Enhancing Endodontic Outcomes with the Synergistic Microbicidal and Activated Root-Cleansing Technique (SMART): A Novel Approach to Root Canal Irrigation
Abstract
1. Introduction
2. Materials and Methods
2.1. AromaRoot Formulation
2.2. Bacterial Reduction Assessment
2.3. Smear Layer Removal Assessment
2.4. Statistical Analysis
3. Results
3.1. Bacterial Reduction Outcomes
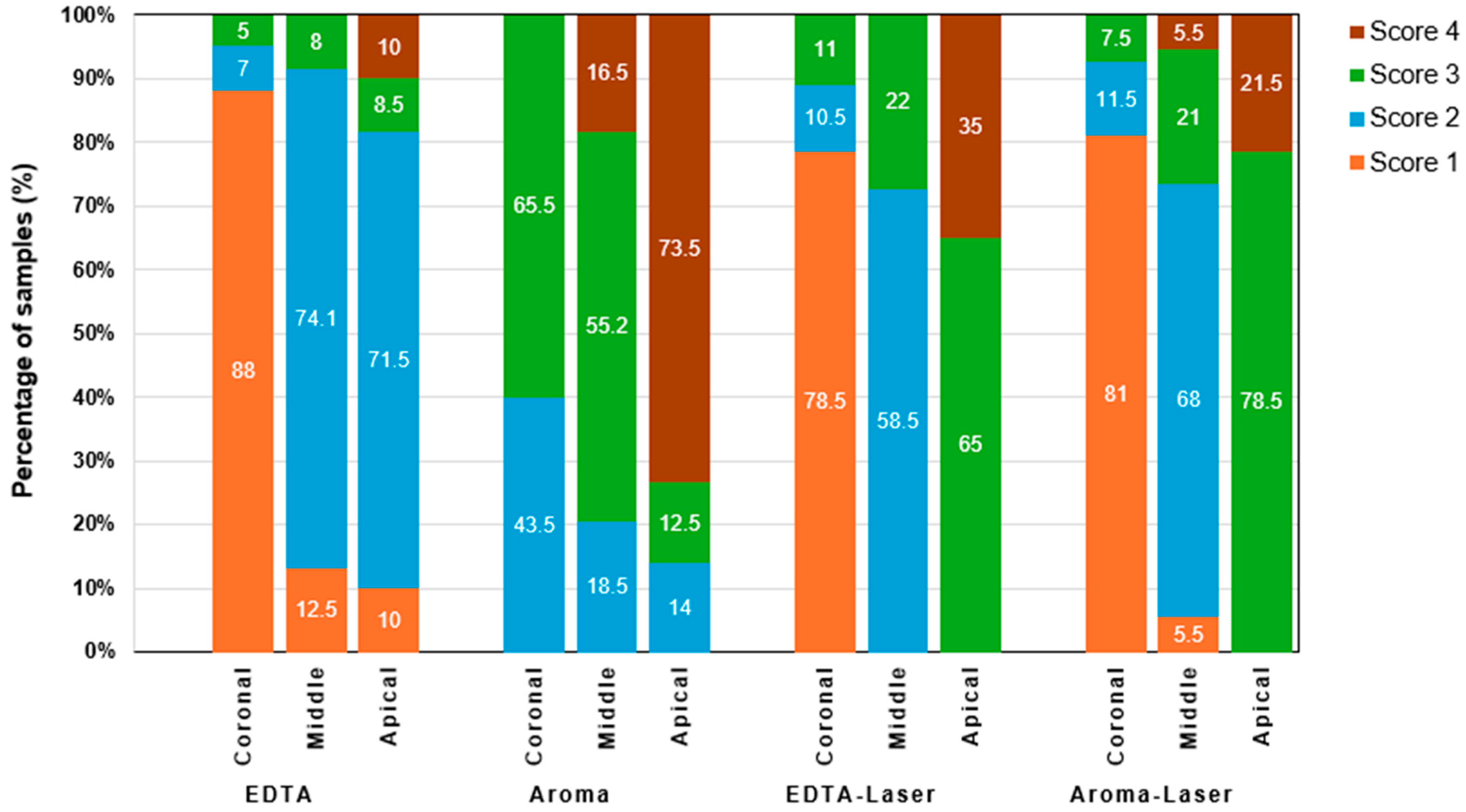
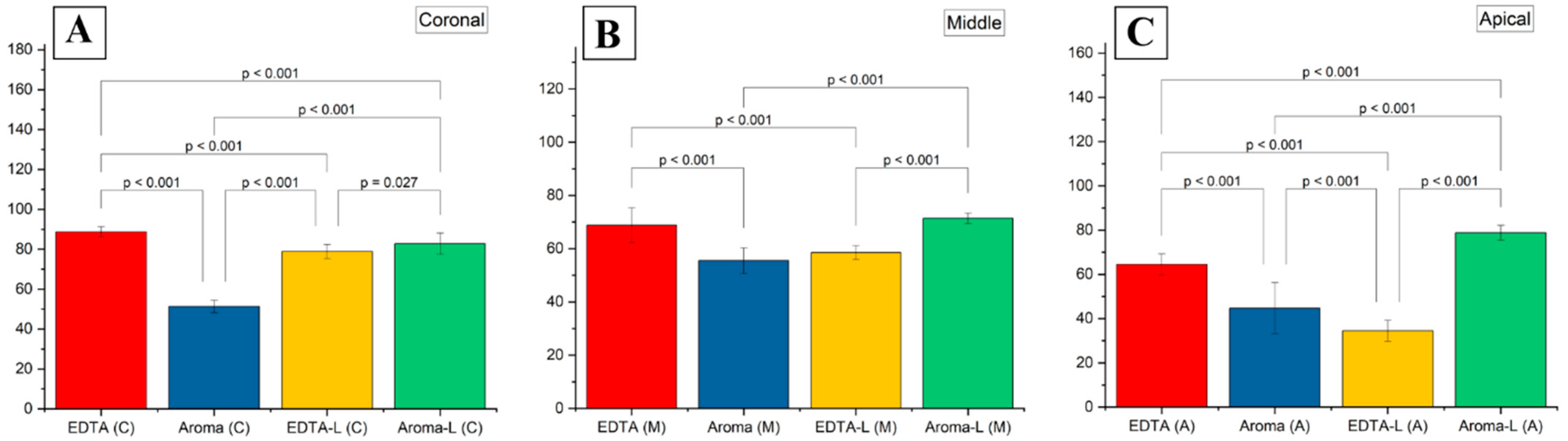
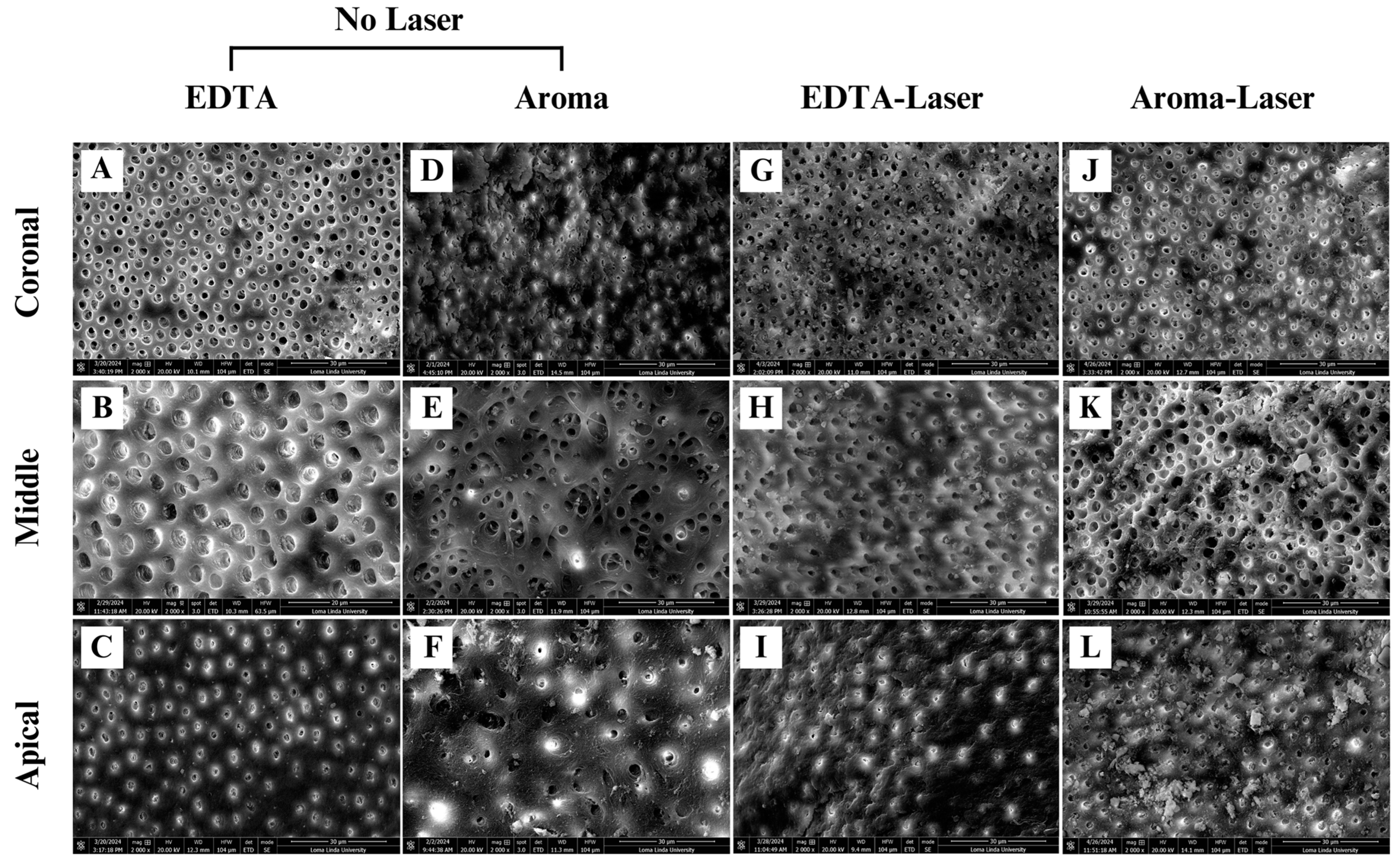
3.2. Smear Layer Removal Outcomes
4. Discussion
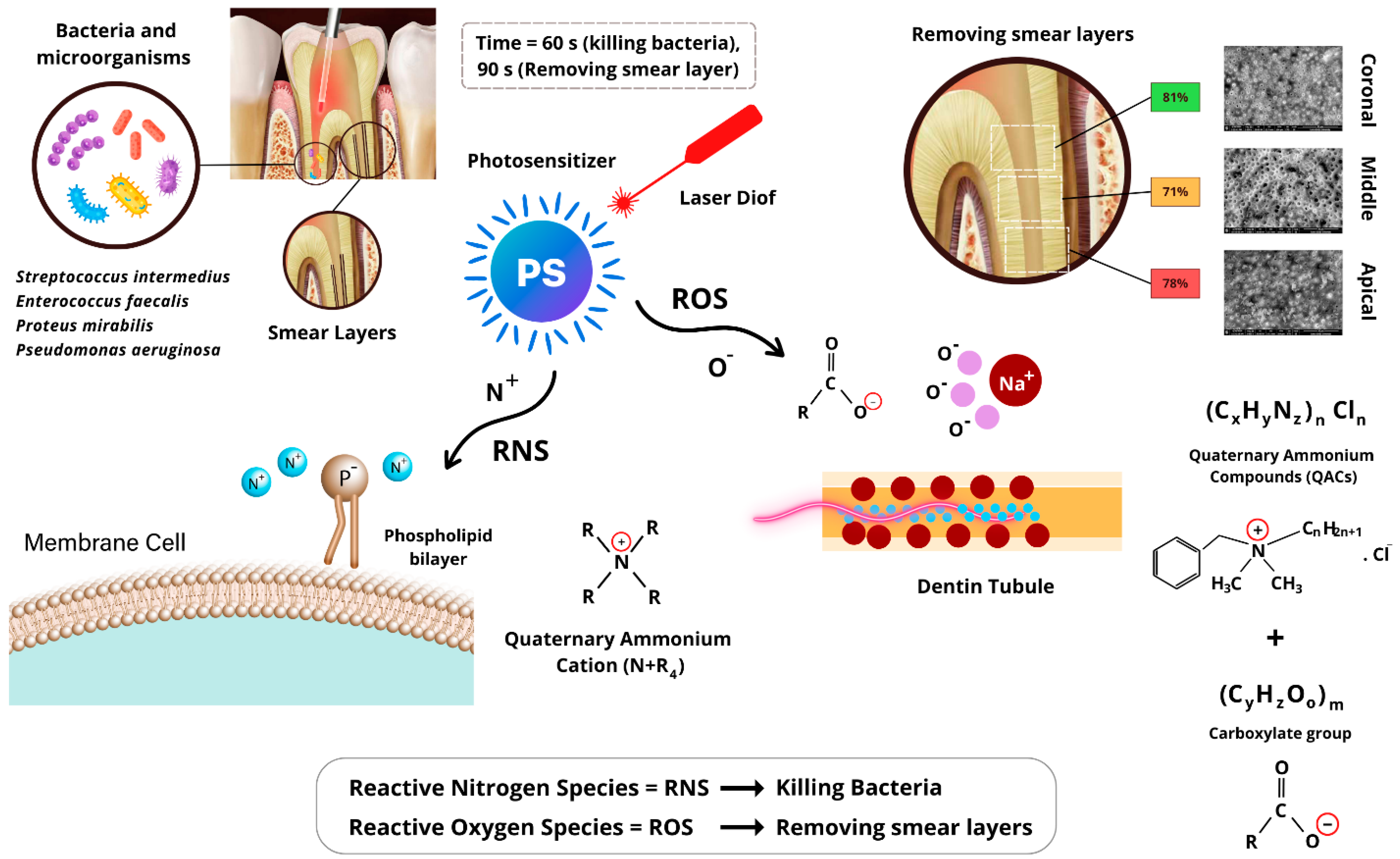
4.1. Mechanism of Action of AromaRoot with Laser Activation
4.2. Bacterial Reduction
4.3. Smear Layer Removal
4.4. Statistical Validation and Clinical Implications
4.5. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EDTA | Ethylenediaminetetraacetic acid |
| NaOCl | Sodium hypochlorite |
| LAI | Laser-activated irrigation |
| QACs | quaternary ammonium compounds |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SMART | Synergistic Microbicidal and Activated Root-cleansing Technique |
References
- Siqueira, J.F. Endodontic infections: Concepts, paradigms, and perspectives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2002, 94, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Hülsmann, M.; Peters, O.A.; Dummer, P.M.H. Mechanical preparation of root canals: Shaping goals, techniques and means. Endod. Top. 2005, 10, 30–76. [Google Scholar] [CrossRef]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in