The Role of Admission Glucose and Inflammatory Markers in Histopathological Features of Atherosclerotic Plaques in Carotid and Femoro-Popliteal Arteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
- -
- NLR = neutrophils/lymphocytes
- -
- MLR = monocytes/lymphocytes
- -
- PLR = platelets/lymphocytes
2.3. Histopathological Characterization of Atherosclerotic Plaque
2.4. Study Endpoint
2.5. Statistical Analysis
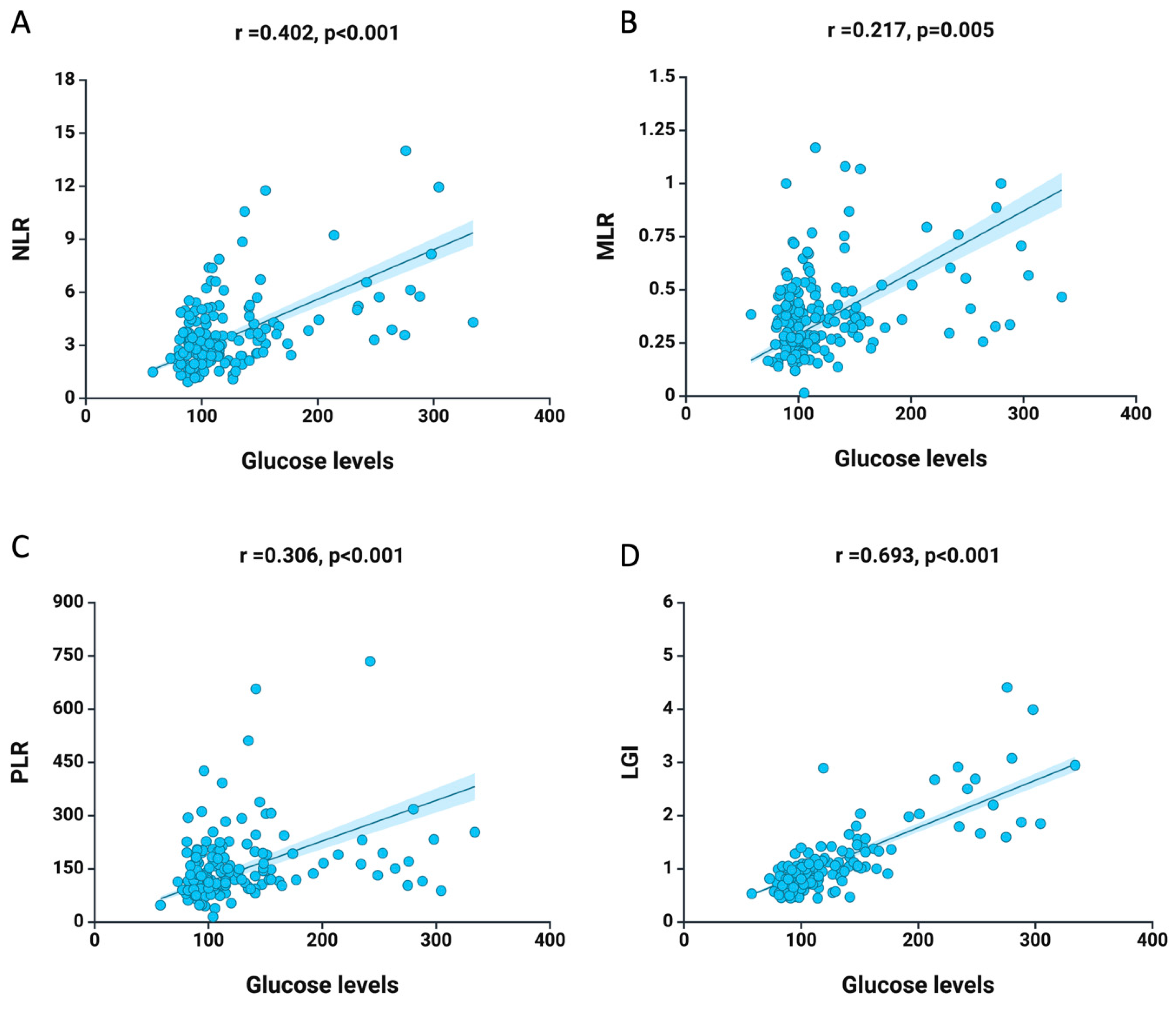
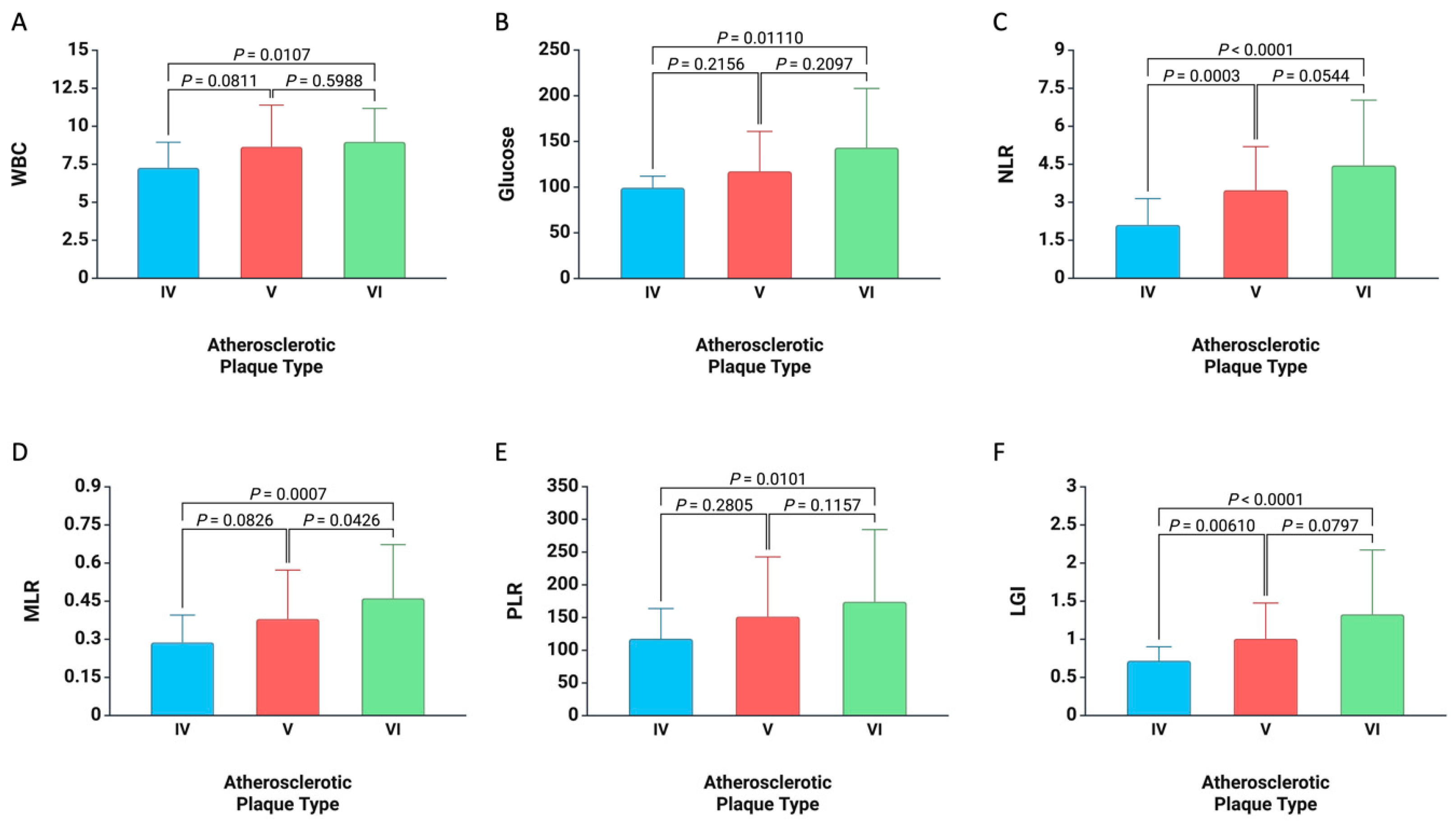
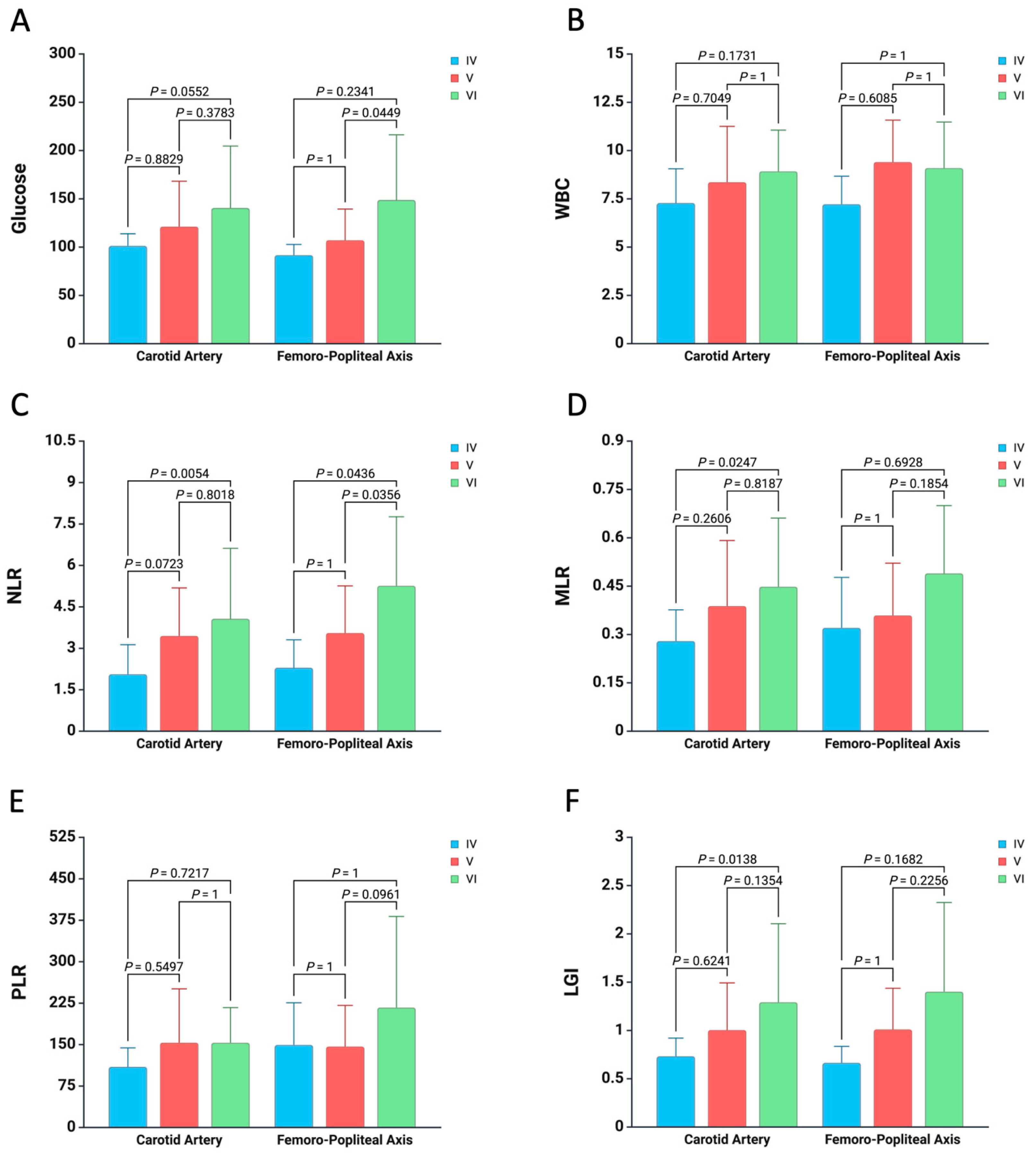
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NLR | neutrophil-to-lymphocyte ratio |
| LMR | lymphocyte-to-monocyte ratio |
| PLR | platelet-to-lymphocyte ratio |
| LGI | leukocyte glucose index |
References
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Fernando, S.; Schwarz, N.; Tan, J.T.; Bursill, C.A.; Psaltis, P.J. Inflammation as a Therapeutic Target in Atherosclerosis. J. Clin. Med. 2019, 8, 1109. [Google Scholar] [CrossRef]
- Stoll, G.; Bendszus, M. Inflammation and Atherosclerosis. Stroke 2006, 37, 1923–1932. [Google Scholar] [CrossRef]
- Libby, P. Inflammation during the Life Cycle of the Atherosclerotic Plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and Atherosclerosis: Signaling Pathways and Therapeutic Intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.-X.; Monaco, C. Therapeutic Strategies Targeting Inflammation and Immunity in Atherosclerosis: How to Proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Sterpetti, A.V. Inflammatory Cytokines and Atherosclerotic Plaque Progression. Therapeutic Implications. Curr. Atheroscler. Rep. 2020, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Moroni, F.; Norata, G.D.; Magnoni, M.; Camici, P.G. Markers of Inflammation Associated with Plaque Progression and Instability in Patients with Carotid Atherosclerosis. Mediat. Inflamm. 2015, 2015, 718329. [Google Scholar] [CrossRef]
- Bhat, T.M.; Afari, M.E.; Garcia, L.A. Neutrophil lymphocyte ratio in peripheral vascular disease: A review. Expert Rev. Cardiovasc. Ther. 2016, 14, 871–875. [Google Scholar] [CrossRef]
