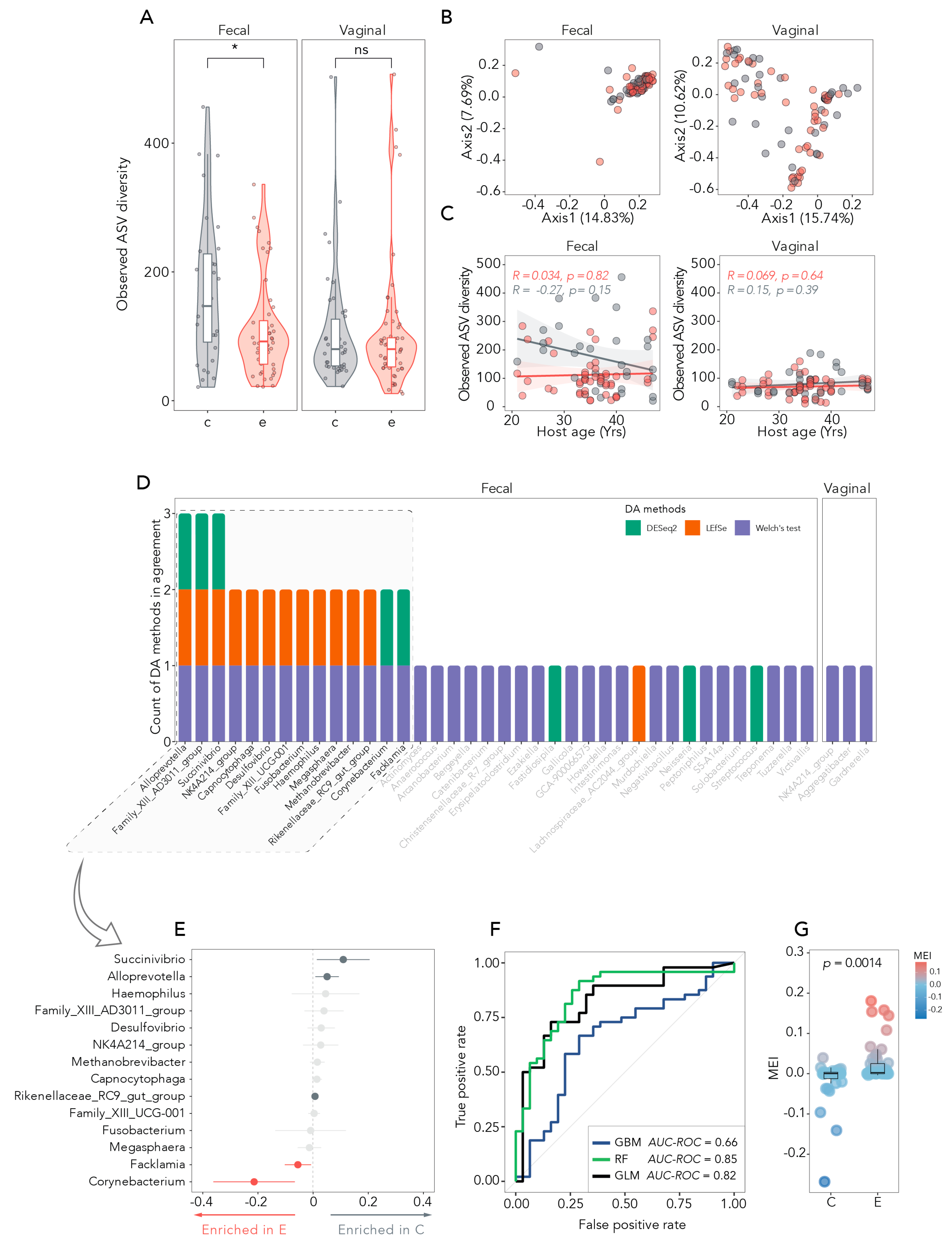
3.2. Microbial Diversity Between and Within Samples
3.3. Taxonomic Relative Abundance in Fecal and Vaginal Samples
3.4. Differentially Abundant Bacteria and Disease Status Prediction
3.5. The MEI as a Measure of Disease-Specific Diversity
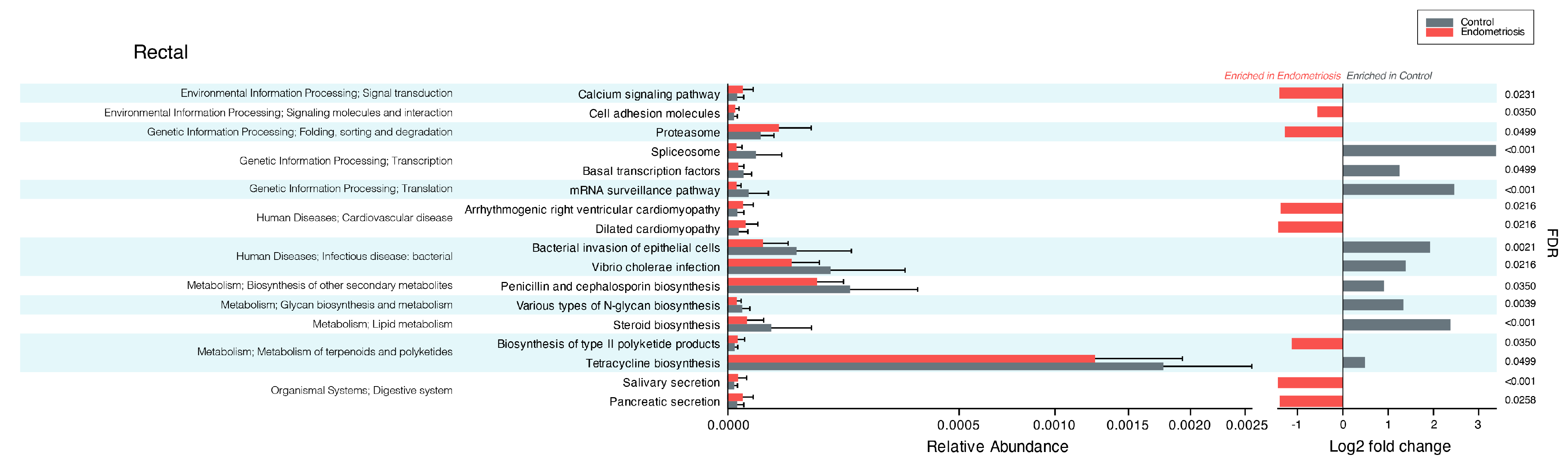
3.6. Results of Functional Microbiome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASV | amplicon sequence variant |
| PCOS | polycystic ovary syndrome |
| PCA | principal component analysis |
| CRP | C-reactive protein |
| MEI | microbial endometriosis index |
References
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Nyholt, D.R.; Low, S.K.; Anderson, C.A.; Painter, J.N.; Uno, S.; Morris, A.P.; MacGregor, S.; Gordon, S.D.; Henders, A.K.; Martin, N.G.; et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat. Genet. 2012, 44, 1355–1359. [Google Scholar] [CrossRef]
- Tai, F.W.; Chang, C.Y.; Chiang, J.H.; Lin, W.C.; Wan, L. Association of Pelvic Inflammatory Disease with Risk of Endometriosis: A Nationwide Cohort Study Involving 141,460 Individuals. J. Clin. Med. 2018, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Iwabe, T.; Harada, T.; Tsudo, T.; Nagano, Y.; Yoshida, S.; Tanikawa, M.; Terakawa, N. Tumor necrosis factor-promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J. Clin. Endocr. Metab. 2000, 85, 824–829. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Altamura, S.; Del Pinto, R.; Pietropaoli, D.; Ferri, C. Oral health as a modifiable risk factor for cardiovascular diseases. Trends Cardiovasc. Med. 2024, 34, 267–275. [Google Scholar] [CrossRef]
- Hicks, C.; Leonardi, M.; Chua, X.Y.; Mari-Breedt, L.; Espada, M.; El-Omar, E.M.; Condous, G.; El-Assaad, F. Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study. BJOG 2025, 132, 326–336. [Google Scholar] [CrossRef]
- Muraoka, A.; Suzuki, M.; Hamaguchi, T.; Watanabe, S.; Iijima, K.; Murofushi, Y.; Shinjo, K.; Osuka, S.; Hariyama, Y.; Ito, M.; et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts. Sci. Transl. Med. 2023, 15, eadd1531. [Google Scholar] [CrossRef]
- Sobstyl, A.; Chalupnik, A.; Mertowska, P.; Grywalska, E. How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 10920. [Google Scholar] [CrossRef]
- Maroun, P.; Cooper, M.J.; Reid, G.D.; Keirse, M.J. Relevance of gastrointestinal symptoms in endometriosis. Aust. N. Z. J. Obs. Gynaecol. 2009, 49, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Ballweg, M.L. Impact of endometriosis on women’s health: Comparative historical data show that the earlier the onset, the more severe the disease. Best Pract. Res. Clin. Obs. Gynaecol. 2004, 18, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Fauconnier, A.; Chapron, C.; Dubuisson, J.B.; Vieira, M.; Dousset, B.; Bréart, G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil. Steril. 