Endodontics. Dent. Clin. N. Am. 2010, 54, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z. Sodium hypochlorite in endodontics: An update review. Int. Dent. J. 2008, 58, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Hülsmann, M.; Heckendorff, M.; Lennon, Á. Chelating agents in root canal treatment: Mode of action and indications for their use. Int. Endod. J. 2003, 36, 810–830. [Google Scholar] [CrossRef] [PubMed]
- Dutner, J.; Mines, P.; Anderson, A. Irrigation Trends among American Association of Endodontists Members: A Web-based Survey. J. Endod. 2012, 38, 37–40. [Google Scholar] [CrossRef]
- Gomes, B.P.F.A.; Ferraz, C.C.R.; ME, V.; Berber, V.B.; Teixeira, F.B.; Souza-Filho, F.J. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int. Endod. J. 2001, 34, 424–428. [Google Scholar] [CrossRef]
- Torabinejad, M.; Khademi, A.A.; Babagoli, J.; Cho, Y.; Ben Johnson, W.; Bozhilov, K.; Kim, J.; Shabahang, S. A New Solution for the Removal of the Smear Layer. J. Endod. 2003, 29, 170–175. [Google Scholar] [CrossRef]
- Stojicic, S.; Zivkovic, S.; Qian, W.; Zhang, H.; Haapasalo, M. Tissue Dissolution by Sodium Hypochlorite: Effect of Concentration, Temperature, Agitation, and Surfactant. J. Endod. 2010, 36, 1558–1562. [Google Scholar] [CrossRef]
- Chia, M.S.Y.; Parolia, A.; Lim, B.S.H.; Jayaraman, J.; Porto, I.C.C.d.M. Effect of QMix irrigant in removal of smear layer in root canal system: A systematic review of in vitro studies. Restor. Dent. Endod. 2020, 45, e28. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Hu, Y.; Huang, F.; Xiao, Y. Quaternary ammonium compounds in dental restorative materials. Dent. Mater. J. 2018, 37, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary Ammonium Salts and Their Antimicrobial Potential: Targets or Nonspecific Interactions? ChemMedChem. 2012, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Ferrer-Luque, C.M.; Espigares-García, M.; Baca, P. Enterococcus faecalis biofilms eradication by root canal irrigants. J. Endod. 2009, 35, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Luque, C.M.; Arias-Moliz, M.T.; González-Rodríguez, M.P.; Baca, P. Antimicrobial Activity of Maleic Acid and Combinations of Cetrimide with Chelating Agents against Enterococcus Faecalis Biofilm. J. Endod. 2010, 36, 1673–1675. [Google Scholar] [CrossRef]
- Almadi, E.M.; Almohaimede, A.A. Natural products in endodontics. Saudi Med. J. 2018, 39, 124–130. [Google Scholar] [CrossRef]
- Vinothkumar, T.S.; Rubin, M.; Balaji, L.; Kandaswamy, D. In vitro evaluation of five different herbal extracts as an antimicrobial endodontic irrigant using real time quantitative polymerase chain reaction. J. Conserv. Dent. 2013, 16, 167–170. [Google Scholar] [CrossRef]
- Gupta-Wadhwa, A.; Wadhwa, J.; Duhan, J. Comparative evaluation of antimicrobial efficacy of three herbal irrigants in reducing intracanal E. faecalis populations: An in vitro study. J. Clin. Exp. Dent. 2016, 8, e230–e235. [Google Scholar] [CrossRef]