- Wijeratne, T.; Menon, R.; Sales, C.; Karimi, L.; Crewther, S. Carotid artery stenosis and inflammatory biomarkers: The role of inflammation-induced immunological responses affecting the vascular systems. Ann. Transl. Med. 2020, 8, 1276. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Anzaldi, M.; Libra, M.; Navolanic, P.M.; Malaponte, G.; Mangano, K.; Quattrocchi, C.; Di Marco, R.; Fiore, V.; Neri, S. Plasma Levels of Inflammatory Biomarkers in Peripheral Arterial Disease: Results of a Cohort Study. Angiology 2016, 67, 870–874. [Google Scholar] [CrossRef]
- Tudurachi, B.-S.; Anghel, L.; Tudurachi, A.; Sascău, R.A.; Stătescu, C. Assessment of Inflammatory Hematological Ratios (NLR, PLR, MLR, LMR and Monocyte/HDL-Cholesterol Ratio) in Acute Myocardial Infarction and Particularities in Young Patients. Int. J. Mol. Sci. 2023, 24, 14378. [Google Scholar] [CrossRef] [PubMed]
- Cosarca, M.C.; Hălmaciu, I.; Muresan, A.V.; Suciu, B.A.; Molnar, C.; Russu, E.; Horvath, E.; Niculescu, R.; Puscasiu, L.; Bacalbaşa, N.; et al. Neutrophil-to-lymphocyte, Platelet-to-lymphocyte and Lymphocyte-to-monocyte Ratios Are Associated with Amputation Rates in Patients with Peripheral Arterial Disease and Diabetes Mellitus Who Underwent Revascularization: A Romanian Regional Center Study. Exp. Ther. Med. 2022, 24, 703. [Google Scholar] [CrossRef]
- Liu, J.; Ao, W.; Zhou, J.; Luo, P.; Wang, Q.; Xiang, D. The Correlation between PLR-NLR and Prognosis in Acute Myocardial Infarction. Am. J. Transl. Res. 2021, 13, 4892–4899. [Google Scholar] [PubMed]
- Wang, H.; Li, L.; Ma, Y. Platelet-to-lymphocyte ratio a potential prognosticator in acute myocardial infarction: A prospective longitudinal study. Clin. Cardiol. 2023, 46, 632–638. [Google Scholar] [CrossRef]
- Song, S.-Y.; Zhao, X.-X.; Rajah, G.; Hua, C.; Kang, R.; Han, Y.; Ding, Y.; Meng, R. Clinical Significance of Baseline Neutrophil-to-Lymphocyte Ratio in Patients with Ischemic Stroke or Hemorrhagic Stroke: An Updated Meta-Analysis. Front. Neurol. 2019, 10, 1032. [Google Scholar] [CrossRef]
- Li, W.; Hou, M.; Ding, Z.; Liu, X.; Shao, Y.; Li, X. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 686983. [Google Scholar] [CrossRef]
- Pasqui, E.; de Donato, G.; Lazzeri, E.; Molino, C.; Galzerano, G.; Giubbolini, M.; Palasciano, G. High Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios Are Associated with a Higher Risk of Hemodialysis Vascular Access Failure. Biomedicines 2022, 10, 2218. [Google Scholar] [CrossRef]