2002, 78, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Seaman, H.E.; Ballard, K.D.; Wright, J.T.; de Vries, C.S. Endometriosis and its coexistence with irritable bowel syndrome and pelvic inflammatory disease: Findings from a national case-control study—Part 2. BJOG 2008, 115, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Kavoussi, S.K.; West, B.T.; Taylor, G.W.; Lebovic, D.I. Periodontal disease and endometriosis: Analysis of the National Health and Nutrition Examination Survey. Fertil. Steril. 2009, 91, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204. [Google Scholar] [CrossRef]
- Huang, L.; Liu, B.; Liu, Z.; Feng, W.; Liu, M.; Wang, Y.; Peng, D.; Fu, X.; Zhu, H.; Cui, Z.; et al. Gut Microbiota Exceeds Cervical Microbiota for Early Diagnosis of Endometriosis. Front. Cell Infect. Microbiol. 2021, 11, 788836. [Google Scholar] [CrossRef]
- Wessels, J.M.; Dominguez, M.A.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. Endometrial microbiota is more diverse in people with endometriosis than symptomatic controls. Sci. Rep. 2021, 11, 18877. [Google Scholar] [CrossRef]
- Perrotta, A.R.; Borrelli, G.M.; Martins, C.O.; Kallas, E.G.; Sanabani, S.S.; Griffith, L.G.; Alm, E.J.; Abrao, M.S. The Vaginal Microbiome as a Tool to Predict rASRM Stage of Disease in Endometriosis: A Pilot Study. Reprod. Sci. 2020, 27, 1064–1073. [Google Scholar] [CrossRef]
- Glockner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef]
- Cassol, I.; Ibañez, M.; Bustamante, J.P. Key features and guidelines for the application of microbial alpha diversity metrics. Sci Rep 2025, 15, 622. [Google Scholar] [CrossRef]
- Bray, J.R.; John, T.C. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Van de Wiel, M.A.; Zeileis, A. A Lego system for conditional inference. Am. Stat. 2006, 60, 257–263. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Ferri, C.; Giannoni, M.; Cominelli, F.; Pizarro, T.T.; Pietropaoli, D. Meta-analysis of oral microbiome reveals sex-based diversity in biofilms during periodontitis. JCI Insight 2024, 9, 171311. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.L. The generalisation of student’s problems when several different population variances are involved. Biometrika 1947, 34, 28–35. [Google Scholar] [CrossRef]
- Nearing, J.T.; Douglas, G.M.; Hayes, M.G.; MacDonald, J.; Desai, D.K.; Allward, N.; Jones, C.M.A.; Wright, R.J.; Dhanani, A.S.; Comeau, A.M.; et al. Author Correction: Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 2022, 13, 777. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S. Microbiome R Package; Bioconductor: Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, Q.Y.; Wang, D.; Zhang, P.C.; Liu, Y.; Niu, C. microbiomeMarker: An R/Bioconductor package for microbiome marker identification and visualization. Bioinformatics 2022, 38, 4027–4029. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J.R. Statistic. Soc. B. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mai, J.; Cao, X.; Burberry, A.; Cominelli, F.; Zhang, L. ggpicrust2: An R package for PICRUSt2 predicted functional profile analysis and visualization. Bioinformatics 2023, 39, btad470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMurdie, P.J.; Holmes, S. Waste not, want not: Why rarefying microbiome data is inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyashira, C.H.; Oliveira, F.R.; Andres, M.P.; Gingold, J.A.; Abrao, M.S. The microbiome and endometriosis. Reprod. Fertil. 2022, 3, R163–R175. [Google Scholar] [CrossRef]
- Ser, H.L.; Au Yong, S.J.; Shafiee, M.N.; Mokhtar, N.M.; Ali, R.A.R. Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review. Microorganisms 2023, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Abdulamir, A.S.; Hafidh, R.R.; Bakar, F.A. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: Inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol. Cancer 2010, 9, 249. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, C. Role of the gut microbiota in the pathogenesis of endometriosis: A review. Front. Microbiol. 2024, 15, 1363455. [Google Scholar] [CrossRef]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed]
- Dicksved, J.; Halfvarson, J.; Rosenquist, M.; Jarnerot, G.; Tysk, C.; Apajalahti, J.; Engstrand, L.; Jansson, J.K. Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. ISME J. 2008, 2, 716–727. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Palombella, V.J.; Sausville, E.A.; Johnson, J.; Destree, A.; Lazarus, D.D.; Maas, J.; Pien, C.S.; Prakash, S.; Elliott, P.J. Proteasome inhibitors: A novel class of potent and effective antitumor agents. Cancer Res. 1999, 59, 2615–2622. [Google Scholar]
- Ilad, R.S.; Fleming, S.D.; Bebington, C.R.; Murphy, C.R. Ubiquitin is associated with the survival of ectopic stromal cells in endometriosis. Reprod. Biol. Endocrinol. 2004, 2, 69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Celik, O.; Hascalik, S.; Elter, K.; Tagluk, M.E.; Gurates, B.; Aydin, N.E. Combating endometriosis by blocking proteasome and nuclear factor-kappaB pathways. Hum. Reprod. 2008, 23, 2458–2465. [Google Scholar] [CrossRef]
- Colombo, G.E.; Mahamat-Saleh, Y.; Armour, M.; Madan, K.; Sabag, A.; Kvaskoff, M.; Missmer, S.A.; Condous, G.; Pathan, F.; Leonardi, M. Non-malignant gynaecological disease and risk of cardiovascular or cerebrovascular disease: A systematic review and meta-analysis. Heart 2025, 111, 402–411. [Google Scholar] [CrossRef] [PubMed]
- AlAshqar, A.; Patzkowsky, K.; Afrin, S.; Wild, R.; Taylor, H.S.; Borahay, M.A. Cardiometabolic Risk Factors and Benign Gynecologic Disorders. Obs. Gynecol. Surv. 2019, 74, 661–673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, M.; Li, D.; Zhang, Z.; Sun, H.; An, M.; Wang, G. Endometriosis induces gut microbiota alterations in mice. Hum. Reprod. 2018, 33, 607–616. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Cheng, M.; Parnell, L.A.; Yin, Y.; Schriefer, A.; Mysorekar, I.U.; Kommagani, R. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum. Reprod. 2019, 34, 1106–1116. [Google Scholar] [CrossRef]