- Mathew, J.; Pathrose, S.; Kottoor, J.; Karaththodiyil, R.; Alani, M.; Mathew, J. Evaluation of an Indigenously Prepared Herbal Extract (EndoPam) as an Antimicrobial Endodontic Irrigant: An Ex Vivo Study. J. Int. Oral. Health 2015, 7, 88–91. [Google Scholar]
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Camagay, A.V.; Kendall, N.; Connolly, M.K. Quaternary Ammonium Compound Toxicity; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Stabholz, A.; Sahar-Helft, S.; Moshonov, J. Lasers in endodontics. Dent. Clin. N. Am. 2004, 48, 809–832. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, N.; Franzen, R.; Meister, J.; Vanweersch, L.; Mir, M. Temperature evolution on human teeth root surface after diode laser assisted endodontic treatment. Lasers Med. Sci. 2005, 20, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Blanken, J.; De Moor, R.J.G.; Meire, M.; Verdaasdonk, R. Laser induced explosive vapor and cavitation resulting in effective irrigation of the root canal. Part 1: A visualization study. Lasers Surg. Med. 2009, 41, 514–519. [Google Scholar] [CrossRef]
- George, R.; Walsh, L.J. Apical Extrusion of Root Canal Irrigants When Using Er:YAG and Er,Cr:YSGG Lasers with Optical Fibers: An In Vitro Dye Study. J. Endod. 2008, 34, 706–708. [Google Scholar] [CrossRef]
- George, R.; Walsh, L.J. Performance assessment of novel side firing safe tips for endodontic applications. J. Biomed. Opt. 2011, 16, 048004. [Google Scholar] [CrossRef]