- Paquissi, F.C. The Role of Inflammation in Cardiovascular Diseases: The Predictive Value of Neutrophil–Lymphocyte Ratio as a Marker in Peripheral Arterial Disease. Ther. Clin. Risk Manag. 2016, 12, 851–860. [Google Scholar] [CrossRef]
- Gary, T.; Pichler, M.; Belaj, K.; Hafner, F.; Gerger, A.; Froehlich, H.; Eller, P.; Rief, P.; Hackl, G.; Pilger, E.; et al. Platelet-to-lymphocyte ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS ONE 2013, 8, e67688. [Google Scholar] [CrossRef]
- Demirdal, T.; Sen, P. The Significance of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio and Lymphocyte-Monocyte Ratio in Predicting Peripheral Arterial Disease, Peripheral Neuropathy, Osteomyelitis and Amputation in Diabetic Foot Infection. Diabetes Res. Clin. Pract. 2018, 144, 118–125. [Google Scholar] [CrossRef] [PubMed]
- van Haelst, S.T.; Haitjema, S.; de Vries, J.P.P.; Moll, F.L.; Pasterkamp, G.; den Ruijter, H.M.; de Borst, G.J. Patients with Diabetes Differ in Atherosclerotic Plaque Characteristics and Have Worse Clinical Outcome after Iliofemoral Endarterectomy Compared with Patients without Diabetes. J. Vasc. Surg. 2017, 65, 414–421.e5. [Google Scholar] [CrossRef]
- Xia, R.; Fan, S.; Jian, H.; Lei, C.; Wendan, M.; Chenxu, W.; Yicheng, F.; Tavengana, G.; Mingfei, J.; Huan, W.; et al. Effect of Fasting Glucose Levels on Carotid Intima-Media Thickness in Premenopausal versus Postmenopausal Women. Arch. Endocrinol. Metab. 2024, 68, e230110. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Hibi, K.; Gohbara, M.; Kataoka, S.; Takano, K.; Akiyama, E.; Matsuzawa, Y.; Saka, K.; Maejima, N.; Endo, M.; et al. Association between Blood Glucose Variability and Coronary Plaque Instability in Patients with Acute Coronary Syndromes. Cardiovasc. Diabetol. 2015, 14, 111. [Google Scholar] [CrossRef]
- Çakır, M.O.; Gören, M.T.; Gören, T. Comparison of Atherosclerotic Plaque Compositions in Diabetic and Non-Diabetic Patients. Cureus 2023, 15, e45721. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, V.P.W.; Peeters, W.; van Lammeren, G.W.; Howard, D.P.J.; de Vries, J.-P.P.M.; Jan de Borst, G.; Redgrave, J.N.; Kemperman, H.; Schalkwijk, C.G.; den Ruijter, H.M.; et al. Type 2 Diabetes Is Not Associated with an Altered Plaque Phenotype among Patients Undergoing Carotid Revascularization. A Histological Analysis of 1455 Carotid Plaques. Atherosclerosis 2014, 235, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Arbănași, E.-M.; Kaller, R.; Mureșan, A.V.; Voidăzan, S.; Arbănași, E.-M.; Russu, E. Impact of COVID-19 Pandemic on Vascular Surgery Unit Activity in Central Romania. Front. Surg. 2022, 9, 883935. [Google Scholar] [CrossRef]