- Jiang, I.; Yong, P.J.; Allaire, C.; Bedaiwy, M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021, 22, 5644. [Google Scholar] [CrossRef]
- Qi, X.Y.; Yun, C.Y.; Pang, Y.L.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, S.; Punjala-Patel, A.; Gavrilova-Jordan, L. Hypothalamic-Pituitary-Ovarian Axis Disorders Impacting Female Fertility. Biomedicines 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes 2023, 15, 2236749. [Google Scholar] [CrossRef] [PubMed]
- Lourenvarsigmao, T.G.B.; Spencer, S.J.; Alm, E.J.; Colombo, A.P.V. Defining the gut microbiota in individuals with periodontal diseases: An exploratory study. J. Oral. Microbiol. 2018, 10, 1487741. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hua, X.; Wu, M.C.; Proll, S.; Valint, D.J.; Reed, S.D.; Guthrie, K.A.; LaCroix, A.Z.; Larson, J.C.; Pepin, R.; et al. Impact of Topical Interventions on the Vaginal Microbiota and Metabolome in Postmenopausal Women: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e225032. [Google Scholar] [CrossRef]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Liu, L.; Liang, L.; Yang, C.; Zhou, Y.; Chen, Y. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes 2021, 13, 1902718. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Tang, Z.; Huang, Y.; Huang, M.; Liu, H.; Ziebolz, D.; Schmalz, G.; Jia, B.; Zhao, J. More Than Just a Periodontal Pathogen -the Research Progress on Fusobacterium nucleatum. Front. Cell Infect. Microbiol. 2022, 12, 815318. [Google Scholar] [CrossRef]
- Rahmati, E.; Martin, V.; Wong, D.; Sattler, F.; Petterson, J.; Ward, P.; Butler-Wu, S.M.; She, R.C. Facklamia Species as an Underrecognized Pathogen. Open Forum Infect. Dis. 2017, 4, ofw272. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D.; Appah, M.; Dean, M.N.; Sesler, C.L.; Factor, S.A.; Molho, E.; Zabetian, C.P.; Standaert, D.G.; Payami, H. Characterizing dysbiosis of gut microbiome in PD: Evidence for overabundance of opportunistic pathogens. npj Parkinsons Dis. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Toh, E.; Xing, Y.; Gao, X.; Jordan, S.J.; Batteiger, T.A.; Batteiger, B.E.; Van Der Pol, B.; Muzny, C.A.; Gebregziabher, N.; Williams, J.A.; et al. Sexual behavior shapes male genitourinary microbiome composition. Cell Rep. Med. 2023, 4, 100981. [Google Scholar] [CrossRef]
- Akiyama, K.; Nishioka, K.; Khan, K.N.; Tanaka, Y.; Mori, T.; Nakaya, T.; Kitawaki, J. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am. J. Reprod. Immunol. 2019, 82, e13147. [Google Scholar] [CrossRef]