- Radcliffe, C.E.; Potouridou, L.; Qureshi, R.; Habahbeh, N.; Qualtrough, A.; Worthington, H.; Drucker, D.B. Antimicrobial activity of varying concentrations of sodium hypochlorite on the endodontic microorganisms Actinomyces israelii, A. naeslundii, Candida albicans and Enterococcus faecalis. Int. Endod. J. 2004, 37, 438–446. [Google Scholar] [CrossRef]
- Poggio, C.; Arciola, C.R.; Dagna, A.; Florindi, F.; Chiesa, M.; Saino, E.; Imbriani, M.; Visai, L. Photoactivated Disinfection (PAD) in Endodontics: An in vitro Microbiological Evaluation. Int. J. Artif. Organs. 2011, 34, 889–897. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N. Clinical Implications and Microbiology of Bacterial Persistence after Treatment Procedures. J. Endod. 2008, 34, 1291–1301.e3. [Google Scholar] [CrossRef]
| Group | Treatment | Mean (%) | SD (%) | Min (%) | Mdn (%) | Max (%) | Post Hoc |
|---|---|---|---|---|---|---|---|
| 1 | 5.25% NaOCl for 3 min | 98.34 | 1.33 | 96.03 | 98.34 | 99.5 | A |
| 2 | 5.25% NaOCl + 30 s and 60 s laser | 98.5 | 1.54 | 97.23 | 98.5 | 99.45 | A, B |
| 3 | AromaRoot + 60 s laser | 98.78 | 1.4 | 97.08 | 98.78 | 99.65 | C |
| 4 | AromaRoot + NaOCl + 60 s laser | 99 | 1.2 | 97.75 | 99 | 99.85 | C |
| Groups | Treatment | Coronal (%) | Middle (%) | Apical (%) |
|---|---|---|---|---|
| 1 | Control group (17% EDTA) | 89 | 69 | 64.5 |
| 2 | AromaRoot | 51.5 | 55.2 | 43 |
| 3 | EDTA with laser | 78.5 | 58.5 | 34 |
| 4 | AromaRoot with laser | 81 | 71.3 | 78.5 |
| Groups | Coronal (Mean ± SD) | Coronal (Min) | Coronal (Max) | Middle (Mean ± SD) | Middle (Min) | Middle (Max) | Apical (Mean ± SD) | Apical (Min) | Apical (Max) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 88.75 ± 2.5 | 84 | 93 | 68.81 ± 6.5 | 58 | 81 | 64.50 ± 4.8 | 57 | 72 |