- Rastogi, A.; Dogra, H.; Jude, E.B. COVID-19 and Peripheral Arterial Complications in People with Diabetes and Hypertension: A Systematic Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102204. [Google Scholar] [CrossRef]
- Noory, E.; Böhme, T.; Salm, J.; Beschorner, U.; Westermann, D.; Zeller, T. Impact of COVID-19 Pandemic on Clinical Care of Peripheral Arterial Disease Patients: A Single-Center Experience. J. Clin. Med. 2023, 12, 890. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Florea, E.; Arbănași, E.-M.; Bartus, R.; Arbănași, E.-M.; Ion, A.P.; Cordoș, B.A.; Halatiu, V.B.; Niculescu, R.; Stoian, A.; et al. Elevated Leukocyte Glucose Index Is Associated with Long-Term Arteriovenous Fistula Failure in Dialysis Patients. J. Clin. Med. 2024, 13, 2037. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.; Tan, S.; Qu, X.; Huang, W.; Cai, J.; You, J.; Fu, X.; He, Y.; Yang, H. The Association between Immunoinflammatory Biomarkers NLR, PLR, LMR and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Clin. Exp. Med. 2025, 25, 39. [Google Scholar] [CrossRef]
- Mindrescu, N.M.; Guja, C.; Jinga, V.; Ispas, S.; Curici, A.; Nelson Twakor, A.; Pantea Stoian, A.M. Interactions between Gut Microbiota and Oral Antihyperglycemic Drugs: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3540. [Google Scholar] [CrossRef]
- Imtiaz, F.; Shafique, K.; Mirza, S.S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil Lymphocyte Ratio as a Measure of Systemic Inflammation in Prevalent Chronic Diseases in Asian Population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef]
- Karaye, K.M.; Sani, M.U. Factors Associated with Poor Prognosis among Patients Admitted with Heart Failure in a Nigerian Tertiary Medical Centre: A Cross-Sectional Study. BMC Cardiovasc. Disord. 2008, 8, 16. [Google Scholar] [CrossRef]
- Pintea-Trifu, M.-L.; Balici, S.-Ş.; Vică, M.L.; Leucuţa, D.-C.; Coman, H.G.; Nemeş, B.; Matei, H.-V. Neutrophil to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, Lymphocyte to Monocyte Ratio and Systemic Inflammatory Index in Sexually Transmitted Diseases. Med. Pharm. Rep. 2024, 97, 162–168. [Google Scholar] [CrossRef]