- Lombardi, F.; Augello, F.R.; Palumbo, P.; Bonfili, L.; Artone, S.; Altamura, S.; Sheldon, J.M.; Latella, G.; Cifone, M.G.; Eleuteri, A.M.; et al. Bacterial Lysate from the Multi-Strain Probiotic SLAB51 Triggers Adaptative Responses to Hypoxia in Human Caco-2 Intestinal Epithelial Cells under Normoxic Conditions and Attenuates LPS-Induced Inflammatory Response. Int. J. Mol. Sci. 2023, 24, 8134. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Sashihara, T.; Hosono, A.; Kaminogawa, S.; Uchida, M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 2011, 63, 205–210. [Google Scholar] [CrossRef]
- Molina, N.M.; Sola-Leyva, A.; Saez-Lara, M.J.; Plaza-Diaz, J.; Tubic-Pavlovic, A.; Romero, B.; Clavero, A.; Mozas-Moreno, J.; Fontes, J.; Altmae, S. New Opportunities for Endometrial Health by Modifying Uterine Microbial Composition: Present or Future? Biomolecules 2020, 10, 593. [Google Scholar] [CrossRef]
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183. [Google Scholar] [CrossRef]
- Ghanei, N.; Rezaei, N.; Amiri, G.A.; Zayeri, F.; Makki, G.; Nasseri, E. The probiotic supplementation reduced inflammation in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J. Funct. Foods 2018, 42, 306–311. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
| Variable | Level | Overall | Control | Endometriosis | p-Value |
|---|---|---|---|---|---|
| 182 | 74 | 108 | |||
| Age (mean (SD)) | - | 35.5 (6.8) | 36.6 (7.2) | 34.9 (6.4) | 0.117 |
| BMI (mean (SD)) | - | 24.4 (3.7) | 24.0 (2.5) | 24.7 (4.4) | 0.249 |
| Sampling site (N (%)) | Rectal | 79 (43.4) | 31 (41.9) | 48 (44.4) | 0.850 |
| Vaginal | 103 (56.6) | 43 (58.1) | 60 (55.6) | ||
| Menstrual Cycle Stage (N (%)) | Follicular | 86 (53.4%) | 33 (50.8%) | 53 (55.2%) | 0.580 |
| Menses | 75 (46.6%) | 32 (49.2%) | 43 (44.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torraco, A.; Di Nicolantonio, S.; Cardisciani, M.; Ortu, E.; Pietropaoli, D.; Altamura, S.; Del Pinto, R. Meta-Analysis of 16S rRNA Sequencing Reveals Altered Fecal but Not Vaginal Microbial Composition and Function in Women with Endometriosis. Medicina 2025, 61, 888. https://doi.org/10.3390/medicina61050888
Torraco A, Di Nicolantonio S, Cardisciani M, Ortu E, Pietropaoli D, Altamura S, Del Pinto R. Meta-Analysis of 16S rRNA Sequencing Reveals Altered Fecal but Not Vaginal Microbial Composition and Function in Women with Endometriosis. Medicina. 2025; 61(5):888. https://doi.org/10.3390/medicina61050888
Chicago/Turabian StyleTorraco, Astrid, Sara Di Nicolantonio, Martina Cardisciani, Eleonora Ortu, Davide Pietropaoli, Serena Altamura, and Rita Del Pinto. 2025. "Meta-Analysis of 16S rRNA Sequencing Reveals Altered Fecal but Not Vaginal Microbial Composition and Function in Women with Endometriosis" Medicina 61, no. 5: 888. https://doi.org/10.3390/medicina61050888
APA StyleTorraco, A., Di Nicolantonio, S., Cardisciani, M., Ortu, E., Pietropaoli, D., Altamura, S., & Del Pinto, R. (2025). Meta-Analysis of 16S rRNA Sequencing Reveals Altered Fecal but Not Vaginal Microbial Composition and Function in Women with Endometriosis. Medicina, 61(5), 888. https://doi.org/10.3390/medicina61050888