| 2 | 51.37 ± 3.2 | 46 | 57 | 55.50 ± 4.8 | 48 | 63 | 44.75 ± 11.5 | 28 | 63 |
| 3 | 78.87 ± 3.7 | 73 | 85 | 58.50 ± 2.6 | 54 | 63 | 34.50 ± 4.8 | 27 | 42 |
| 4 | 82.75 ± 5.3 | 74 | 92 | 71.37 ± 1.96 | 68 | 75 | 78.75 ± 3.4 | 73 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foroughi, M.; Abolmaali, S.; Abedi, H.; Ravenel, T. Enhancing Endodontic Outcomes with the Synergistic Microbicidal and Activated Root-Cleansing Technique (SMART): A Novel Approach to Root Canal Irrigation. Medicina 2025, 61, 874. https://doi.org/10.3390/medicina61050874
Foroughi M, Abolmaali S, Abedi H, Ravenel T. Enhancing Endodontic Outcomes with the Synergistic Microbicidal and Activated Root-Cleansing Technique (SMART): A Novel Approach to Root Canal Irrigation. Medicina. 2025; 61(5):874. https://doi.org/10.3390/medicina61050874
Chicago/Turabian StyleForoughi, Max, Sara Abolmaali, Hamid Abedi, and Theodore Ravenel. 2025. "Enhancing Endodontic Outcomes with the Synergistic Microbicidal and Activated Root-Cleansing Technique (SMART): A Novel Approach to Root Canal Irrigation" Medicina 61, no. 5: 874. https://doi.org/10.3390/medicina61050874
APA StyleForoughi, M., Abolmaali, S., Abedi, H., & Ravenel, T. (2025). Enhancing Endodontic Outcomes with the Synergistic Microbicidal and Activated Root-Cleansing Technique (SMART): A Novel Approach to Root Canal Irrigation. Medicina, 61(5), 874. https://doi.org/10.3390/medicina61050874