- Staicovici, S.; Sârbu, V.; Gheorghe, E.; Deacu, S.; Vlase, I.; Ispas, S.; Chirila, S.; Nelson-Twakor, A. Effectiveness of Continuing Post-Surgery Antibiotic Prophylaxis in Reducing Nosocomial Infections—A Literature Review. Chirurgia 2023, 118, 358–369. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, Y.; Wu, J.; Li, C.; Zheng, L.; Zhu, B.; Min, Y.; Ling, T.; Liu, X. Association between Systemic Inflammatory Indicators with the Survival of Chronic Kidney Disease: A Prospective Study Based on NHANES. Front. Immunol. 2024, 15, 1365591. [Google Scholar] [CrossRef]
- Bajari, R.; Tak, S. Predictive Prognostic Value of Neutrophil-Lymphocytes Ratio in Acute Coronary Syndrome. Indian Heart J. 2017, 69 (Suppl. 1), S46–S50. [Google Scholar] [CrossRef]
- Li, X.; Weber, N.C.; Cohn, D.M.; Hollmann, M.W.; DeVries, J.H.; Hermanides, J.; Preckel, B. Effects of Hyperglycemia and Diabetes Mellitus on Coagulation and Hemostasis. J. Clin. Med. 2021, 10, 2419. [Google Scholar] [CrossRef]
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, A.J.G.; Maggio, M.; Dixit, V.D.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric Acid and Inflammatory Markers. Eur. Heart J. 2006, 27, 1174–1181. [Google Scholar] [CrossRef]
- Herisson, F.; Heymann, M.F.; Chétiveaux, M.; Charrier, C.; Battaglia, S.; Pilet, P.; Rouillon, T.; Krempf, M.; Lemarchand, P.; Heymann, D.; et al. Carotid and femoral atherosclerotic plaques show different morphology. Atherosclerosis 2011, 216, 348–354. [Google Scholar] [CrossRef]
- Cunnane, E.M.; Mulvihill, J.J.E.; Barrett, H.E.; Hennessy, M.M.; Kavanagh, E.G.; Walsh, M.T. Mechanical properties and composition of carotid and femoral atherosclerotic plaques: A comparative study. J. Biomech. 2016, 49, 3697–3704. [Google Scholar] [CrossRef]
- Liao, M.; Liu, L.; Bai, L.; Wang, R.; Liu, Y.; Zhang, L.; Han, J.; Li, Y.; Qi, B. Correlation between novel inflammatory markers and carotid atherosclerosis: A retrospective case-control study. PLoS ONE 2024, 19, e0303869. [Google Scholar] [CrossRef]
- Corriere, T.; Di Marca, S.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Stancanelli, B.; Malatino, L. Neutrophil-to-Lymphocyte Ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 23–27. [Google Scholar] [CrossRef]
- Song, J.E.; Hwang, J.I.; Ko, H.J.; Park, J.Y.; Hong, H.E.; Kim, A.S. Exploring the Correlation between Systemic Inflammatory Markers and Carotid Atherosclerosis Indices in Middle-Aged Adults: A Cross-Sectional Study. J. Cardiovasc. Dev. Dis. 2024, 11, 73. [Google Scholar] [CrossRef]
| Variables | All Patients n = 165 | |
|---|---|---|
| Age, mean ± SD | 65.68 ± 8.58 | |
| Male, no. (%) | 119 (72.12%) | |
| Female, no. (%) | 46 (27.88%) | |
| Comorbidities and Risk factors, no. (%) | ||
| Hypertension | 141 (85.45%) | |
| Atrial Fibrillation | 18 (10.9%) | |
| Diabetes | 54 (32.72%) | |
| Ischemic Heart Disease | 96 (58.18%) | |
| Chronic Heart Failure | 48 (29.09%) | |
| Chronic Kidney Disease | 11 (6.67%) | |
| Active Smoking | 61 (36.96%) | |
| Dyslipidemia | 44 (26.67%) | |
| Obesity | 37 (22.42%) | |
| Artery Site Harvest and Atherosclerotic Type, no. (%) | ||
| Carotid Artery | 119 (72.12%) | |
| Femoro-Popliteal Axis | Common Femoral Artery | 19 (11.51%) |
| Superficial Femoral Artery | 22 (13.33%) | |
| Popliteal Artery | 5 (3.03%) | |
| Atherosclerotic Plaque | Type IV | 20 (12.12%) |
| Type V | 93 (56.36%) | |
| Type VI | 52 (31.52%) | |
| Chronic Medication, No. (%) | ||
| No treatment, no. (%) | 9 (5.45%) | |
| Only Anticoagulant, no. (%) | 7 (4.24%) | |
| Only Antiaggregant, no. (%) | 68 (41.21%) | |
| Double Therapy, no. (%) | 81 (49.1%) | |
| Variables | Carotid Artery n = 119 | Femoro-Popliteal Axis n = 46 | p Value | |
|---|---|---|---|---|
| Age, mean ± SD | 66.33 ± 7.96 | 64.02 ± 9.91 | 0.117 | |
| Male, no. (%) | 79 (66.38%) | 40 (86.95%) | 0.008 | |
| Female, no. (%) | 40 (33.62%) | 6 (13.05%) | ||
| Comorbidities and Risk factors, no. (%) | ||||
| Hypertension | 104 (87.39%) | 46 (100%) | 0.255 | |
| Atrial Fibrillation | 11 (9.24%) | 7 (15.22%) | 0.270 | |
| Diabetes | 40 (33.61%) | 14 (30.43%) | 0.696 | |
| Ischemic Heart Disease | 66 (55.46%) | 30 (65.22%) | 0.255 | |
| Chronic Heart Failure | 30 (25.21%) | 18 (39.13%) | 0.078 | |
| Chronic Kidney Disease | 10 (8.40%) | 1 (2.17%) | 0.150 | |
| Active Smoking | 47 (39.49%) | 14 (30.43%) | 0.322 | |
| Dyslipidemia | 24 (20.17%) | 20 (43.48%) | 0.002 | |
| Obesity | 28 (23.53%) | 9 (19.56%) | 0.629 | |
| Atherosclerotic Plaque | Type IV | 16 (13.44%) | 4 (8.69%) | 0.659 |
| Type V | 67 (56.30%) | 26 (56.52%) | ||
| Type VI | 36 (30.25%) | 16 (34.78%) | ||
| Laboratory Data, mean ± SD | ||||
| Hemoglobin g/dL | 13.53 ± 1.71 | 12.91 ± 2.46 | 0.094 | |
| Hematocrit % | 40.39 ± 4.92 | 39.09 ± 7.11 | 0.273 | |
| WBC | 8.41 ± 2.61 | 9.03 ± 2.21 | 0.075 | |
| Creatinine (mg/dL) | 0.98 ± 0.33 | 0.89 ± 0.19 | 0.137 | |
| BUN (mg/dL) | 41.29 ± 20.38 | 34.74 ± 10.48 | 0.159 | |
| Glucose (mg/dL) | 125.18 ± 51.92 | 118.44 ± 48.94 | 0.114 | |
| Neutrophils × 103/uL | 5.63 ± 2.35 | 6.25 ± 1.97 | 0.423 | |
| Lymphocytes × 103/uL | 1.85 ± 0.71 | 1.90 ± 0.65 | 0.020 | |
| Monocyte × 103/uL | 0.65 ± 0.28 | 0.69 ± 0.24 | 0.450 | |
| PLT × 103/uL | 240.72 ± 82.86 | 284.61 ± 114.83 | 0.028 | |
| NLR | 3.45 ± 2.02 | 4.04 ± 2.21 | 0.121 | |
| MLR | 0.39 ± 0.21 | 0.40 ± 0.19 | 0.518 | |
| PLR | 147.66 ± 82.72 | 172.47 ± 119.63 | 0.246 | |
| LGI | 1.07 ± 0.62 | 1.08 ± 0.62 | 0.685 | |
| Chronic Medication, No. (%) | ||||
| No treatment, no. (%) | 4 (3.36%) | 5 (10.87%) | <0.001 | |
| Only Anticoagulant, no. (%) | 2 (1.68%) | 5 (10.87%) | ||
| Only Antiaggregant, no. (%) | 65 (54.62%) | 3 (6.52%) | ||
| Double Therapy, no. (%) | 48 (40.33%) | 33 (71.74%) | ||
| Variables | Cut-Off | AUC | Std. Error | 95% CI | Sensitivity | Specificity | p Value |
|---|---|---|---|---|---|---|---|
| Unstable Atherosclerotic Plaque | |||||||
| WBC | 9.03 | 0.592 | 0.048 | 0.499–0.685 | 53.8% | 68.1% | 0.053 |
| Glucose | 134.75 | 0.614 | 0.050 | 0.515–0.712 | 42.3% | 82.3% | 0.024 |
| NLR | 2.79 | 0.667 | 0.043 | 0.582–0.752 | 80.8% | 49.6% | <0.001 |
| MLR | 0.29 | 0.651 | 0.045 | 0.562–0.740 | 82.7% | 43.4% | 0.001 |
| PLR | 122.08 | 0.625 | 0.046 | 0.535–0.715 | 71.2% | 55.8% | 0.006 |
| LGI | 0.95 | 0.650 | 0.048 | 0.556–0.743 | 65.4% | 62.8% | 0.002 |
| Variables | Unstable Atherosclerotic Plaque | ||
|---|---|---|---|
| OR | 95% CI | p Value | |
| Female | 0.98 | 0.46–2.09 | 0.968 |
| Atrial Fibrillation | 3.71 | 1.36–10.17 | 0.010 |
| Ischemic Heart Disease | 1.80 | 0.88–3.67 | 0.106 |
| Diabetes | 1.61 | 0.79–3.25 | 0.185 |
| Active Smoking | 1.45 | 0.72–2.89 | 0.300 |
| Obesity | 2.11 | 0.97–4.56 | 0.057 |
| WBC | 1.25 * | 0.91–1.73 | 0.184 |
| Glucose | 1.73 * | 1.23–2.43 | 0.002 |
| NLR | 1.81 * | 1.25–2.61 | 0.002 |
| MLR | 1.89 * | 1.18–3.02 | 0.007 |
| PLR | 1.34 * | 0.96–1.86 | 0.083 |
| LGI | 1.84 * | 1.26–2.68 | 0.001 |
| Variables | Unstable Atherosclerotic Plaque | |||
|---|---|---|---|---|
| OR * | 95% CI | p Value | ||
| Glucose | Model 1 | 1.74 | 1.22–2.48 | 0.002 |
| Model 2 | 1.75 | 1.21–2.52 | 0.003 | |
| Model 3 | 1.58 | 1.06–2.35 | 0.023 | |
| NLR | Model 1 | 1.81 | 1.25–2.61 | 0.002 |
| Model 2 | 1.85 | 1.26–2.72 | 0.002 | |
| Model 3 | 1.78 | 1.19–2.66 | 0.005 | |
| MLR | Model 1 | 1.88 | 1.18–3.02 | 0.008 |
| Model 2 | 1.82 | 1.13–2.95 | 0.014 | |
| Model 3 | 1.78 | 1.09–2.92 | 0.021 | |
| LGI | Model 1 | 1.84 | 1.26–2.68 | 0.002 |
| Model 2 | 1.88 | 1.27–2.78 | 0.002 | |
| Model 3 | 1.75 | 1.17–2.64 | 0.007 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coșarcă, M.C.; Șincaru, S.V.; Horváth, E.; Sala, D.T.; Lazăr, N.A.; Szanto, L.A.; Harpa, M.M.; Carașcă, C.; Ráduly, G.; Bândea, P.; et al. The Role of Admission Glucose and Inflammatory Markers in Histopathological Features of Atherosclerotic Plaques in Carotid and Femoro-Popliteal Arteries. Medicina 2025, 61, 879. https://doi.org/10.3390/medicina61050879
Coșarcă MC, Șincaru SV, Horváth E, Sala DT, Lazăr NA, Szanto LA, Harpa MM, Carașcă C, Ráduly G, Bândea P, et al. The Role of Admission Glucose and Inflammatory Markers in Histopathological Features of Atherosclerotic Plaques in Carotid and Femoro-Popliteal Arteries. Medicina. 2025; 61(5):879. https://doi.org/10.3390/medicina61050879
Chicago/Turabian StyleCoșarcă, Mircea Cătălin, Suzana Vasilica Șincaru, Emőke Horváth, Daniela Tatiana Sala, Nicolae Alexandru Lazăr, Ludovic Alexandru Szanto, Marius Mihai Harpa, Cosmin Carașcă, Gergő Ráduly, Paula Bândea, and et al. 2025. "The Role of Admission Glucose and Inflammatory Markers in Histopathological Features of Atherosclerotic Plaques in Carotid and Femoro-Popliteal Arteries" Medicina 61, no. 5: 879. https://doi.org/10.3390/medicina61050879
APA StyleCoșarcă, M. C., Șincaru, S. V., Horváth, E., Sala, D. T., Lazăr, N. A., Szanto, L. A., Harpa, M. M., Carașcă, C., Ráduly, G., Bândea, P., & Mureșan, V. A. (2025). The Role of Admission Glucose and Inflammatory Markers in Histopathological Features of Atherosclerotic Plaques in Carotid and Femoro-Popliteal Arteries. Medicina, 61(5), 879. https://doi.org/10.3390/medicina61050879






